Synthesis of Water-Dispersed Ferrecene/Phenylboronic Acid-Modified Bifunctional Gold Nanoparticles and the Application in Biosensing
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Preparation of Fc-MBA-AuNPs
2.3. Procedure for Avidin Detection
3. Results and Discussion
3.1. Characterization of Fc–MBA–AuNPs
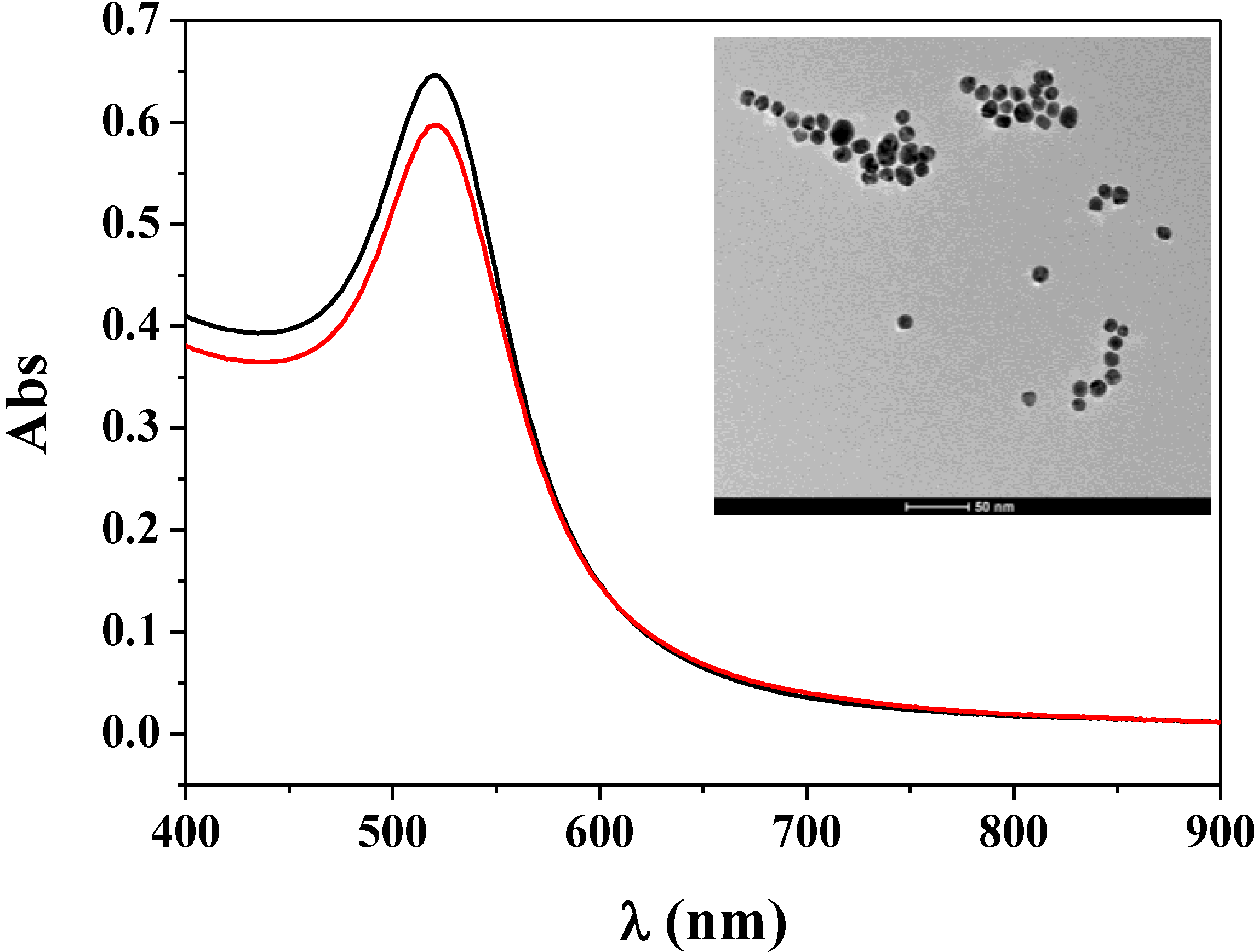
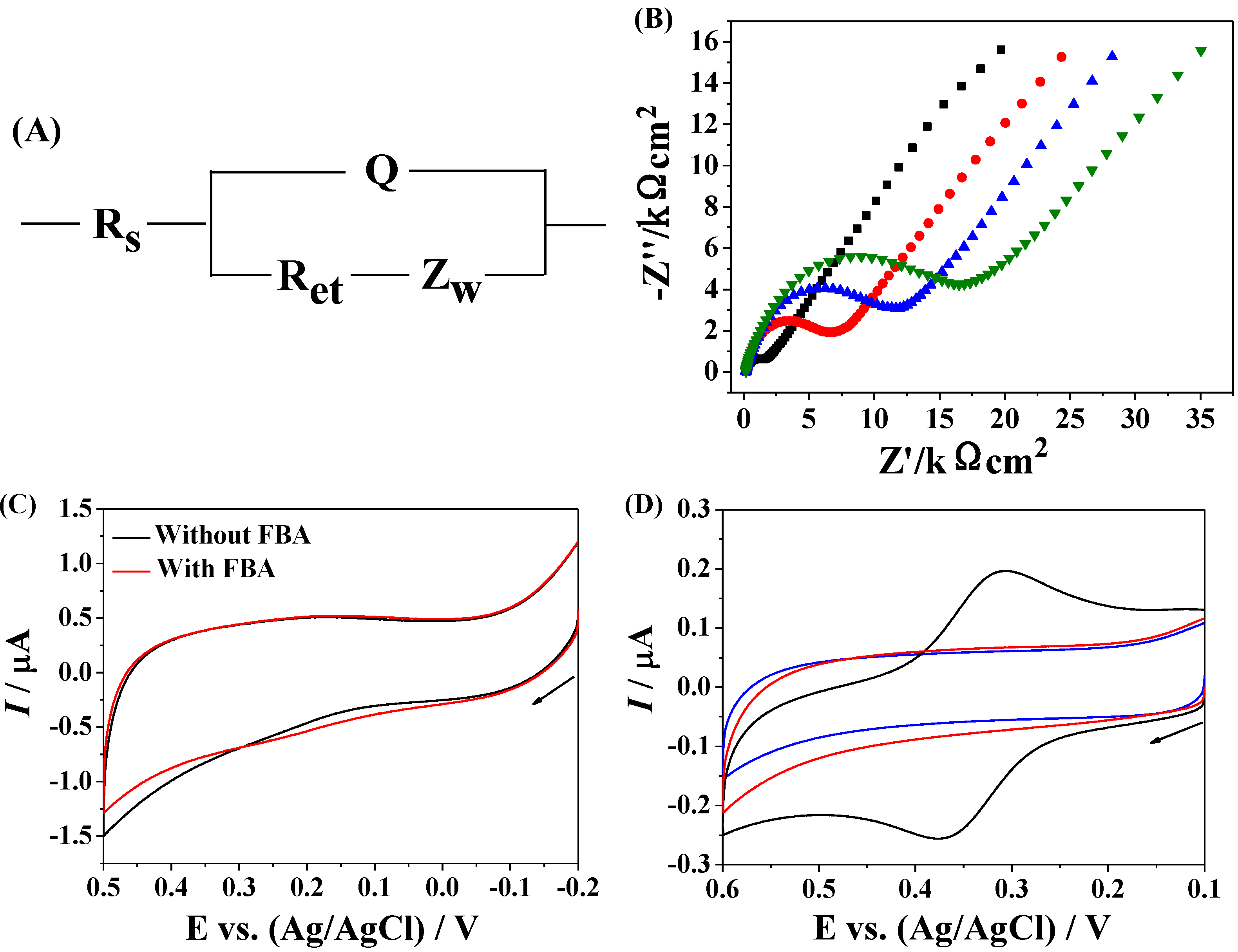
3.2. Application of Fc–MBA–AuNPs in Biosensing


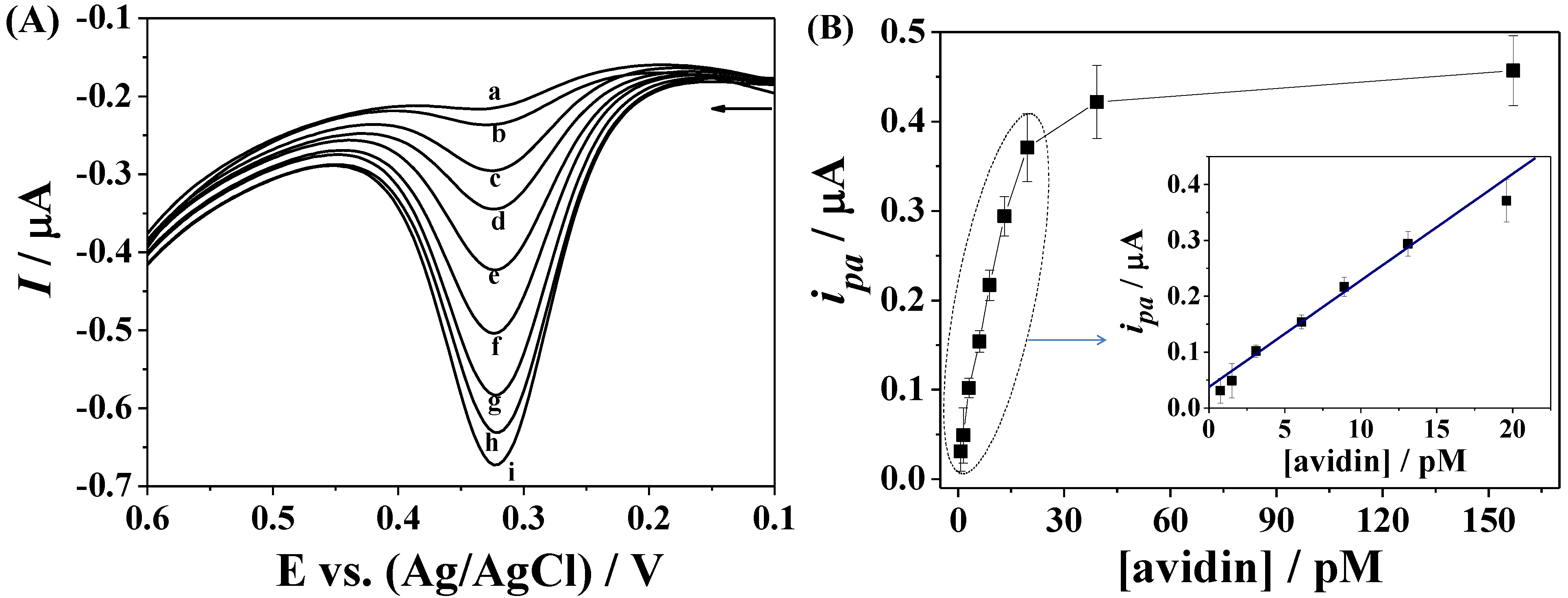
3.3. Amplified Voltammetric Determination of Avidin
3.4. Dependence on Avidin Concentration
4. Conclusions/Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patolsky, F.; Lichtenstein, A.; Willner, I. Electrochemical transduction of liposome-amplified DNA sensing. Angew. Chem. Int. Ed. 2000, 39, 940–943. [Google Scholar] [CrossRef]
- Xia, N.; Ma, F.; Zhao, F.; He, Q.; Du, J.; Li, S.; Chen, J.; Liu, L. Comparing the performances of electrochemical sensors using p-aminophenol redox cycling by different reductants on gold electrodes modified with self-assembled monolayers. Electrochim. Acta 2013, 109, 348–354. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef]
- Yu, S.; Wei, Q.; Du, B.; Wu, D.; Li, H.; Yan, L.; Ma, H.; Zhang, Y. Label-free immunosensor for the detection of kanamycin using Ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens. Bioelectron. 2013, 48, 224–229. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Xiong, P.; Gan, N.; Cui, H.; Zhou, J.; Cao, Y.T.; Hu, F.T.; Li, T.H. Incubation-free electrochemical immunoassay for diethylstilbestrol in milk using gold nanoparticle-antibody conjugates for signal amplification. Microchim. Acta 2014, 181, 453–462. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, H.; Du, B.; Ma, H.; Wu, D.; Wei, Q. A silver–palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol. Biosens. Bioelectron. 2013, 49, 14–19. [Google Scholar] [CrossRef]
- Liu, L.; Du, J.; Li, S.; Yuan, B.; Han, H.; Jing, M.; Xia, N. Amplified voltammetric detection of dopamine using ferrocene-capped gold nanoparticle/streptavidin conjugates. Biosens. Bioelectron. 2013, 41, 730–735. [Google Scholar] [CrossRef]
- Lai, W.; Zhuang, J.; Tang, J.; Chen, G.; Tang, D. One-step electrochemical immunosensing for simultaneous detection of two biomarkers using thionine and ferrocene as distinguishable signal tags. Microchim. Acta 2012, 178, 357–365. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Tu, Q.; Guo, Q.; Zarui, C.S.; Momand, J.; Sun, X.Z.; Zhou, F. Capture of p53 by electrodes modified with consensus DNA duplexes and amplified voltammetric detection using ferrocene-capped gold nanoparticle/streptavidin conjugates. Anal. Chem. 2008, 80, 769–774. [Google Scholar] [CrossRef]
- Deng, D.; Shi, Y.; Feng, H.; Chen, Q.; Li, D.; Liu, L. Label-free electrochemical sensing platform for the detection of protease. Int. J. Electrochem. Sci. 2013, 8, 6933–6940. [Google Scholar]
- Liu, G.; Wang, J.; Wunschel, D.S.; Lin, Y. Electrochemical proteolytic beacon for detection of matrix metalloproteinase activities. J. Am. Chem. Soc. 2006, 128, 12382–12383. [Google Scholar] [CrossRef]
- Egawa, Y.; Seki, T.; Takahashi, S.; Anzai, J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C 2011, 31, 1257–1264. [Google Scholar]
- Wang, X.; Xia, N.; Liu, L. Boronic acid-based approach for separation and immobilization of glycoproteins and its application in sensing. Int. J. Mol. Sci. 2013, 14, 20890–20912. [Google Scholar]
- Jiang, Y.; Ma, Y. A fast capillary electrophoresis method for separation and quantification of modified nucleosides in urinary samples. Anal. Chem. 2009, 81, 6474–6480. [Google Scholar]
- Liu, L.; Xia, N.; Liu, H.; Kang, X.; Liu, X.; Xue, C.; He, X. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar]
- Hazot, P.; Delair, T.; Elaïssari, A.; Chapel, J.-P.; Pichot, C. Functionalization of poly(N-ethylmethacrylamide) thermosensitive particles by phenylboronic acid. Colloid Polym. Sci. 2002, 280, 637–646. [Google Scholar] [CrossRef]
- Rahman, M.M.; Elaїssari, A. Nucleic acid sample preparation for in vitro molecular diagnosis: From conventional techniques to biotechnology. Drug Discov.Today 2012, 17, 1199–1207. [Google Scholar] [CrossRef]
- Rahman, M.M.; Elaїssari, A. Multi-stimuli responsive magnetic core-shell particles: Synthesis, characterization and specific RNA recognition. J. Colloid Sci. Biotechnol. 2012, 1, 3–15. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Liu, S.; Du, Z.; Li, P.; Li, F. Sensitive colorimetric visualization of dihydronicotinamide adenine dinucleotide based on anti-aggregation of gold nanoparticles via boronic acid–diol binding. Biosens. Bioelectron. 2012, 35, 443–446. [Google Scholar] [CrossRef]
- Falabella, J.B.; Cho, T.J.; Ripple, D.C.; Hackley, V.A.; Tarlov, M.J. Characterization of gold nanoparticles modified with single-stranded DNA using analytical ultracentrifugation and dynamic light scattering. Langmuir 2010, 26, 12740–12747. [Google Scholar]
- Xia, N.; Deng, D.; Zhang, L.; Yuan, B.; Jing, M.; Du, J.; Liu, L. Sandwich-type electrochemical biosensor for glycoproteins detection based on dual-amplification of boronic acid-gold nanoparticles and dopamine-gold nanoparticles. Biosens. Bioelectron. 2013, 43, 155–159. [Google Scholar] [CrossRef]
- Grubisha, D.S.; Lipert, R.J.; Park, H.-Y.; Driskell, J.; Porter, M.D. Femtomolar detection of prostate-specific antigen: An immunoassay based on surface-enhanced raman scattering and immunogold labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef]
- Kannagi, R.; Izawa, M.; Koike, T.; Miyazaki, K.; Kimura, N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004, 95, 377–384. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer construction of protein architectures through avidin–biotin and lectin–sugar interactions for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 1749–1758. [Google Scholar] [CrossRef]
- Liu, L.; Xia, N.; Du, J.; He, Q.; Qin, Y.; Li, H.; Li, C. A simple and rapid method for probing of isomerization of glucose to fructose with ferroceneboronic acid. Int. J. Electrochem. Sci. 2013, 8, 9163–9170. [Google Scholar]
- Liu, S.; Wollenberger, U.; Katterle, M.; Scheller, F.W. Ferroceneboronic acid-based amperometric biosensor for glycated hemoglobin. Sens. Actuators B 2006, 113, 623–629. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Z. Multiplexed electrochemical immunoassay of biomarkers using chitosan nanocomposites. Biosens. Bioelectron. 2014, 55, 343–349. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xing, Y.; Liu, L.; Zhao, D.; Yang, Y.; Chu, X. Synthesis of Water-Dispersed Ferrecene/Phenylboronic Acid-Modified Bifunctional Gold Nanoparticles and the Application in Biosensing. Materials 2014, 7, 5554-5564. https://doi.org/10.3390/ma7085554
Xing Y, Liu L, Zhao D, Yang Y, Chu X. Synthesis of Water-Dispersed Ferrecene/Phenylboronic Acid-Modified Bifunctional Gold Nanoparticles and the Application in Biosensing. Materials. 2014; 7(8):5554-5564. https://doi.org/10.3390/ma7085554
Chicago/Turabian StyleXing, Yun, Lin Liu, Danqing Zhao, Yixin Yang, and Xiaoran Chu. 2014. "Synthesis of Water-Dispersed Ferrecene/Phenylboronic Acid-Modified Bifunctional Gold Nanoparticles and the Application in Biosensing" Materials 7, no. 8: 5554-5564. https://doi.org/10.3390/ma7085554



