Liquid Crystalline Network Composites Reinforced by Silica Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Glass and Isotropic Transition Temperatures
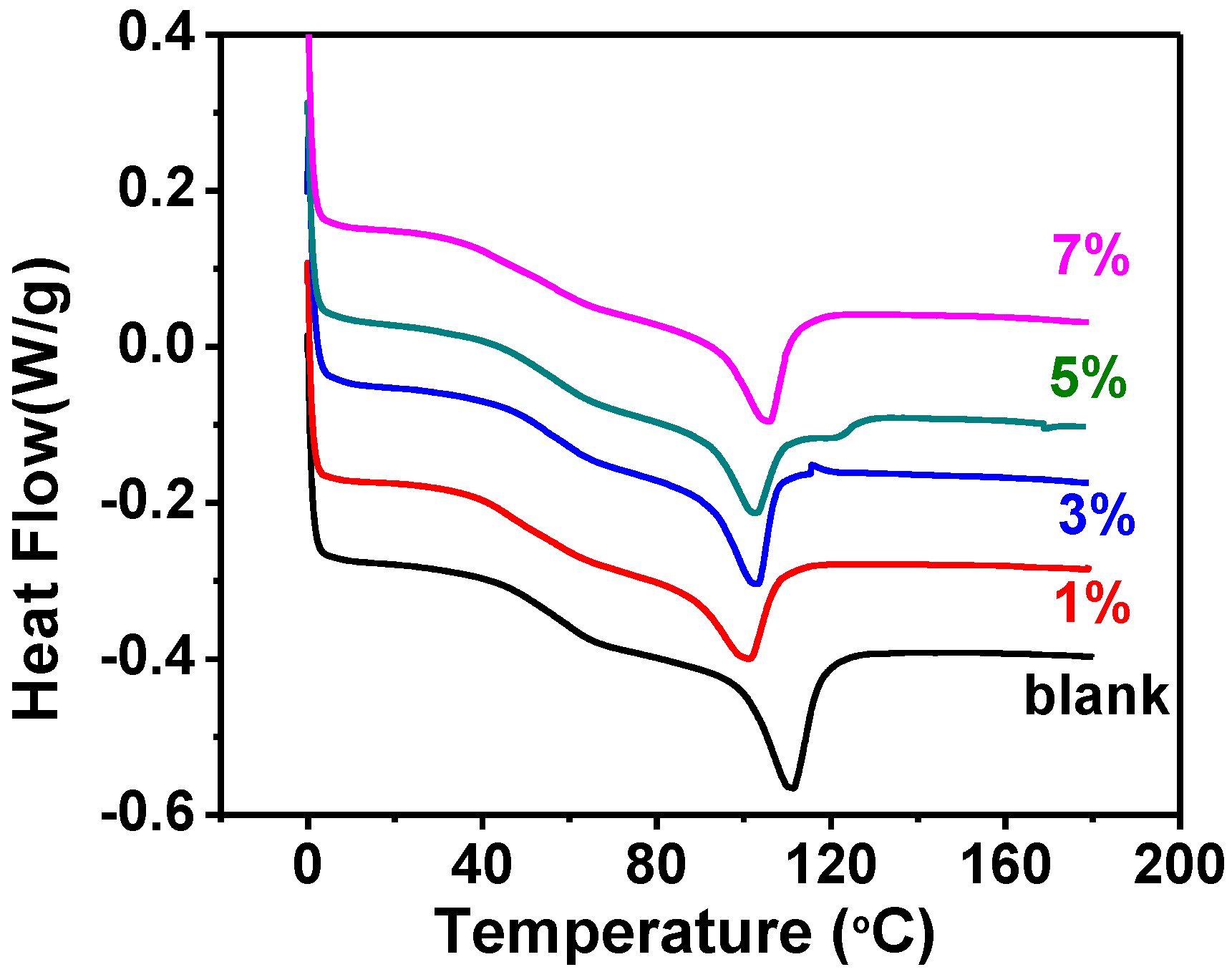
| Sample | Tg (°C) | Ti (°C) | Td (°C) | Ti enthalpy (J/g) | Weight at 800 °C (%) |
|---|---|---|---|---|---|
| blank | 60.2 | 114.3 | 329.5 | 7.93 | 0 |
| SNP 1% | 46.1 | 104.1 | 360.1 | 6.05 | 1.49 |
| SNP 3% | 54.4 | 105.2 | 340.4 | 6.64 | 3.12 |
| SNP 5% | 57.4 | 108.2 | 357.3 | 7.20 | 3.87 |
| SNP 7% | 56.8 | 107.9 | 356.2 | 7.06 | 5.92 |
2.2. Thermal Stability
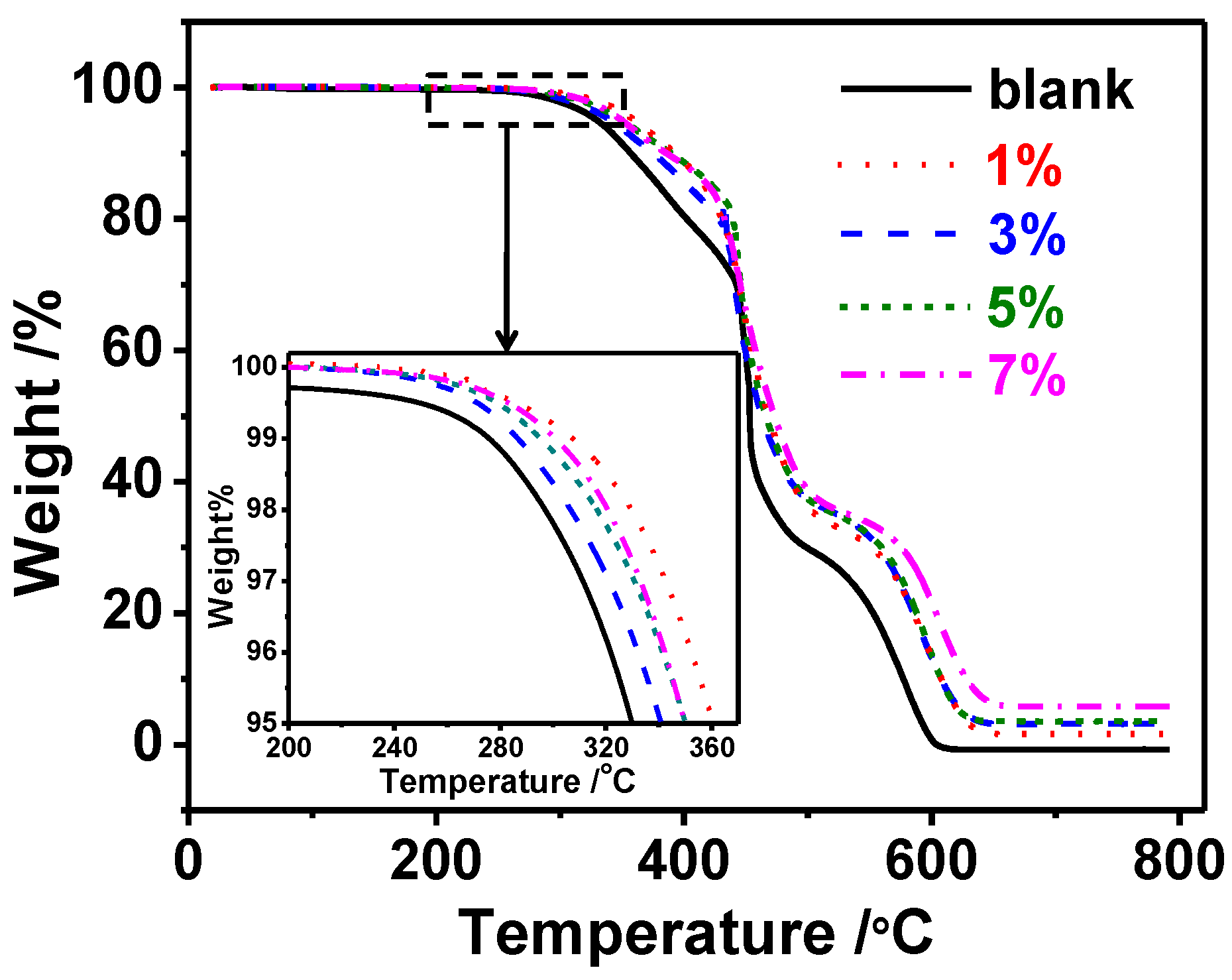
| Temperature (°C) | Blank | SNP 1% | SNP 3% | SNP 5% | SNP 7% |
|---|---|---|---|---|---|
| 30 | 3.636 | 2.674 | 3.063 | 3.208 | 2.494 |
| 90 | 0.0454 | 0.0608 | 0.0557 | 0.0501 | 0.0447 |
| 160 | 0.0151 | 0.0182 | 0.0143 | 0.0199 | 0.0178 |
2.3. Dynamic Mechanical Properties of the Composites
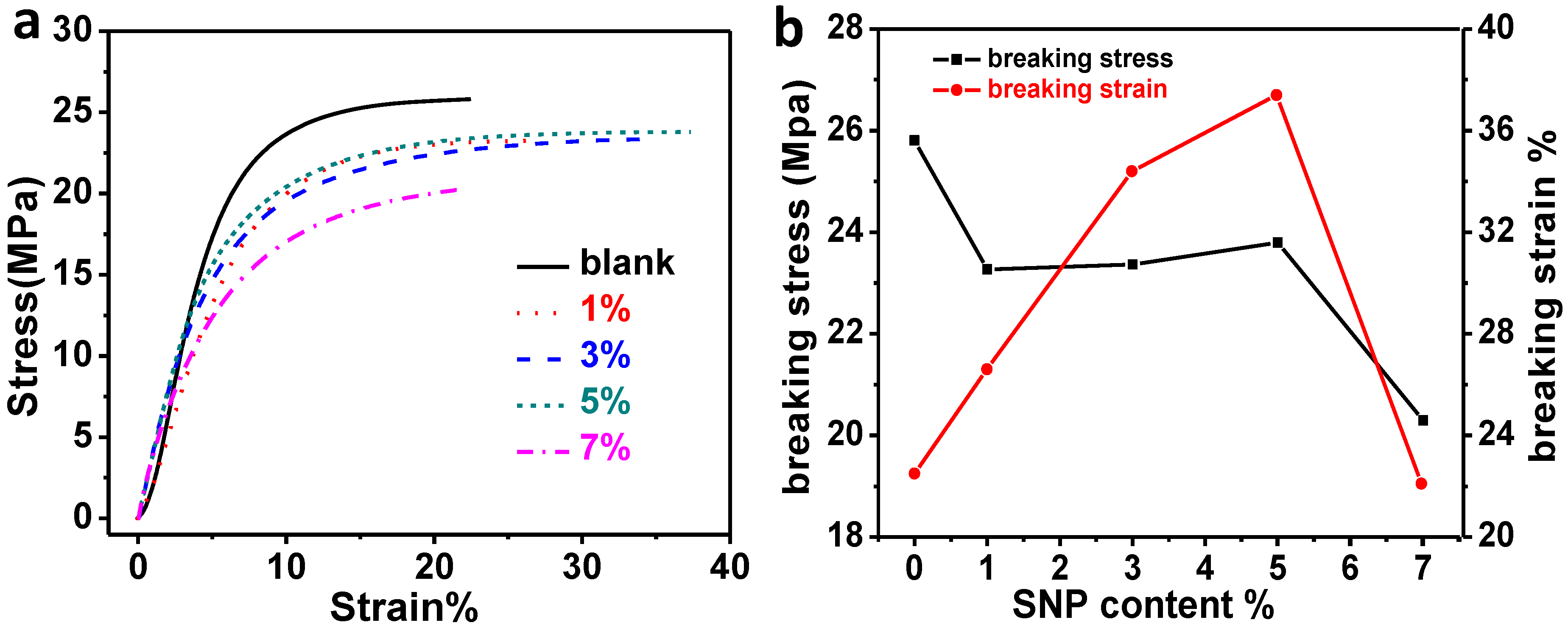
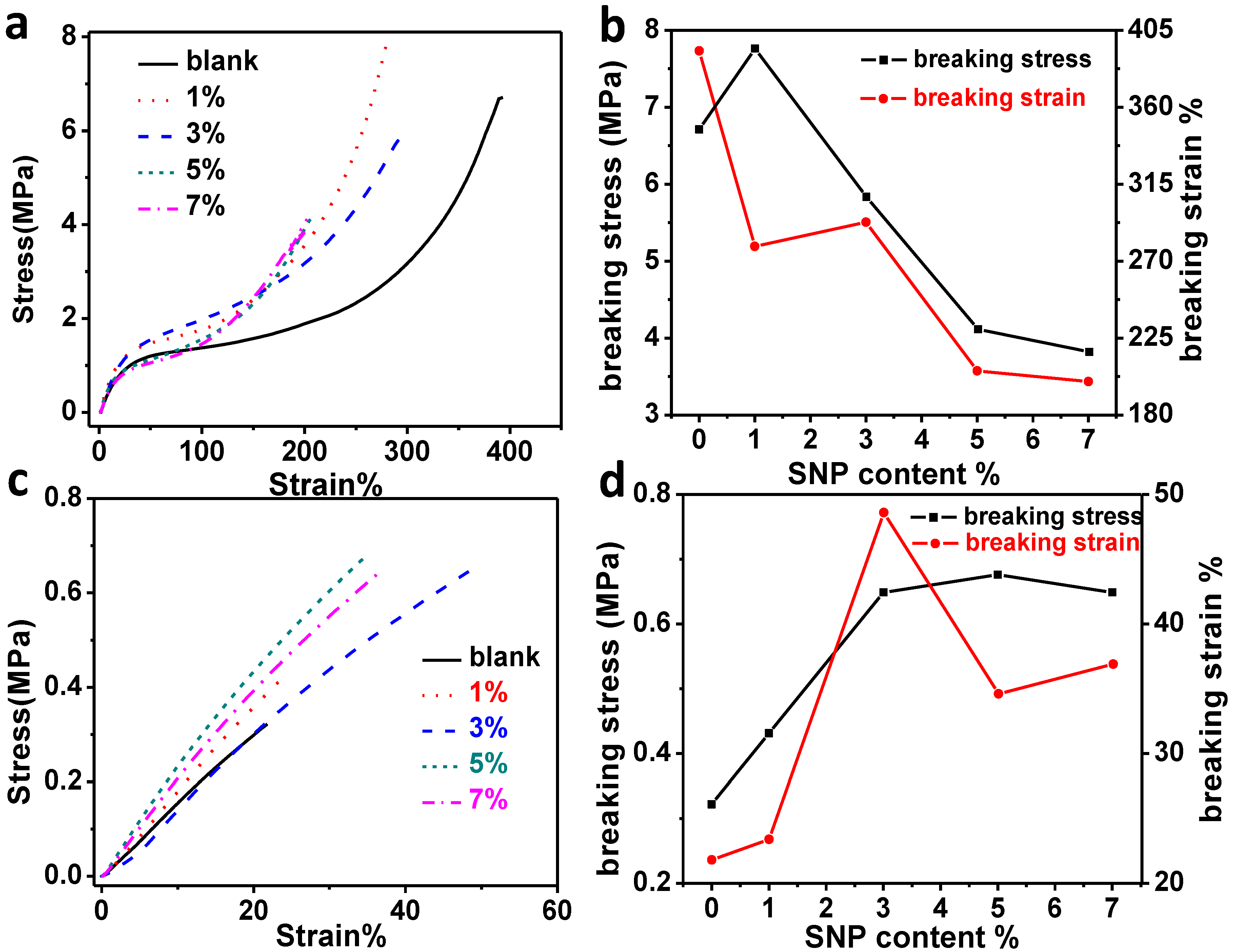
2.4. Morphology Investigation of Fractured Surface
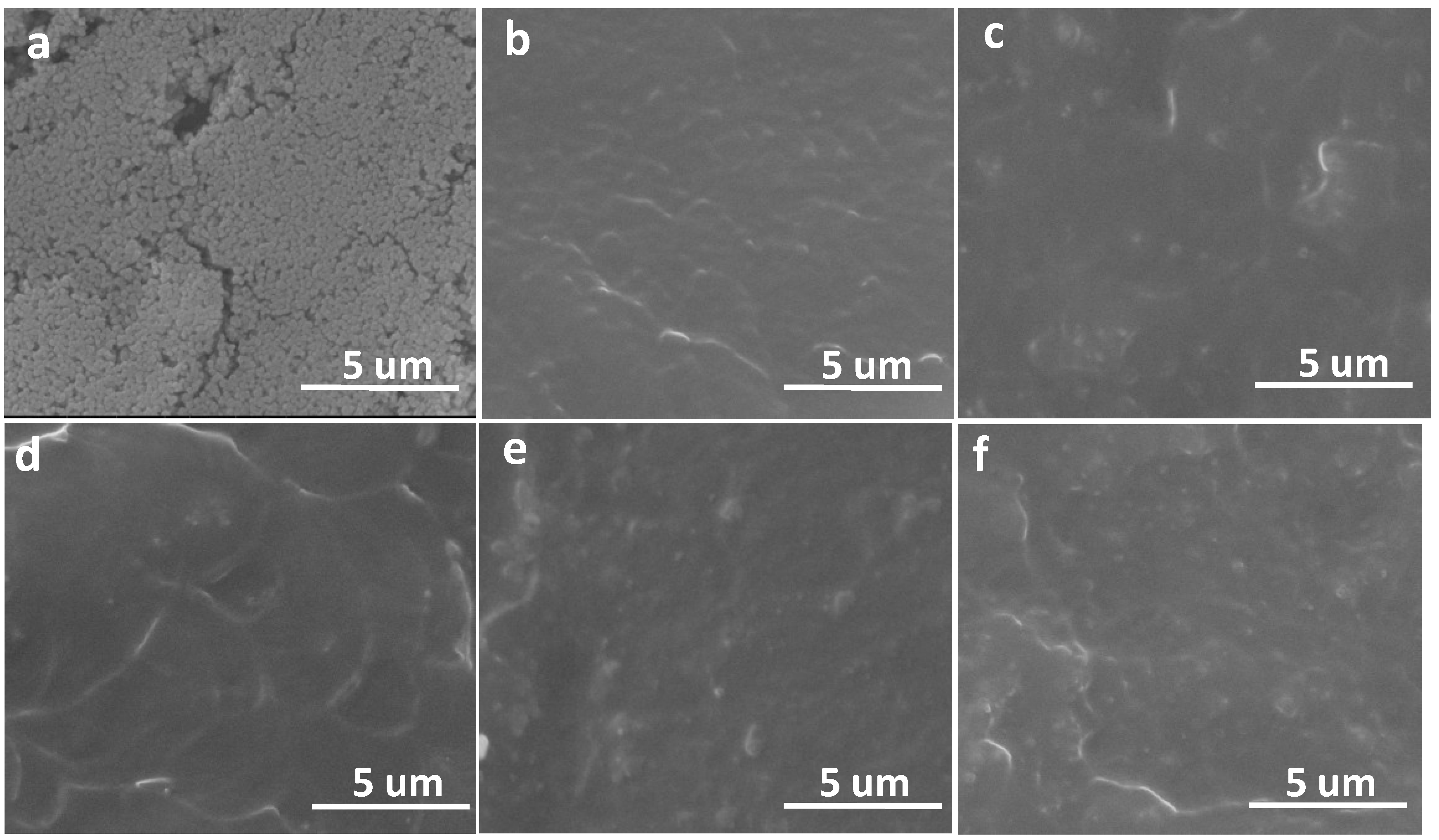
2.5. Thermal Actuation and Shape Memory

3. Experimental Section
3.1. Preparation of SiO2 Nanoparticles
3.2. Preparation of xLCNs/SNP Film

3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Warner, M.; Terentjev, E.M. Liquid Crystal Elastomers; Oxford University Press: Oxford, UK, 2003; Volume 120. [Google Scholar]
- Küpfer, J.; Finkelmann, H. Liquid crystal elastomers: Influence of the orientational distribution of the crosslinks on the phase behaviour and reorientation processes. Macromol. Chem. Phys. 1994, 195, 1353–1367. [Google Scholar] [CrossRef]
- Hogan, P.; Tajbakhsh, A.; Terentjev, E. Uv manipulation of order and macroscopic shape in nematic elastomers. Phys. Rev. E 2002, 65, 041720. [Google Scholar] [CrossRef]
- Ji, Y.; Marshall, J.E.; Terentjev, E.M. Nanoparticle-liquid crystalline elastomer composites. Polymers 2012, 4, 316–340. [Google Scholar] [CrossRef]
- Marshall, J.E.; Gallagher, S.; Terentjev, E.M.; Smoukov, S.K. Anisotropic colloidal micromuscles from liquid crystal elastomers. J. Am. Chem. Soc. 2013, 136, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, D.L.; Keller, P.; Naciri, J.; Pink, R.; Jeon, H.; Shenoy, D.; Ratna, B.R. Liquid crystal elastomers with mechanical properties of a muscle. Macromolecules 2001, 34, 5868–5875. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Terentjev, E. Spontaneous thermal expansion of nematic elastomers. Eur. Phys. J. E 2001, 6, 181–188. [Google Scholar] [CrossRef]
- Yamada, M.; Kondo, M.; Mamiya, J.-i.; Yu, Y.; Kinoshita, M.; Barrett, C.J.; Ikeda, T. Photomobile polymer materials: Towards light-driven plastic motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef]
- Amigó-Melchior, A.; Finkelmann, H. A concept for bifocal contact—or intraocular lenses: Liquid single crystal hydrogels (“LSCH”). Polym. Adv. Technol. 2002, 13, 363–369. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Fischl, T.; Stubenrauch, M.; Wurmus, H.; Hoffmann, M.; Finkelmann, H. Photo-crosslinked side-chain liquid-crystalline elastomers for microsystems. Macromol. Chem. Phys. 2009, 210, 1671–1677. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Fischl, T.; Stubenrauch, M.; Albrecht, A.; Wurmus, H.; Hoffmann, M.; Finkelmann, H. Liquid-crystalline elastomer microvalve for microfluidics. Adv. Mater. 2011, 23, 4526–4530. [Google Scholar] [CrossRef] [PubMed]
- Torras, N.; Zinoviev, K.E.; Esteve, J.; Sánchez-Ferrer, A. Liquid-crystalline elastomer micropillar array for haptic actuation. J. Mater. Chem. C 2013, 1, 5183–5190. [Google Scholar] [CrossRef]
- Finkelmann, H.; Kim, S.T.; Palffy-Muhoray, P.; Taheri, B. Tunable mirrorless lasing in cholesteric liquid crystalline elastomers. Adv. Mater. 2001, 13, 1069–1072. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Huang, Y.Y.; Terentjev, E.M. Dissolving and aligning carbon nanotubes in thermotropic liquid crystals. Langmuir 2011, 27, 13254–13260. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Huang, Y.Y.; Rungsawang, R.; Terentjev, E.M. Dispersion and alignment of carbon nanotubes in liquid crystalline polymers and elastomers. Adv. Mater. 2010, 22, 3436–3440. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K. Influence of non-mesogenic impurities on a nematic to isotropic phase transition. Liq. Cryst. 1997, 22, 239–243. [Google Scholar] [CrossRef]
- Kaiser, A.; Winkler, M.; Krause, S.; Finkelmann, H.; Schmidt, A.M. Magnetoactive liquid crystal elastomer nanocomposites. J. Mater. Chem. 2009, 19, 538–543. [Google Scholar] [CrossRef]
- Haberl, J.M.; Sánchez-Ferrer, A.; Mihut, A.M.; Dietsch, H.; Hirt, A.M.; Mezzenga, R. Liquid-crystalline elastomer-nanoparticle hybrids with reversible switch of magnetic memory. Adv. Mater. 2013, 25, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Domenici, V.; Zupančič, B.; Laguta, V.V.; Belous, A.G.; V’yunov, O.I.; Remškar, M.; Zalar, B. PbTiO3 nanoparticles embedded in a liquid crystalline elastomer matrix: Structural and ordering properties. J. Phys. Chem. C 2010, 114, 10782–10789. [Google Scholar] [CrossRef]
- Domenici, V.; Conradi, M.; Remškar, M.; Viršek, M.; Zupančič, B.; Mrzel, A.; Chambers, M.; Zalar, B. New composite films based on MoO3-x nanowires aligned in a liquid single crystal elastomer matrix. J. Mater. Sci. 2011, 46, 3639–3645. [Google Scholar] [CrossRef]
- Haberl, J.M.; Sánchez-Ferrer, A.; Mihut, A.M.; Dietsch, H.; Hirt, A.M.; Mezzenga, R. Strain-induced macroscopic magnetic anisotropy from smectic liquid-crystalline elastomer-maghemite nanoparticle hybrid nanocomposites. Nanoscale 2013, 5, 5539–5548. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.-F.; Shiu, M.-C. Epoxy composites reinforced by different size silica nanoparticles. J. Appl. Polym. Sci. 2010, 115, 2648–2653. [Google Scholar] [CrossRef]
- Alzina, C.; Sbirrazzuoli, N.; Mija, A. Epoxy-amine based nanocomposites reinforced by silica nanoparticles. Relationships between morphologic aspects, cure kinetics, and thermal properties. J. Phys. Chem. C 2011, 115, 22789–22795. [Google Scholar]
- Poulin, P.; Raghunathan, V.; Richetti, P.; Roux, D. On the dispersion of latex particles in a nematic solution. I. Experimental evidence and a simple model. J. Phys. II 1994, 4, 1557–1569. [Google Scholar]
- Anderson, V.; Terentjev, E. Cellular solid behaviour of liquid crystal colloids 2. Mechanical properties. Eur. Phys. J. E 2001, 4, 21–28. [Google Scholar] [CrossRef]
- Halder, S.; Ghosh, P.K.; Goyat, M.S.; Ray, S. Ultrasonic dual mode mixing and its effect on tensile properties of SiO2-epoxy nanocomposite. J. Adhes. Sci. Technol. 2013, 27, 111–124. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Kuo, M.; Huang, J.; Chen, M. On the peek composites reinforced by surface-modified nano-silica. Mater. Sci. Eng.: A 2007, 458, 158–169. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Z.; Yang, Y.; Qin, B.; Zhang, X.; Tao, L.; Wei, Y.; Ji, Y. Liquid Crystalline Network Composites Reinforced by Silica Nanoparticles. Materials 2014, 7, 5356-5365. https://doi.org/10.3390/ma7075356
Li Z, Yang Y, Qin B, Zhang X, Tao L, Wei Y, Ji Y. Liquid Crystalline Network Composites Reinforced by Silica Nanoparticles. Materials. 2014; 7(7):5356-5365. https://doi.org/10.3390/ma7075356
Chicago/Turabian StyleLi, Zhen, Yang Yang, Benye Qin, Xiaoyong Zhang, Lei Tao, Yen Wei, and Yan Ji. 2014. "Liquid Crystalline Network Composites Reinforced by Silica Nanoparticles" Materials 7, no. 7: 5356-5365. https://doi.org/10.3390/ma7075356




