Improving the Efficiency of Organic Solar Cells upon Addition of Polyvinylpyridine
Abstract
:1. Introduction
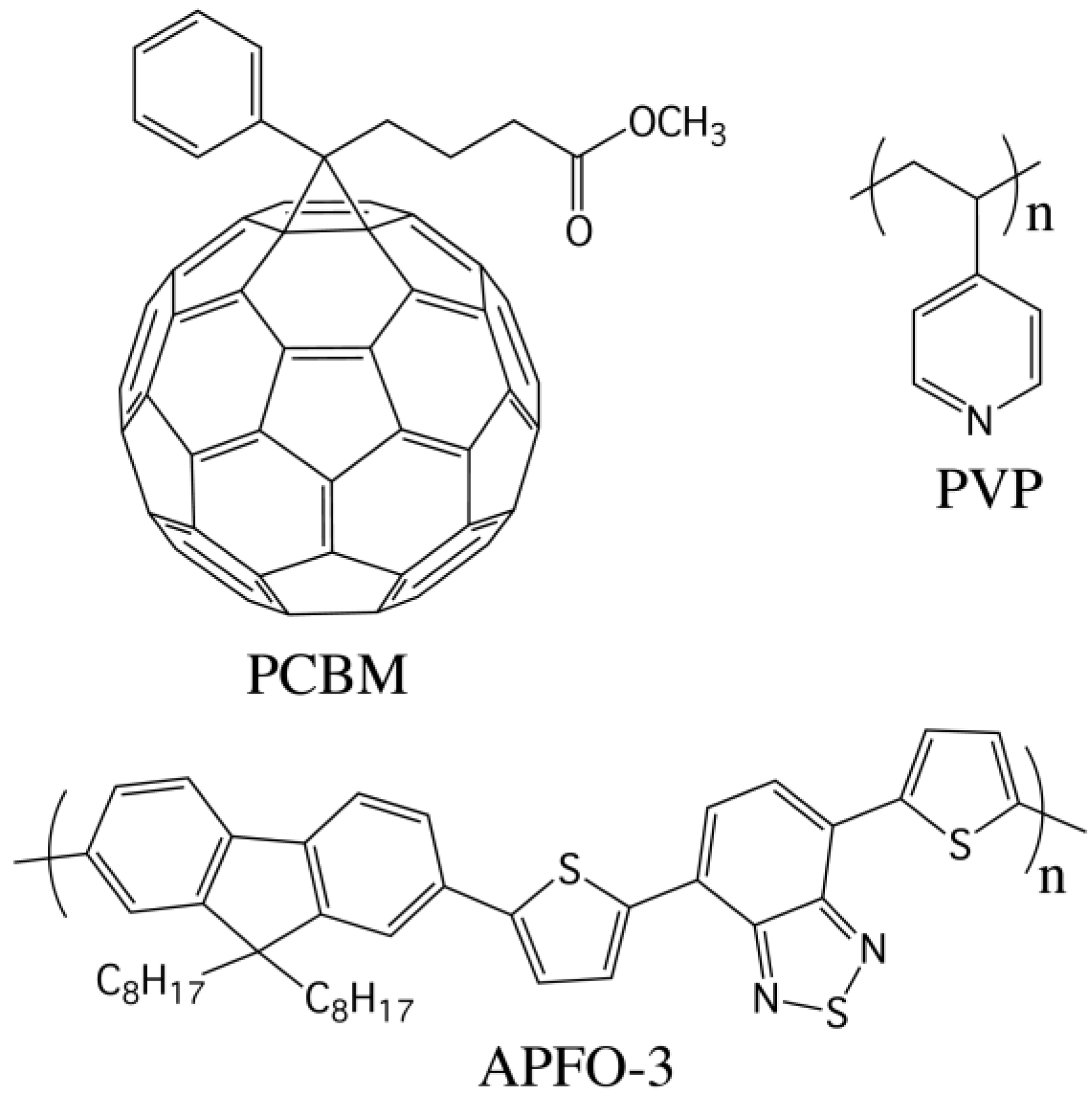
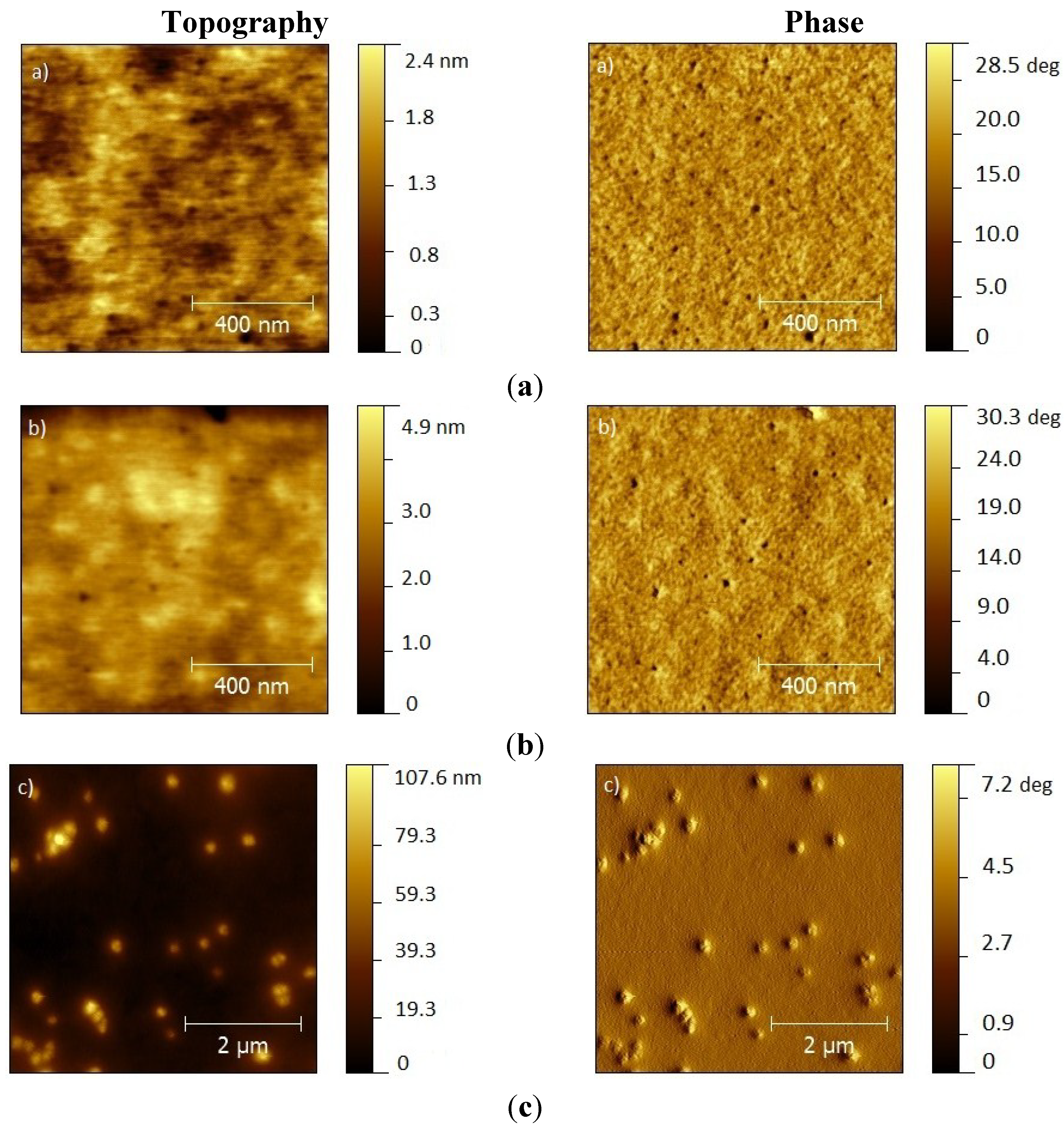
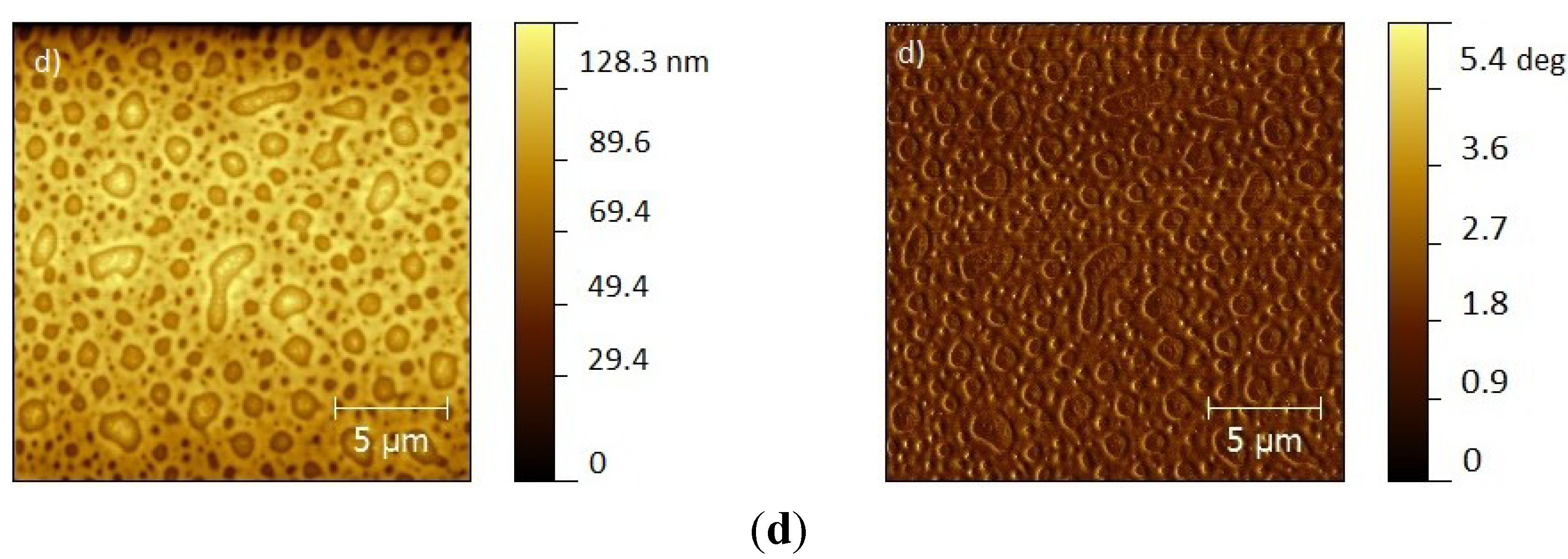
2. Results and Discussion

3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Kippelen, B.; Brédas, J.-L. Organic photovoltaics. Energy Environ. Sci. 2009, 2, 251–261. [Google Scholar] [CrossRef]
- Pivrikas, A.; Stadler, A.; Neugebauer, H.; Sariciftci, N.S. Substituting the postproduction treatment for bulk-heterojunction solar cells using chemical additives. Org. Electron. 2008, 9, 775–782. [Google Scholar] [CrossRef]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-processed small-molecule solar cells with 6.7% efficiency. Nat. Mater. 2012, 11, 44–48. [Google Scholar] [CrossRef]
- Yang, C.H.; Qiao, J.; Sun, Q.J.; Jiang, K.J.; Li, Y.L.; Li, Y.F. Improvement of the performance of polymer/C60 photovoltaic cells by small-molecule doping. Synth. Met. 2003, 137, 1521–1522. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Pivrikas, A.; Neugebauer, H.; Sariciftci, N.S. Influence of processing additives to nano-morphology and efficiency of bulk-heterojunction solar cells: A comparative review. Sol. Energy 2011, 85, 1226–1237. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, W.; Mukherjee, S.; Ade, H.; Kramer, E.J.; Bazan, G.C. High-molecular-weight insulating polymers can improve the performance of molecular solar cells. Adv. Mater. 2014, 26, 4168–4172. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Zhang, F.; Inganäs, O.; Andersson, M.R. Synthesis and properties of alternating polyfluorene copolymers with redshift absorption for use in solar cells. Synth. Met. 2003, 135, 137–138. [Google Scholar] [CrossRef]
- Svensson, M.; Zhang, F.; Veenstra, S.C.; Verhees, W.J.H.; Hummelen, J.C.; Kroon, J.M.; Inganäs, O.; Andersson, M.R. High-performance polymer solar cells of an alternating polyfluorene copolymer and a fullerene derivative. Adv. Mater. 2003, 15, 988–991. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future-Bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Najari, A.; Berrouard, P.; Beaupré, S.; Aïch, B.R.; Tao, Y.; Leclerc, M. A thieno[3,4-c]pyrrole-4,6-dione-based copolymer for efficient solar cells. J. Am. Chem. Soc. 2010, 132, 5330–5331. [Google Scholar] [CrossRef] [PubMed]
- Bjorstrom, C.M.; Magnusson, K.O.; Moons, E. Control of phase separation in blends of polyfluorene (co)polymers and the C60-derivative PCBM. Synth. Met. 2005, 152, 109–112. [Google Scholar] [CrossRef]
- Nilsson, S.; Bernasik, A.; Budkowski, A.; Moons, E. Morphology and phase segregation of spin-casted films of polyfluorene/PCBM blends. Macromolecules 2007, 40, 8291–8301. [Google Scholar] [CrossRef]
- Bernasik, A.; Rysz, J.; Budkowski, A.; Kowalski, K.; Camra, J.; Jedlinski, J. Three-dimensional information on the phase domain structure of thin films of polymer blends revealed by secondary ion mass spectrometry. Macromol. Rapid Commun. 2001, 22, 829–834. [Google Scholar] [CrossRef]
- Raczkowska, J.; Cyganik, P.; Budkowski, A.; Bernasik, A.; Rysz, J.; Raptis, I.; Czuba, P.; Kowalski, K. Composition effects in polymer blends spin-cast on patterned substrates. Macromolecules 2005, 38, 8486–8493. [Google Scholar] [CrossRef]
- Björström, C.M.; Bernasik, A.; Rysz, J.; Budkowski, A.; Nilsson, S.; Svensson, M.; Andersson, M.R.; Magnusson, K.O.; Moons, E. Multilayer formation in spin-coated thin films of low-bandgap polyfluorene:PCBM blends. J. Phys. Condens. Matter 2005, 17, L529–L534. [Google Scholar] [CrossRef]
- Svanström, C.M.B.; Rysz, J.; Bernasik, A.; Budkowski, A.; Zhang, F.; Inganäs, O.; Andersson, M.R.; Magnusson, K.O.; Benson-Smith, J.J.; Nelson, J.; Moons, E. Device performance of APFO-3/PCBM solar cells with controlled morphology. Adv. Mater. 2009, 21, 4398–4403. [Google Scholar] [CrossRef]
- Mihailetchi, V.D.; Blom, P.W.M.; Hummelen, J.C.; Rispens, M.T. Cathode dependence of the open-circuit voltage of polymer:fullerene bulk heterojunction solar cells. J. Appl. Phys. 2003, 94, 6849–6854. [Google Scholar] [CrossRef]
- Tong, T.; Babatope, B.; Admassie, S.; Meng, J.; Akwogu, O.; Akande, W.; Soboyejo, W.O. Adhesion in organic electronic structures. J. Appl. Phys. 2009, 106, 083708. [Google Scholar] [CrossRef]
- Yang, G.H.; Kang, E.T.; Neoh, K.G.; Zhang, Y.; Tan, K.L. Electroless deposition of copper on polyimide films modified by surface graft copolymerization with nitrogen-containing vinyl monomers. Colloid Polym. Sci. 2001, 279, 745–753. [Google Scholar] [CrossRef]
- Chan-Park, M.B.; Tan, S.S. Thermal graft copolymerization of 4-vinyl pyridine on polyimide to improve adhesion to copper. Int. J. Adhesi. Adhesi. 2002, 22, 471–475. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.; Meira, R.; Ferreira, Q.; Charas, A.; Morgado, J. Improving the Efficiency of Organic Solar Cells upon Addition of Polyvinylpyridine. Materials 2014, 7, 8189-8196. https://doi.org/10.3390/ma7128189
Rodrigues R, Meira R, Ferreira Q, Charas A, Morgado J. Improving the Efficiency of Organic Solar Cells upon Addition of Polyvinylpyridine. Materials. 2014; 7(12):8189-8196. https://doi.org/10.3390/ma7128189
Chicago/Turabian StyleRodrigues, Rita, Rui Meira, Quirina Ferreira, Ana Charas, and Jorge Morgado. 2014. "Improving the Efficiency of Organic Solar Cells upon Addition of Polyvinylpyridine" Materials 7, no. 12: 8189-8196. https://doi.org/10.3390/ma7128189




