1. Introduction
The Department of Defense (DOD) relies on multiple-component protective coating systems to maintain the operational readiness of military aircraft. Current high-performance aerospace coating systems (pretreatment, primer and topcoat) for DOD applications center on the use of hexavalent chromium (Cr(VI)) in both the pretreatment and primer layers. The pretreatment coating is a conversion coat that is Cr(VI)-based and the primer contains Cr(VI)-based inhibitors (e.g., strontium chromate (SrCrO4)). The pretreatment and primer are coated with a polyurethane topcoat to complete the aerospace coating. This DOD aerospace coating has been developed over many years to meet the strenuous challenges of corrosion protection, adhesion and weathering encountered by military platforms. DOD aerospace platforms such as the F-18, F-16, F-22, Joint Strike Fighter, MV-22, CV-22, H-60, C-141, C-130, C-5 and P-3 Orion aircraft use these coatings for corrosion protection and to ensure mission readiness.
Chromate conversion coatings (CCCs) and Cr(VI) primers are effective at inhibiting corrosion, because they are capable of “self-healing”. The soluble Cr(VI) present in CCCs and to a lesser degree in Cr(VI) primers acts as a reservoir for mobile Cr(VI). These mobile Cr(VI) species are capable of migrating to defects and inhibiting further corrosion by creating a passivating layer at the defect site [
1,
2,
3,
4,
5,
6]. Cr(VI) compounds are known carcinogens and are toxic to both humans and the environment [
7]. Chronic inhalation of Cr(VI) compounds increases the risk for lung cancer. Soluble species can cause or exacerbate contact dermatitis. Ingestion can cause irritation and ulcers of the stomach and intestine and Cr(VI) is transported into cells via the “sulfate transport” mechanism [
8]. Due to its toxicity and persistence in the environment, Cr(VI) is highly regulated. Therefore, alternative non-toxic pretreatment coatings are under development to replace Cr(VI) with a more benign material.
Recent work on alternatives to Cr(VI) coatings have produced surface pretreatments or conversion coats such as Trivalent Chromium Pretreatment (TCP), Prekote and Alodine 5200/5700
® [
9,
10,
11,
12,
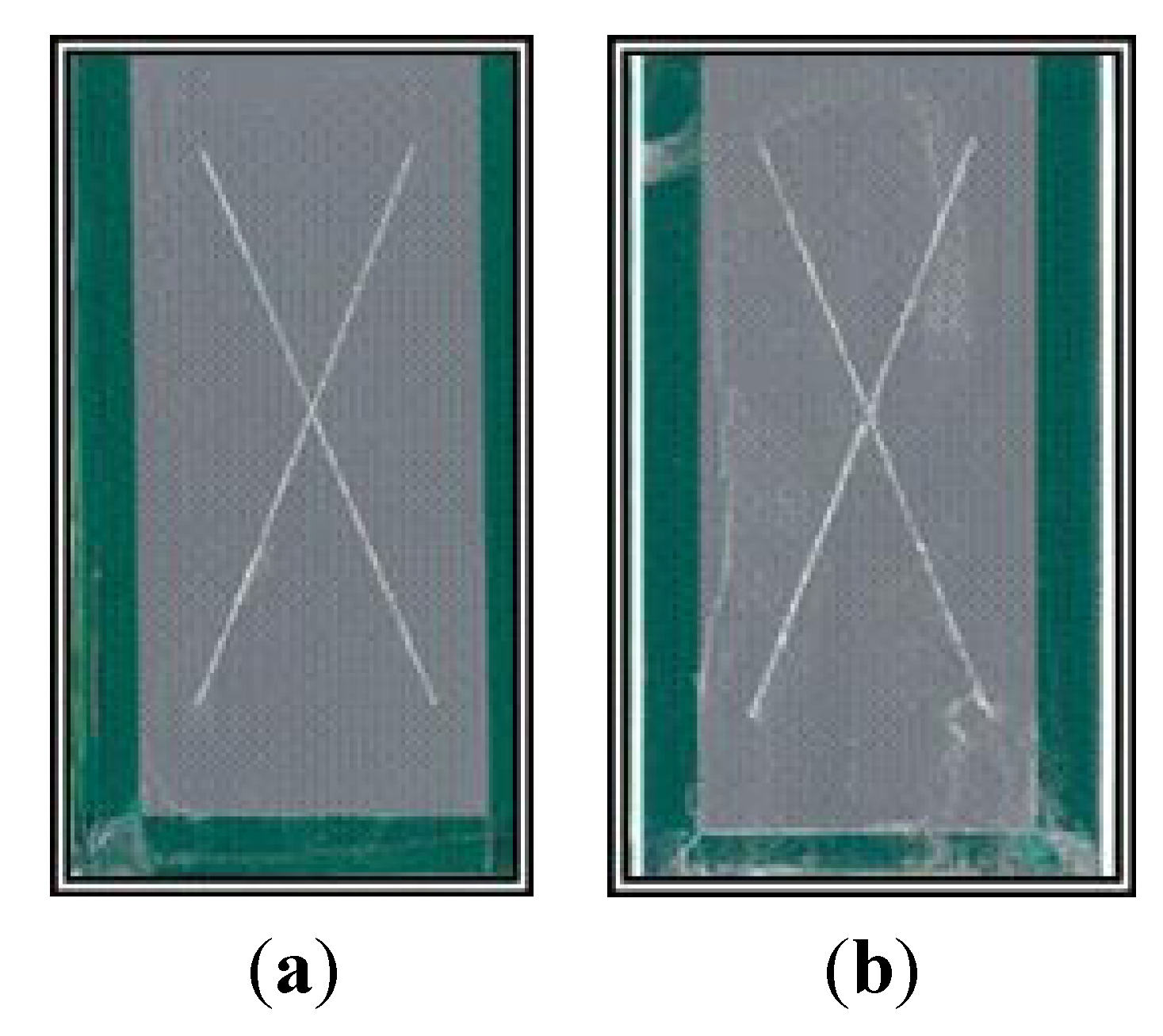
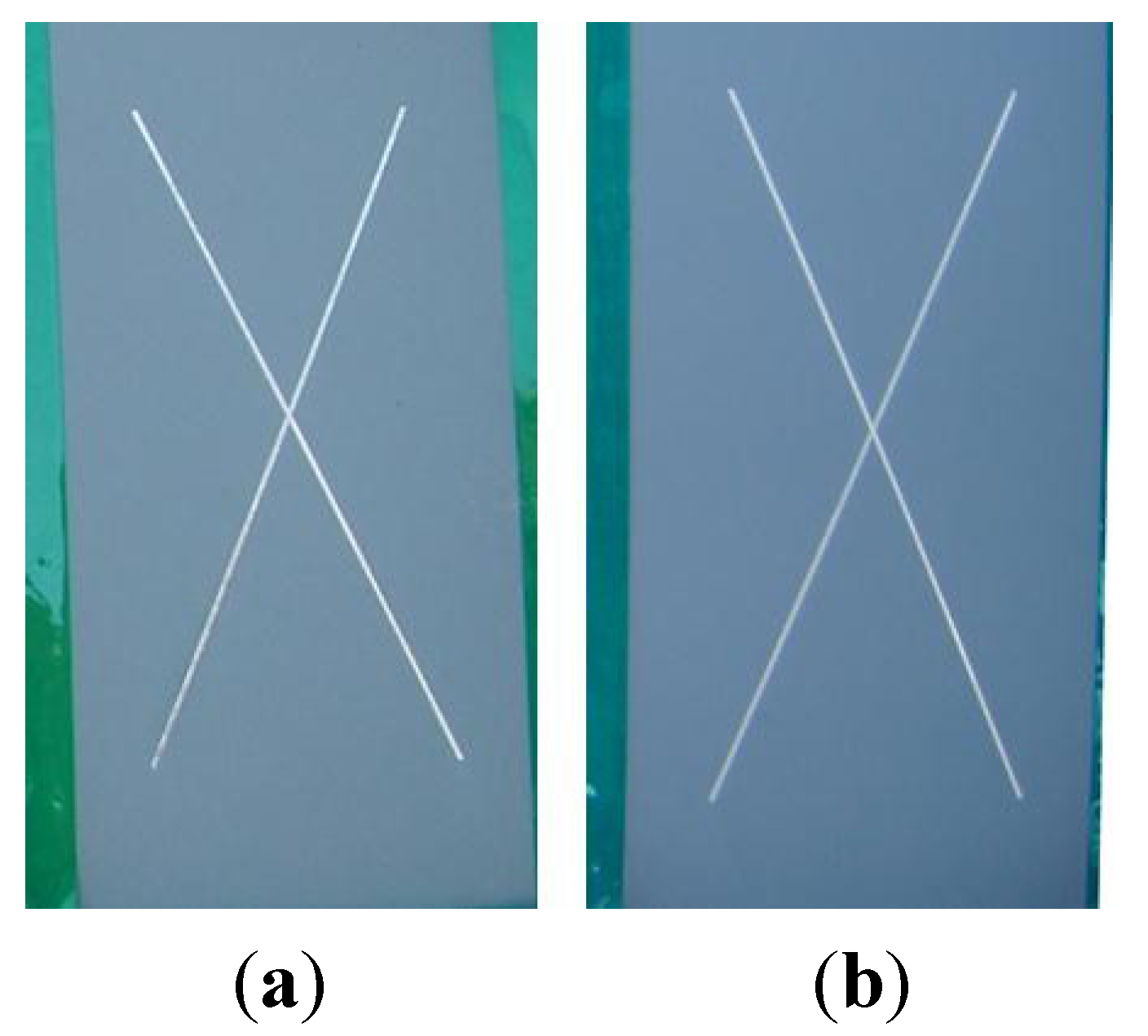
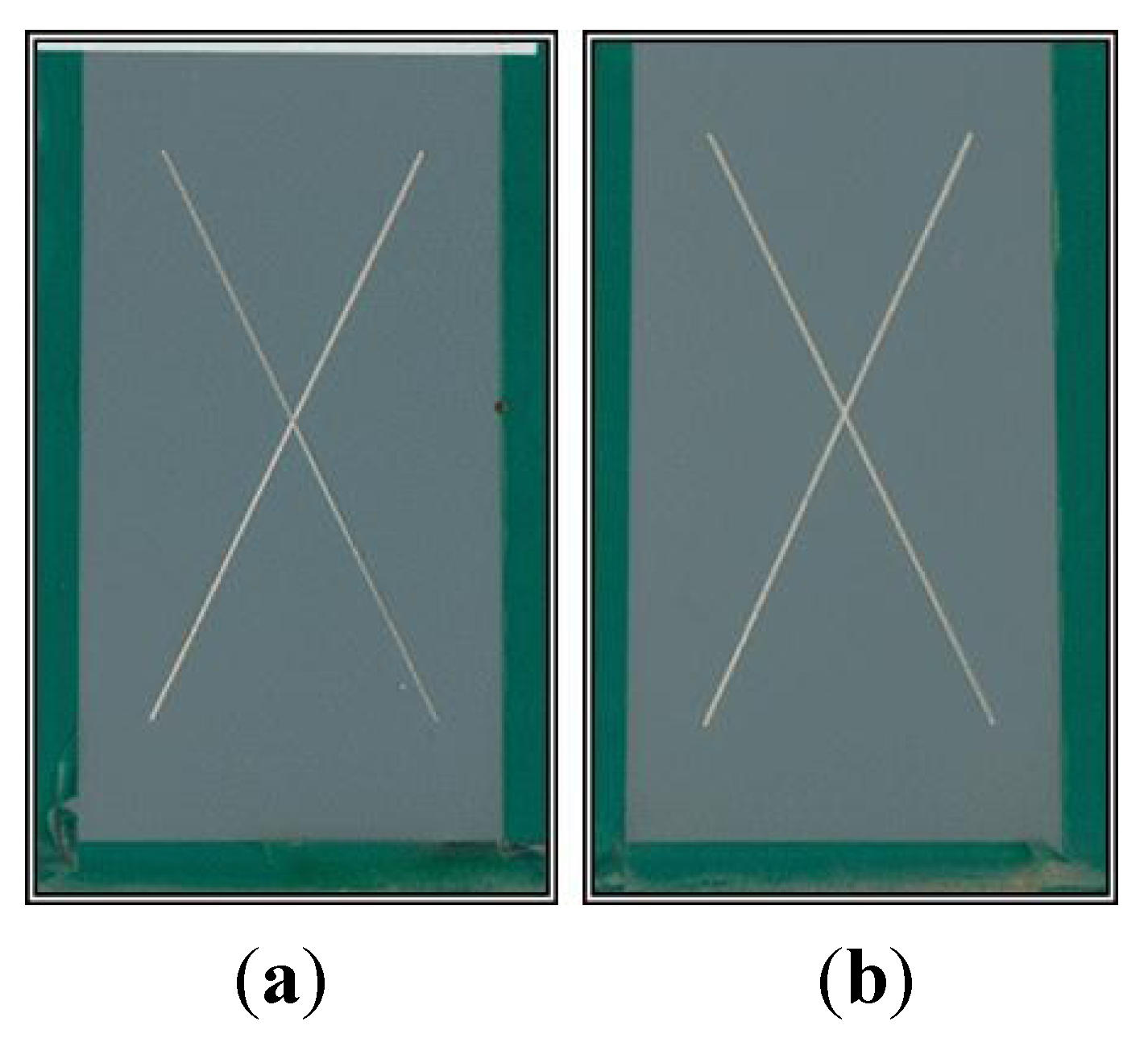
13]. These systems have been used as alternatives to CCCs with varying degrees of success. When they are combined with non-Cr(VI) epoxy primers, these alternatives have not performed as well in aerospace applications as coatings containing Cr(VI). Typically, these alternatives have been unable to meet the minimum standard of 2000 h of neutral salt spray (NSS) exposure before failing. The failure modes include corrosion, blistering or delamination of the coating.
Over the past 25 years, published evidence that EAPs especially, polyaniline (PANI) can inhibit corrosion has come from the pioneering work of Mengoli [
14] and DeBerry [
15]. Mengoli showed that EAP coatings such as PANI deposited onto iron anodes by electropolymerization of aniline resulted in an adherent and corrosion inhibiting film. Further work by DeBerry in 1985 showed that PANI films electrochemically deposited from an aniline monomer solution onto stainless steel provided corrosion protection from sulfuric acid environments. These works and others demonstrated that the PANI film provided anodic protection, thus maintaining a native passive film on the steel [
15,
16]. There are numerous laboratory studies in which EAPs deposited onto metals exhibit corrosion inhibition under a variety of corrosive environments [
17,
18,
19]. Additionally, EAPs and chromium species undergo oxidation at similar potentials, which means that corrosive species that normally react with the chromium coatings will likely also react with EAPs [
20].
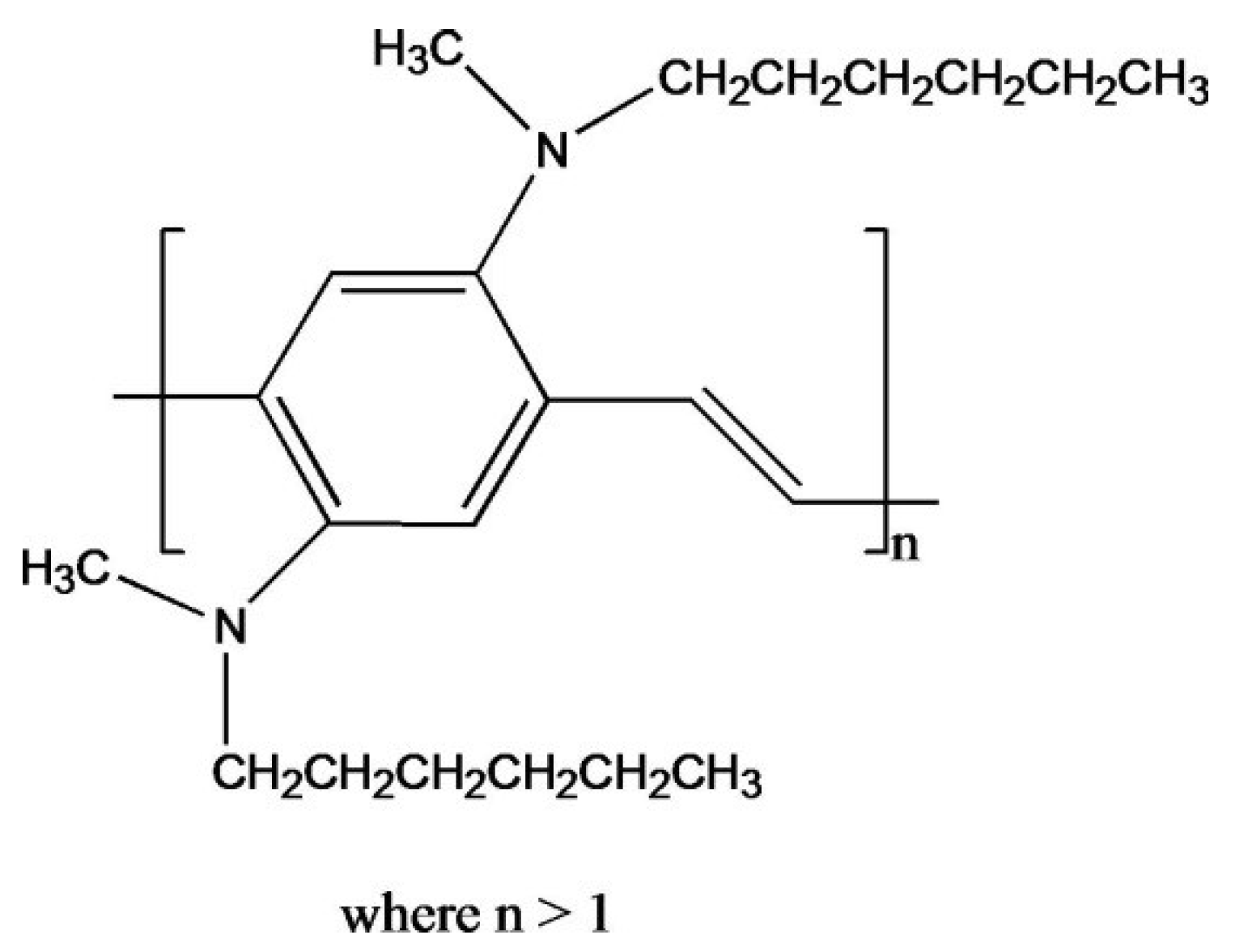
Poly(2,5-bis(
N-methyl-
N-hexylamino)phenylene vinylene) (BAM-PPV),
Figure 1, has been examined as a replacement for CCC’s on AA2024-T3 alloys. Neutral salt spray (NSS) exposure testing at the Naval Air Warfare Center Weapons Division (NAWCWD), China Lake, California has shown that BAM-PPV coatings deposited onto AA2024-T3 can prevent corrosion ≥336 h. A DOD CCC replacement requires a minimum of 336 h of NSS exposure testing without evidence of corrosion, blistering or delamination to be considered as an alternative [
21,
22,
23].
Figure 1.
Structure of BAM-PPV.
Figure 1.
Structure of BAM-PPV.
Studies in which advanced electrochemical techniques were used to examine BAM-PPV’s corrosion protection mechanism have been published previously. Scanning vibrating electrode technique (SVET) and electrochemical noise method (ENM) measurements showed that BAM-PPV films may passivate the metal surface in Dilute Harrison Solution (DHS) [
24].
Published studies using SVET experiments performed on BAM-PPV coatings on AA2024-T3 coupons showed that all current flow was forced to occur only within the defect area. The magnitude of the oxidation current increased with time, reaching 80 μA/cm
2 after 6 h and 10 min of immersion in DHS. The corresponding optical micrograph with overlaid current density vectors, published in [
24], indicated that the oxidation current was localized at the visually corroded area of the defect, while the reduction current appeared to occur both within the defect and to some extent at the polymer film surface. This observation may be due to the polymer undergoing oxidation/doping. Thus, rendering itself sufficiently conductive to mediate electron transfer from the metal/polymer interface to polymer/solution interface. The oxidation current then slowly decreased and reached 10 μA/cm
2 after 24 h of immersion. The corresponding electronic or electrochemical interaction between the polymer and the metal provides evidence for passivation of the metal.
Thin film BAM-PPV coatings (film thickness 0.4 μm) on AA2024-T3 coupons were measured using ENM and the magnitudes of the measured noise resistances were quite low (1 × 10
4–1 × 10
5 Ω) [
24]. Cross-scratched samples of BAM-PPV coated samples showed an initial value of about 5 × 10
4 Ω noise resistance on the first three days of immersion in DHS. The resistance dropped to 1 × 10
4 Ω by the tenth day of immersion and maintained this steady resistance through 42 days. For the non-scratched sample the average initial noise resistance (1 × 10
5 Ω) was higher than that of cross-scratched samples. The fluctuating noise resistance as a function of immersion time may due to the formation/breakdown activities of a passive oxide film at the metal/BAM-PPV polymer interface. Some corrosion products were found after 7 days of immersion. By day 30 the samples had significant buildup of corrosion indicating complete coating failure.
Electrochemical impedance spectroscopy (EIS) measurements on the stability of BAM-PPV films at various pH’s (Tris, pH 8.1, and acetate, pH 4.5, buffer solutions) at room temperature showed that BAM-PPV was robust without evidence of delamination or blistering [
22,
23]. The BAM-PPV films also exhibited purely capacitive behavior with a slightly higher pore resistance (
Rpo) than that observed for the bare AA2024-T3 substrate. In the case of the bare AA2024-T3 substrate, a thin oxide layer is present that strongly adheres to the metal surface and acts as a protective passivation layer. The higher pore resistances measured for the BAM-PPV coated AA2024-T3 substrate suggest that the polymer layer also contributes to the electrical properties at the aluminum/liquid junction. In effect, the polymer coating appears to function as a resistor in series with the oxide layer on the aluminum. The stability of this additional layer is indicated by the persistently higher
Rpo values obtained with the BAM-PPV coated panels
versus the bare aluminum. This evidence suggests that the electronic properties of the BAM-PPV layer are not significantly altered by the buffer solution over the course of the experiments. Further EIS studies on the resistive nature of the BAM-PPV coating
versus CCC on AA2024-T3 coupons showed that the impedances at low frequencies do not significantly change within the first six months of exposure to the 0.5 M NaCl solution. The total resistances do not deviate significant from 1 × 10
4 to 1 × 10
5 Ω regardless of the coating.
The previously published electrochemical results were encouraging and demonstrated that BAM-PPV warranted further investigation as a practical coating replacement for Cr(VI)-based systems. The performance of BAM-PPV aerospace coatings in adhesion, accelerated weathering and outdoor exposure tests are reported here. These results, in combination with the previously reported work, show that BAM-PPV coatings perform similarly to Cr(VI)-based systems in both laboratory and field scale testing, and because of their high performance, BAM-PPV coatings provide a good alternative to Cr(VI)-based coatings.