Phosphated Cellulose as an Efficient Biomaterial for Aqueous Drug Ranitidine Removal
Abstract
:1. Introduction
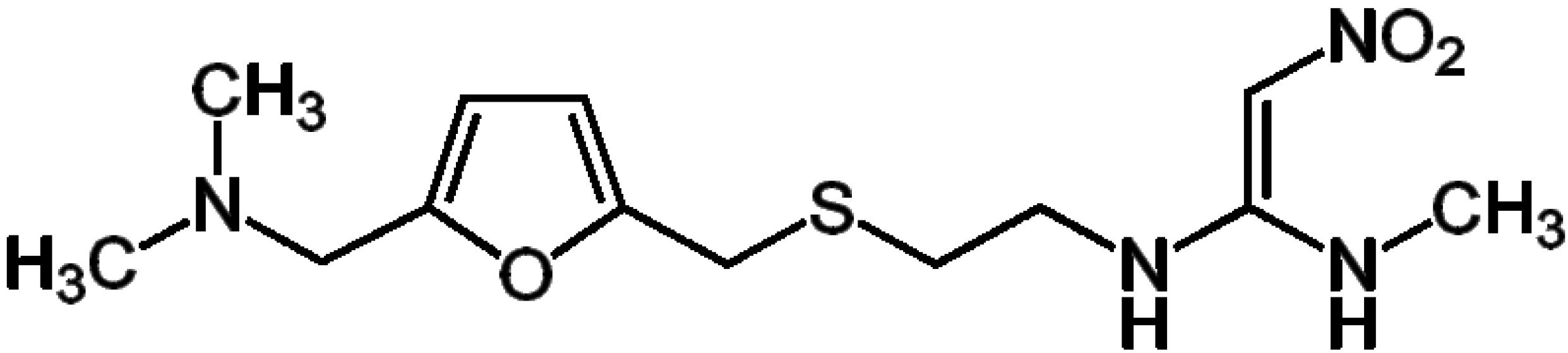
2. Results and Discussion
2.1. Characterization
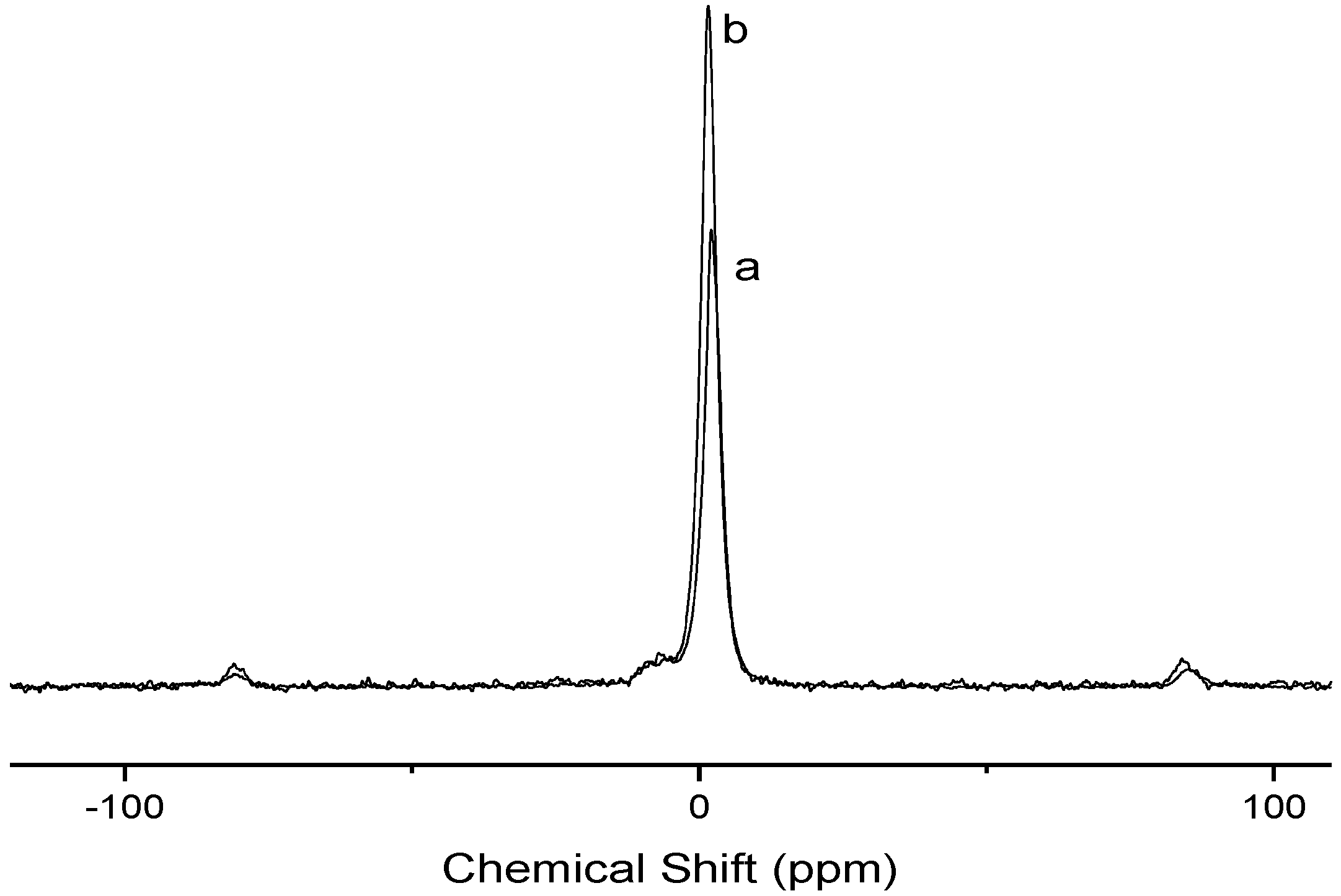

| Element | Pure cellulose | Cel-P-4 | Cel-P-10 |
|---|---|---|---|
| C | 72.1 | 63.9 | 60.8 |
| O | 27.9 | 35.7 | 36.4 |
| Na | - | 0.2 | 1.6 |
| P | - | 0.2 | 1.2 |
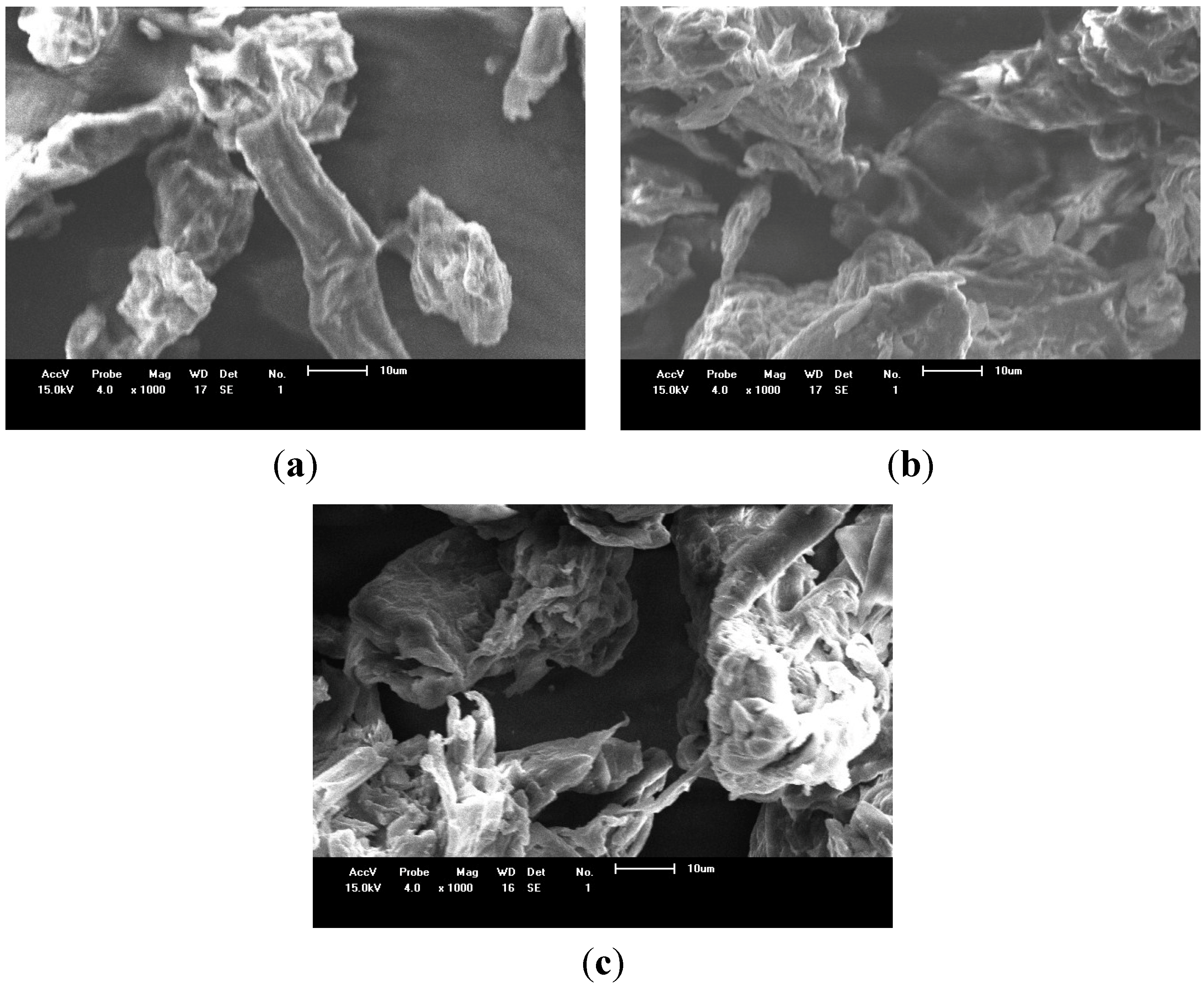
2.2. Ion Exchange
| Adsorbents | Ion exchange capacities (meq·g−1) | Deviation |
|---|---|---|
| Pure cellulose | 0.0119 | 0.0002 |
| Cel-P4 | 0.0121 | 0.0007 |
| Cel-P10 | 0.0124 | 0.0002 |
2.3. Sorption
2.3.1. Influence of pH
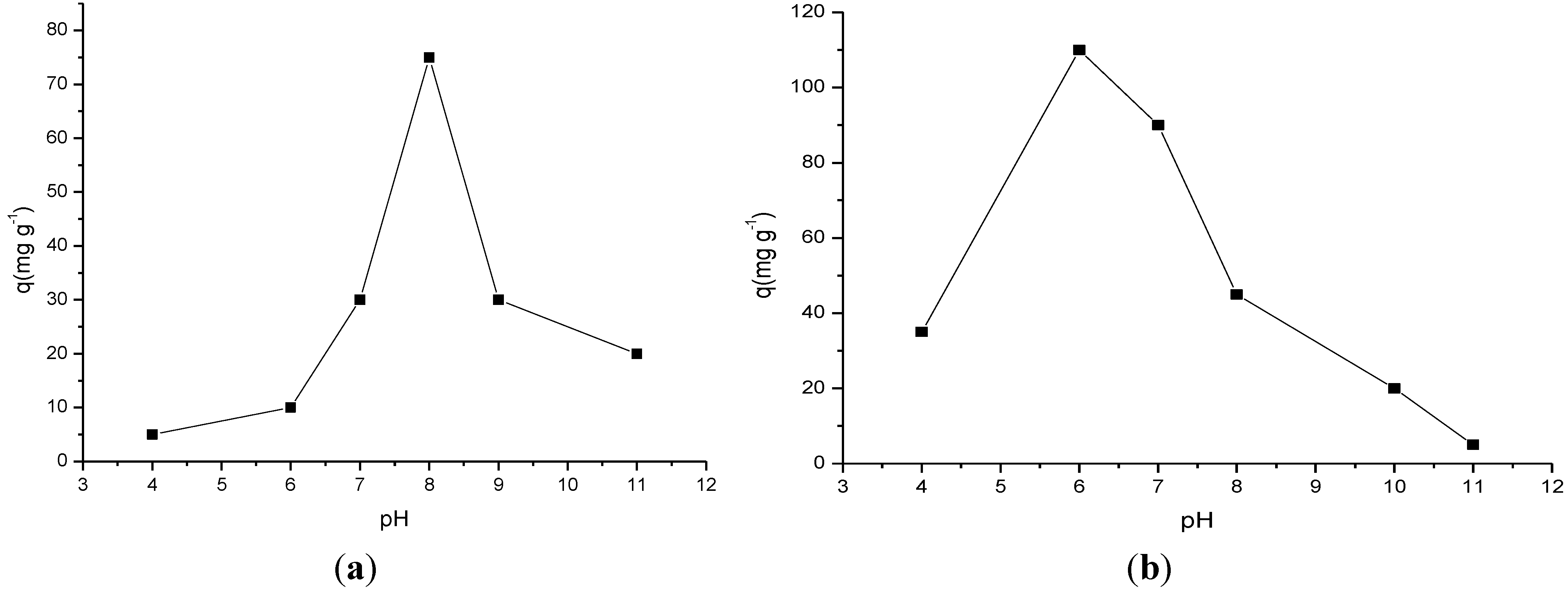
2.3.2. Influence of Time

2.3.3. Influence of Temperature and Concentration
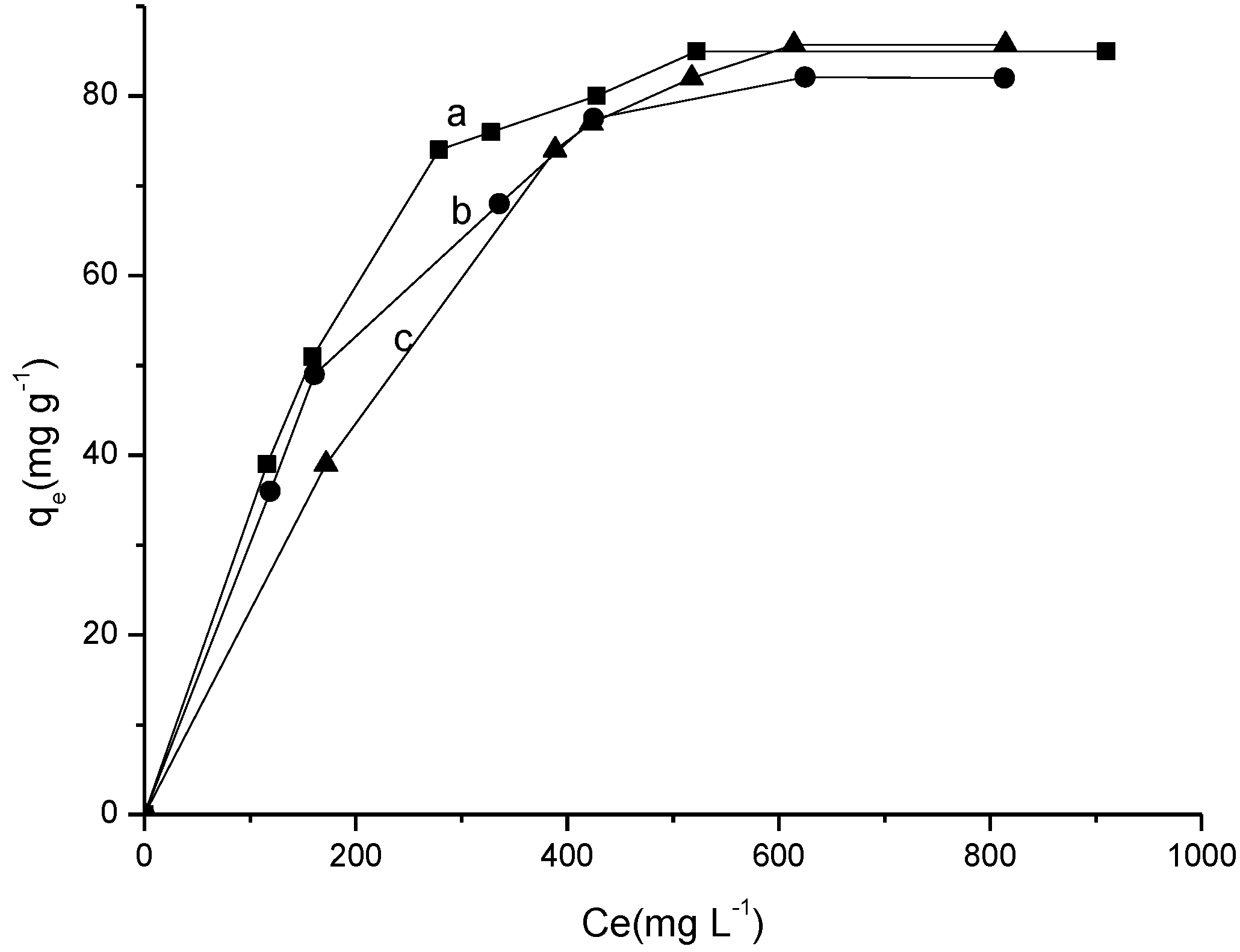
| Temperature (K) | Langmuir model | Freundlich model | Temkin model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL | qm | R2 | RL | KF | nf | R2 | KT | nt | R2 | |
| 298 | 0.243 | 125.0 | 0.9771 | 0.008 | 7.210 | 2.577 | 0.8270 | 6.18 × 10−30 | 0.042 | 0.8417 |
| 308 | 0.296 | 104.0 | 0.9871 | 0.005 | 5.272 | 2.342 | 0.9151 | 7.82 × 10−35 | 0.040 | 0.9375 |
| 318 | 0.183 | 125.0 | 0.9414 | 0.009 | 2.769 | 1.872 | 0.8814 | 5.69 × 10−59 | 0.031 | 0.8959 |
2.3.4. Biopolymer/Ranitidine Interaction
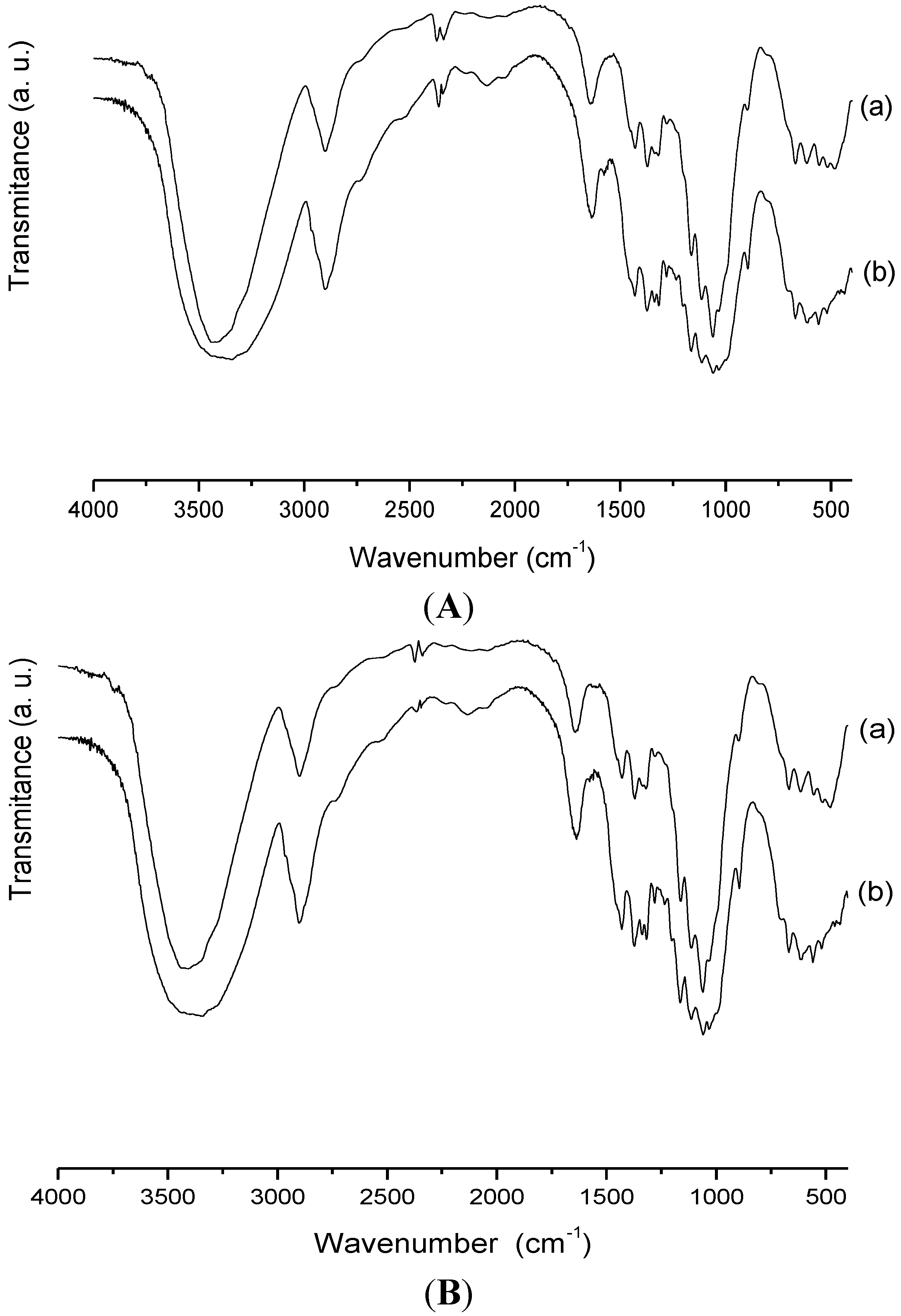
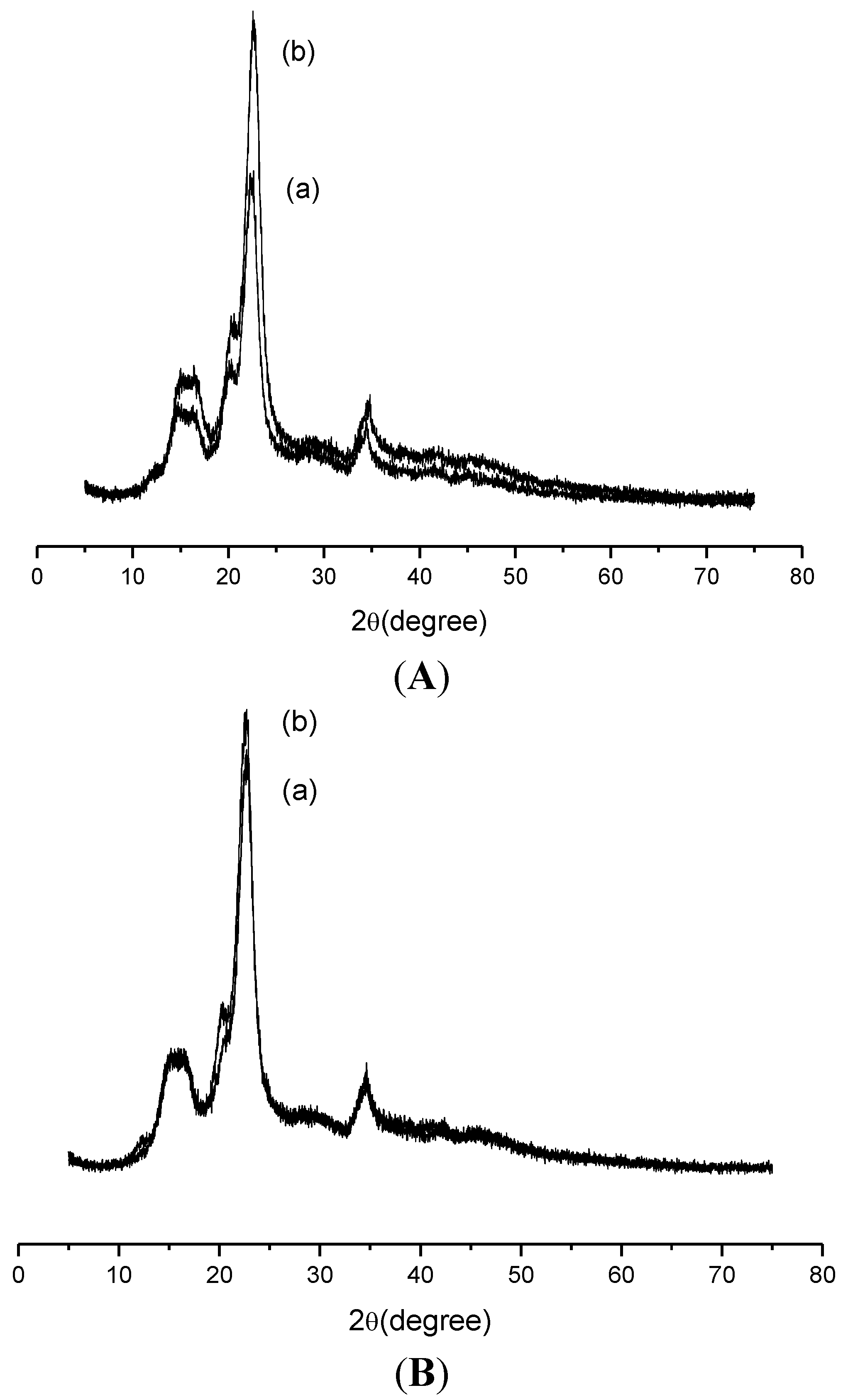
3. Experimental Section
3.1. Reagents
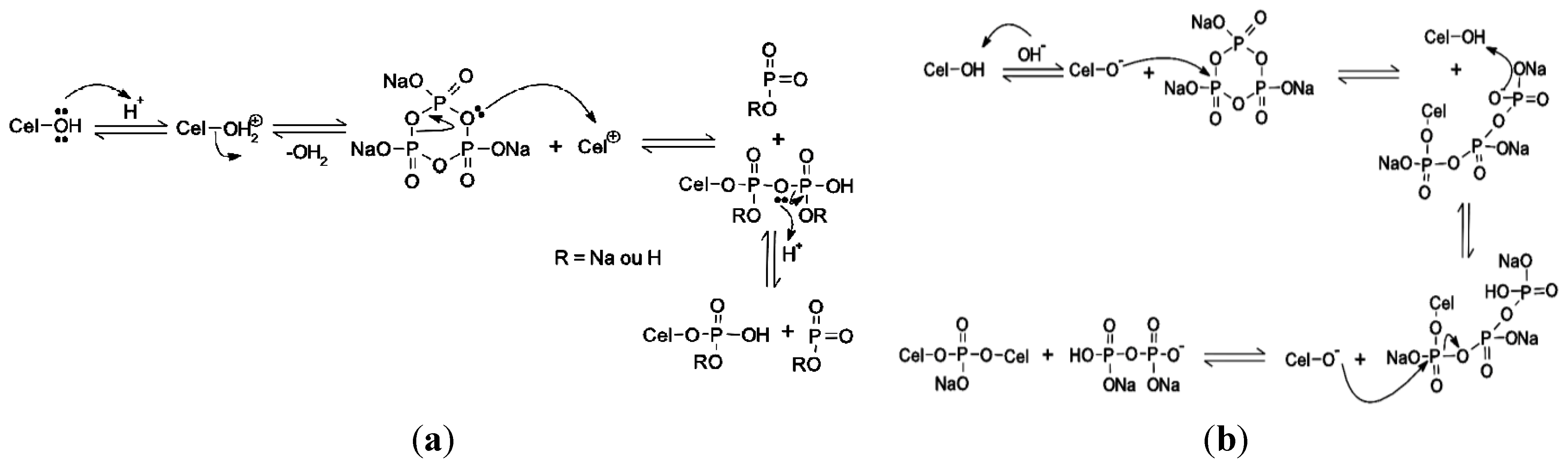
3.2. Synthesis of Phosphated Cellulose
3.3. Characterization
3.4. Ion Exchange Capacity
3.5. Sorption
3.5.1. Influence of pH
3.5.2. Influence of Time
3.5.3. Influence of Concentration and Temperature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bergheim, M.; Gieré, R.K. Biodegradability and ecotoxicitiy of tramadol, ranitidine, and their photoderivatives in the aquatic environment. Environ. Sci. Pollut. Res. 2012, 19, 72–85. [Google Scholar] [CrossRef]
- Shen, R.; Andrews, S.A. Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection. Water Res. 2011, 45, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Pomati, F.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, C. Effects of a complex mixture of therapeutic drugs at environmental levels on human embryonic cells. Environ. Sci. Techol. 2006, 40, 2442–2447. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Yeh, D.H.; Trotz, M.A. Removing pharmaceuticals and endocrine-disrupting compounds wastewater by photocatalysis. Technol. Biotechnol. 2007, 82, 121–134. [Google Scholar] [CrossRef]
- Purkait, M.K.; de DasGupta, S. Adsorption of eosin dye on activated carbon and its surfactant based desorption. J. Environ. Manag. 2005, 76, 135–142. [Google Scholar] [CrossRef]
- Kobya, M. Removel of Cr(IV) from aqueous solutions by adsorption onto hazelnut shell activated carbon: Kinetic and equilibrium studies. Bioresour. Technol. 2004, 91, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Kargı, F.; Pamukoglu, M.Y. Adsorbent supplemented biological treatment of pre-treated landfill leachate by fed-batch operation. Bioresour. Technol. 2004, 94, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Kondo, K.; Ohto, K.; Inoue, K.; Baba, Y. Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React. Funct. Polym. 2008, 68, 376–383. [Google Scholar] [CrossRef]
- Granja, P.L.; de Jéso, B.; Bareille, R.; Rouais, F.; Baquey, C.; Barbosa, M.A. Cellulose phosphates as biomaterials. In vitro biocompatibility studies. React. Funct. Polym. 2006, 66, 728–739. [Google Scholar] [CrossRef]
- Magoso, H.A.; Fattori, N.; Arenas, L.T.; Gushikem, Y. New promising composite materials useful in the adsorption of Cu(II) in ethanol based on cellulose acetate. Cellulose 2012, 19, 913–923. [Google Scholar] [CrossRef]
- Oshima, T.; Taguchi, S.; Ohe, K.; Baba, Y. Phosphorylated bacterial cellulose for adsorption of proteins. Carbohydr. Polym. 2011, 83, 953–958. [Google Scholar] [CrossRef]
- Mucalo, M.R.; Kato, K.; Yokogawa, Y. Phosphorylated cellulose-based substrates as potential adsorbents for bone morphogenetic proteins in biomedical applications: A protein adsorption screening study using cytochrome C as a bone morphogenetic protein mimic. Colloids Surf. B Biointerfaces 2009, 71, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wanrosli, W.D.; Rohaizu, R.; Ghazali, A. Synthesis and characterization of cellulose phosphate from oil palm empty fruit bunches microcrystalline cellulose. Carbohydr. Polym. 2011, 84, 262–267. [Google Scholar] [CrossRef]
- Kim, S.S.; Jeong, W.Y.; Shin, B.C.; Oh, S.Y.; Kim, H.W.; Rhee, J.M. Behavior of CHO cells on phosphated cellulose membranes. J. Biomed. Mater. Res. 1998, 40, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Igura, M.; Okazaki, M. Cadmium sorption characteristics of phosphorylated sago starch-extraction residue. J. Hazard. Mater. 2010, 178, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Sulflet, D.M.; Chitanu, G.C.; Popa, V.I. Phosphorylation of polysaccharides: New results on synthesis and characterization of phosphorylated cellulose. React. Funct. Polym. 2006, 66, 1240–1249. [Google Scholar] [CrossRef]
- Granja, O.L.; Pouységu, L.; Pétraud, M.; de Jéso, B.; Baquey, C.; Barbosa, M.A. Cellulose phosphates as biomaterials. I. Synthesis and characterization of highly phosphorylated cellulose gels. J. Appl. Polym. Sci. 2001, 82, 3341–3353. [Google Scholar] [CrossRef]
- Paiva, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry; Saunders College: Orlando, FL, USA, 1996. [Google Scholar]
- Silverstein, R.M.; Webmaster, F.X. Identificação Espectrométrica de Compostos Orgânicos, 6th ed.; Livros Técnicos Cientificos (LTC): Rio de Janeiro, Brazil, 2000. (In Portuguese) [Google Scholar]
- Sang, Y.; Prakash, O.; Seib, P.A. Characterization of phosphorylated cross-linked resistant starch by 31P nuclear magnetic resonance (31P NMR) spectroscopy. Carbohydr. Polym. 2007, 67, 201–212. [Google Scholar] [CrossRef]
- Ott, E.; Spurlin, H.M.; Grafflin, M.W. Cellulose and Cellulose Derivatives; Interscience Publishers: New York, NY, USA, 1954. [Google Scholar]
- Daud, W.R.W.; Kassim, M.H.M.; Mohamded, M.A.S. Cellulose phosphate from oil palm biomass as potential biomaterials. BioResource 2011, 6, 1719–1740. [Google Scholar]
- Suzuki, S.; Suzuki, F.; Kanie, Y.; Tsujitani, K.; Hirai, A.; Kaji, H.; Horii, F. Structure and crystallization of sub-elementary fibrils of bacterial cellulose isolated by using a fluorescent brightening agent. Cellulose 2012, 19, 713–727. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Voorwald, H.C.J.; Cioffi, M.O.H.; Pereira, M.L.C.S. Preparação e caracterização de materiais híbridos celulose/NbOPO4·nH2O a partir de celulose branqueada de bagaço de cana-de-açúcar. Polímeros 2012, 22, 88–95. (In Portuguese) [Google Scholar] [CrossRef]
- Lim, S.; Seib, P.A. Preparation and pasting properties of wheat and corn starch phosphates. Cereal Chem. 1993, 70, 137–144. [Google Scholar]
- Liu, Z.; Wang, H.; Li, Z.; Lu, X.; Zhang, X.; Zhang, S.; Zhou, K. Characterization of the regenerated cellulose films in ionic liquids and rheological properties of the solutions. Mater. Chem. Phys. 2011, 128, 220–227. [Google Scholar] [CrossRef]
- Guaratini, C.C.I.; Zanoni, V.B. Corantes têxteis. Quím. Nova 2000, 23, 71–78. (In Portuguese) [Google Scholar] [CrossRef]
- Bezerra, R.D.S.; Silva, M.M.F.; Morais, A.I.S.; Santos, M.R.M.C.; Airoldi, C.; Silva Filho, E.C. Natural cellulose for ranitidine drug removal from aqueous solution. J. Environ. Chem. Eng. 2013, 2, 605–611. [Google Scholar] [CrossRef]
- Santana, S.A.A.; Vieira, A.P.; Silva Filho, E.C.; Melo, J.C.P.; Airoldi, C. Immobilization of ethylenesulfide on babassu coconut epicarp and mesocarp for divalent cation sorption. J. Hazard. Mater. 2010, 174, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.; Sharma, A. Adsorption of Pb(II) from aqueous solution by Azadirachta indica (Neem) leaf powder. J. Hazard. Mater. 2004, 113, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Kalavatht, M.H.; Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. J. Colloid Interface Sci. 2005, 292, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.M.F. Über die adsorption in lösungen. Phys. Chem. 1906, 57A, 385–470. (In German) [Google Scholar]
- Zheng, H.; Liu, D.; Zheng, Y.; Liang, S.; Liu, Z. Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J. Hazard. Mater. 2009, 167, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Temkim, M.J.; Pyzhev, V. Recent modifications to Lagmuir isotherms. Acta Phys. 1940, 12, 217–222. [Google Scholar]
- Vieira, A.P.; Santana, S.A.A.; Bezerra, C.W.B.; Silva, H.A.S.; Chaves, J.A.P.; de Melo, J.C.P.; Silva Filho, E.C.; Airoldi, C. Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp. J. Hazard. Mater. 2009, 166, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.D.; Aramrego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Elsevier: Oxford, UK, 1988. [Google Scholar]
- Deetae, P.; Shobsngob, S.; Varanyanond, W.; Chinachoti, P.; Naivikul, O.; Varavinit, S. Preparation, pasting properties and freeze-thaw stability of due modified crosslink-phosphorylated rice starch. Carbohydr. Polym. 2008, 73, 351–358. [Google Scholar] [CrossRef]
- Rungrodnimitchai, S. Rapid preparation of biosorbents with high ion exchange capacity from rice straw and bagasse for removal of heavy metals. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloester stoffe, Kungliga Svenska Vetenskapsakad. Handle 1898, 24, 1–39. (In Swedish) [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, R.D.S.; Silva, M.M.F.; Morais, A.I.S.; Osajima, J.A.; Santos, M.R.M.C.; Airoldi, C.; Filho, E.C.S. Phosphated Cellulose as an Efficient Biomaterial for Aqueous Drug Ranitidine Removal. Materials 2014, 7, 7907-7924. https://doi.org/10.3390/ma7127907
Bezerra RDS, Silva MMF, Morais AIS, Osajima JA, Santos MRMC, Airoldi C, Filho ECS. Phosphated Cellulose as an Efficient Biomaterial for Aqueous Drug Ranitidine Removal. Materials. 2014; 7(12):7907-7924. https://doi.org/10.3390/ma7127907
Chicago/Turabian StyleBezerra, Roosevelt D. S., Márcia M. F. Silva, Alan I. S. Morais, Josy A. Osajima, Maria R. M. C. Santos, Claudio Airoldi, and Edson C. Silva Filho. 2014. "Phosphated Cellulose as an Efficient Biomaterial for Aqueous Drug Ranitidine Removal" Materials 7, no. 12: 7907-7924. https://doi.org/10.3390/ma7127907





