3.2. Characterization and Catalytic Activities of Several Single Metallic Oxides
Several single metallic oxides which were found pure from XRD investigation were tested under the same reaction conditions as mentioned previously. Since the reaction condition was oxygen deficient, oxygenate products and CO were also examined during the reaction. CO2 selectivity was calculated based on the reaction products detected by GC, which are CO2, CO, formic acid, acrolein, acetaldehyde, and ethanol. In fact, more oxidation products may still exist with low concentrations and the real selectivity may be lower. However, the CO2 selectivity calculated here is still suitable to compare catalytic activities of the catalysts working under the same conditions and being calculated by the same way.
Propylene conversion and CO
2 selectivity of the investigated oxides are shown in
Table 1 and
Table 2, respectively. Amongst these catalysts, NiO was only studied at temperatures below 350 °C due to its low thermal resistance. At higher temperatures, it was observed that NiO particles were broken-up, resulting in blocking of the reactor. The same observation was also seen with MnO
2 at temperatures above 450 °C. However, the instability of these catalysts at high temperatures was only due to mechanical reason since TGA/DTA and XRD results indicated no change in phase compositions of the samples at the reaction temperatures. Therefore, their mechanically instability will not significantly influence their catalytic activities.
The results from
Table 1 show that the propylene conversions of the catalysts almost reach to a maximum value at a certain temperature. MnO
2 and Co
3O
4 exhibit high conversions from 250 °C on, whereas NiO shows a high conversion at 350 °C, V
2O
5 and CeO
2 at 400 °C and SnO
2, ZnO at 450 °C. For Al
2O
3, ZrO
2 and CuO, propylene conversion still stays very low even at high temperatures up to 500 °C. Co
3O
4 and NiO catalysts result in the highest propylene conversion. MnO
2 and CeO
2 exhibited only a slightly lower conversion.
Table 1.
Propylene conversion (%) of several oxides at different reaction temperatures.
Table 1.
Propylene conversion (%) of several oxides at different reaction temperatures.
| Samples | 200 °C | 250 °C | 300 °C | 350 °C | 400 °C | 450 °C | 500 °C |
|---|
| Al2O3 (118 m2/g) | 2.67 | 2.34 | 2.25 | 2.42 | 2.77 | 3.78 | 4.69 |
| CeO2 (33 m2/g) | 2.85 | 3.30 | 13.09 | 15.31 | 22.41 | 24.52 | 25.44 |
| Co3O4 (11 m2/g) | 5.69 | 28.78 | 29.42 | 29.74 | 30.00 | 32.97 | 41.67 |
| NiO (11 m2/g) | 5.68 | 4.70 | 6.95 | 29.45 | - | - | - |
| SnO2 (9 m2/g) | 2.91 | 2.48 | 3.27 | 4.47 | 8.28 | 16.87 | 17.67 |
| TiO2 (54 m2/g) | 1.95 | 3.05 | 2.70 | 4.35 | 11.68 | 17.06 | 18.18 |
| V2O5 (4 m2/g) | 2.93 | 2.57 | 4.21 | 12.25 | 22.69 | 19.58 | 19.73 |
| ZrO2 (52 m2/g) | 2.92 | 2.04 | 2.32 | 2.59 | 3.01 | 4.14 | 6.02 |
| MnO2 (6 m2/g) | 5.40 | 21.77 | 22.72 | 23.48 | 23.17 | 24.22 | - |
| ZnO (14 m2/g) | 3.99 | 3.75 | 3.71 | 4.11 | 8.86 | 23.16 | 33.04 |
| CuO (2 m2/g) | 0.29 | 5.17 | 5.82 | 6.73 | 6.55 | 8.08 | 9.36 |
Table 2.
CO2 selectivity (%) of some metal oxides at different reaction temperatures.
Table 2.
CO2 selectivity (%) of some metal oxides at different reaction temperatures.
| Samples | 250 °C | 300 °C | 350 °C | 400 °C | 450 °C | 500 °C |
|---|
| Al2O3 | - | - | - | - | 24.4 | 46.72 |
| CeO2 | - | 100 | 86.54 | 89.07 | 89.02 | 89.18 |
| Co3O4 | 94.71 | 94.38 | 93.95 | 80.56 | 76.16 | 39.31 |
| NiO | 100 | 100 | 91.78 | - | - | - |
| SnO2 | - | - | 34.68 | 71.39 | 70.47 | 71.64 |
| TiO2 | - | - | 41.61 | 39.80 | 30.66 | 21.83 |
| V2O5 | - | 29.61 | 20.22 | 29.09 | 31.18 | 34.08 |
| ZrO2 | - | - | - | - | 24.44 | 36.63 |
| MnO2 | 98.47 | 99.32 | 90.54 | 92.03 | 90.86 | - |
| ZnO | - | - | 22.51 | 31.11 | 46.52 | 76.07 |
| CuO | - | - | 25.88 | 17.00 | 34.05 | 43.41 |
These catalysts also possess rather high CO
2 selectivity as seen from
Table 2. However, CO
2 selectivity of Co
3O
4 decreases dramatically at high temperatures due to the formation of more CO (selectivity at 500 °C is 61%). Oxygenated products were observed in the oxidation of propylene on all investigated catalysts but especially found in the reactions with V
2O
5, SnO
2, TiO
2, ZnO since these catalysts are well known catalysts for partial oxidation of hydrocarbons. Oxygenated products may also be formed when using CuO and ZrO
2 but because CuO and ZrO
2 exhibited low conversions, the amount of formed oxygenated products may be too low to be detected. For V
2O
5, SnO
2, TiO
2, ZnO, CO
2 selectivity was low at 350–400 °C because these temperatures are optimal for partial oxidation to form oxygenate products (selectivity of oxygenate products may reach about 30%). When increasing temperature, CO
2 selectivity on these catalysts increased significantly as the formed oxygenated products were also completely oxidized to CO
2. Amongst the investigated catalysts, CeO
2, and especially MnO
2 exhibit quite constant and highest CO
2 selectivity at all examined temperatures. When BET surface areas (
Table 1) were taken into account, it is clear that high surface area oxides such as Al
2O
3, TiO
2, ZrO
2 exhibited low activity, they are only suitable to be supports. Oppositely, low surface area oxides such as MnO
2, Co
3O
4, NiO, CeO
2 exhibited good activity and suitability to act as active phases. Therefore, if surface areas of highly active oxides are increased, the activities of the catalysts will be improved.
In general, MnO
2, CeO
2 and Co
3O
4 are the most promising catalysts to convert propylene under oxygen deficient conditions. CeO
2 has a high ability to convert propylene with high CO
2 selectivity at all investigated reaction temperatures due to a high OSC as discussed in literature [
16]. Co
3O
4 has a high propylene conversion at low temperatures but also a low CO
2 selectivity at high temperatures. MnO
2 shows high activity for both propylene conversion and CO
2 selectivity but it is mechanically unstable at high temperatures. Therefore, in the following investigation, catalyst mixtures containing CeO
2 will be focused on.
3.3. Characterization and Catalytic Activities of Mixtures of CeO2
The catalytic activity of mixtures of CeO2 and SnO2 with different compositions was investigated since it was expected that the addition of the high conductive semiconductor SnO2 on the highly active catalyst CeO2 will improve the reaction due to the increase of the available lattice oxygen, which may act as an oxidizing agent at high temperatures. However, no development of catalytic activity has been observed with the mixtures of CeO2 and SnO2.
Although catalytic activity of ZrO
2 was low as seen from the previous section, the catalytic activities of CeO
2–ZrO
2 chemical mixtures were also studied since the literature reports that ZrO
2 is able to modify the sub-lattice oxygen in the CeO
2–ZrO
2 mixed oxides, generating defective structures and highly mobile oxygen atoms in the lattice which can be released even at moderate temperatures [
12,
13,
34]. Therefore, the activities of these chemical mixtures are expected to be increased. Moreover, ZrO
2 exhibited higher surface area than CeO
2 as shown in
Table 1, thus, the addition of ZrO
2 may help to increase surface area of the catalysts to improve their activities.
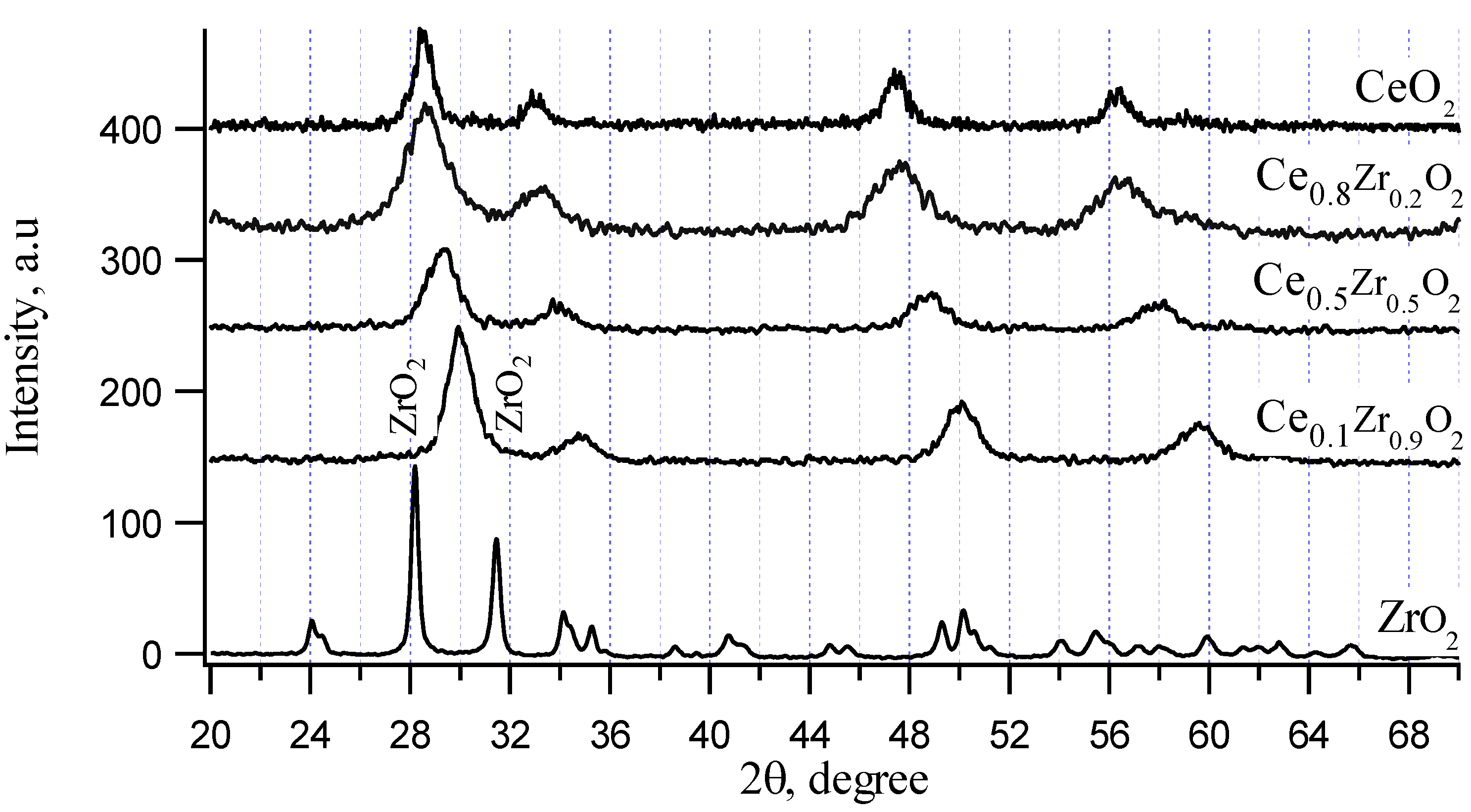
XRD patterns of CeO
2–ZrO
2 chemical mixtures are shown in
Figure 1. CeO
2 exhibits a cubic structure (a, b, c parameter is 5.4 nm) represented by 2θ = 28.6° and 33.1°, ZrO
2 calcined at 550 °C (temperature lower than 1170 °C) exhibits a monoclinic structure represented by 2θ = 28.2° and 31.6°. The evidence of solid solution formation is given by the shift of ceria reflections to higher values for Ce
0.8Zr
0.2O
2 and Ce
0.5Zr
0.5O
2 samples, where Zr (atomic radius is 160 pm) replaced for Ce (atomic radius is 181.8 pm) in the cubic structure of CeO
2. In the case of sample with higher Zr ratio, it has been reported in the literature [
35] that a compound Ce
0.25Zr
0.75O
2 shows the strongest XRD reflection at 2θ = 30°. In our work, the sample Ce
0.1Zr
0.9O
2 also showed a cubic structure with the strongest XRD reflection at 2θ = 30°. Besides, single ZrO
2 monoclinic phase still existed, which represented by small peaks at 2θ = 28.2° and 31.6° as observed in XRD pattern of this sample. Here, it should be noticed that ZrO
2 also exist as cubic structure (a, b, c parameter is 5.1 nm) when synthesized at high temperature (more than 2370 °C). Thus, cubic structure of ZrO
2 is very close to that of CeO
2 with almost the same parameters. The strongest XRD reflection of this cubic ZrO
2 is at 2θ = 30°. Therefore, in the presence of CeO
2, ZrO
2 cubic structure may already be formed at low temperature because a few percentage of other oxide may stabilize cubic ZrO
2. Since the structure of cubic ZrO
2 and cubic CeO
2 are very similar (very close a, b, c parameters and strongest XRD reflections) and the content of CeO
2 in Ce
0.1Zr
0.9O
2 sample was small, XRD patterns of Ce
0.1Zr
0.9O
2 sample looked like that of pure cubic CeO
2 with 2θ shifted to 30° but the existed phase could be mainly assigned for cubic ZrO
2.
Figure 1.
X-ray patterns of CeO2-ZrO2 chemical mixtures.
Figure 1.
X-ray patterns of CeO2-ZrO2 chemical mixtures.
All investigated CeO2–ZrO2 chemical mixtures possess surface areas around 50 m2/g, which are almost equal to those of pure ZrO2 (52 m2/g). Pure CeO2 possesses a little lower surface area (33 m2/g).
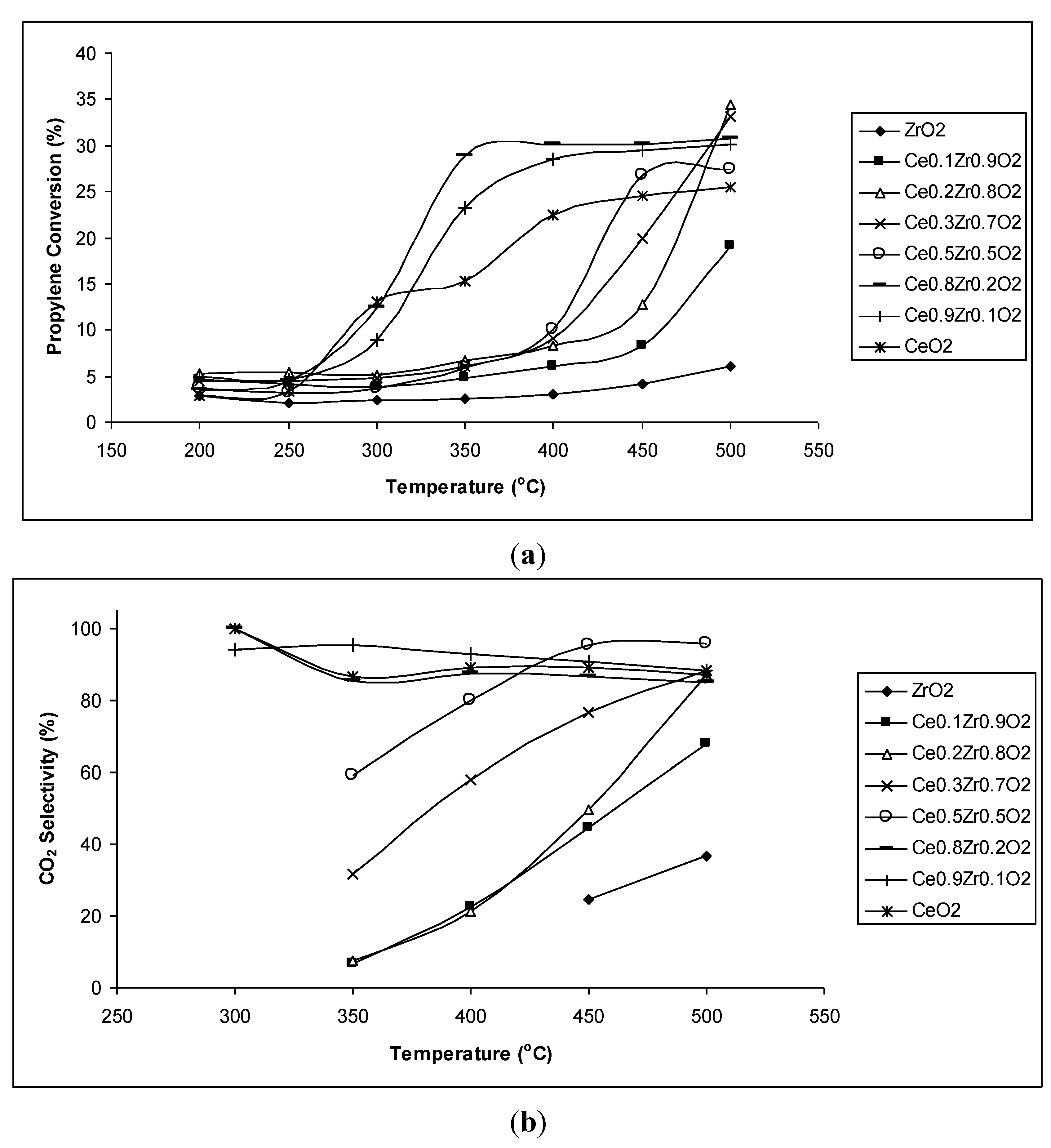
The propylene conversions of CeO
2–ZrO
2 chemical mixtures presented in
Figure 2a indicate that the samples containing a small content of ZrO
2 (10%–20% mol, such as Ce
0.9Zr
0.1O
2 and Ce
0.8Zr
0.2O
2) exhibit high propylene conversions, even at low temperature (350 °C). Their propylene conversions are even higher than that of the most active pure components CeO
2, thus, a synergy effect has occurred. Meanwhile, the samples with higher ZrO
2 content only reach high conversions at high temperatures (450 °C, 500 °C). Their conversions, however, are higher than that of the least active pure component (ZrO
2).
Figure 2.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of CeO2–ZrO2 chemical mixtures at different reaction temperatures.
Figure 2.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of CeO2–ZrO2 chemical mixtures at different reaction temperatures.
Figure 2b shows CO
2 selectivity of CeO
2–ZrO
2 chemical mixtures at different reaction temperatures. It can be seen that CO
2 selectivity of all CeO
2–ZrO
2 chemical mixtures is much higher than that of the least active pure component (ZrO
2). However, only samples with low ZrO
2 content (Ce
0.9Zr
0.1O
2 and Ce
0.8Zr
0.2O
2) exhibit comparable and quite constant high CO
2 selectivity. The high ZrO
2 content samples possess low CO
2 selectivity at temperatures ranging from 350–400 °C since these temperatures were favorable for partial oxidation to form oxygenated products. Combining propylene conversion and CO
2 selectivity, it is clear that Ce
0.9Zr
0.1O
2 and Ce
0.8Zr
0.2O
2 samples exhibit the highest activity for the complete oxidation of propylene into the nontoxic product CO
2. The activity of these catalysts even increased significantly when the reaction was performed under the oxygen sufficient condition (molar ratio of C
3H
6/O
2 was 1/4). The propylene conversions under the oxygen sufficient condition at 500 °C on Ce
0.9Zr
0.1O
2 and Ce
0.8Zr
0.2O
2 catalysts were 77.10% and 85.42%, respectively. The CO
2 selectivity under this condition was above 97%. The catalytic activity of Ce
xZr
1−xO
2 for the oxidation of propylene under oxygen excess conditions have been reported by D. Homsi
et al. [
36], who found no enhancement of activity of the Ce
0.75Zr
0.25O
2 solid solution compared to that of pure CeO
2. That result is a bit different with the result of this work as Ce
0.8Zr
0.2O
2 exhibited higher activity than that of pure CeO
2. The reason may be the different synthesis methods, which may result in different properties of the final products. The catalyst in our work was prepared by solgel synthesis, which may allow better access of zirconium into the structure of ceria, leading to the increase of mobile oxygen as seen from TPR-H
2 results (
Table 3). Oppositely, the catalysts in D. Homsi’s work were prepared by precipitation, the method allows worse mixing of components than sol-gel method. Moreover, surface area of our Ce
0.8Zr
0.2O
2 was higher than that of our CeO
2 while it is opposite in D. Homsi’s work. Therefore, both catalytic activity and reduction ability of Ce
0.75Zr
0.25O
2 was not higher than that of CeO
2 in D. Homsi’s work. Besides, different reaction conditions (oxygen deficient in our work and oxygen excess in D. Homsi’s work) may also influenced since the advance properties of a solid solution will be more presented under oxygen deficient conditions.
Table 3.
Quantity of hydrogen consumed (mL/g) at different reduction peaks in TPR-H2 profiles of pure CeO2, ZrO2, Co3O4 and some potential CeO2 chemical mixtures.
Table 3.
Quantity of hydrogen consumed (mL/g) at different reduction peaks in TPR-H2 profiles of pure CeO2, ZrO2, Co3O4 and some potential CeO2 chemical mixtures.
| | Samples | CeO2 (33 m2/g) | ZrO2 (52 m2/g) | Co3O4 (11 m2/g) | Ce0.9Zr0.1O2 (42 m2/g) | Ce0.8Zr0.2O2 (46 m2/g) | 20% CeO2–80% Co3O4 (45 m2/g) |
|---|
| Temp. (°C) | |
|---|
| 279 | – | – | – | – | – | 28.97 |
| 316 | – | – | – | 9.55 | – | – |
| 364 | – | – | – | – | – | 12.21 |
| 375 | – | – | – | 2.84 | – | – |
| 430 | – | – | 250.54 | – | – | – |
| 474 | 4.62 | – | – | – | – | – |
| 503 | – | – | – | – | – | 101.25 |
| 531 | – | – | – | 18.16 | – | – |
| 536 | – | – | – | – | 10.29 | – |
| 580 | – | – | 39.25 | – | – | – |
| 623 | – | 0.91 | – | – | – | – |
| 625 | – | – | – | – | 12.04 | – |
| 642 | – | 4.36 | – | – | – | – |
| 688 | – | – | – | 2.89 | – | – |
| 694 | 6.23 | – | – | – | – | – |
| Total | 10.85 | 5.27 | 289.79 | 33.44 | 22.33 | 142.43 |
The reason for the enhancement in catalytic activity of Ce
0.9Zr
0.1O
2 and Ce
0.8Zr
0.2O
2 samples, thus, may be the formation of the solid solution Ce
1−xZr
xO
2 in the chemical mixtures of CeO
2–ZrO
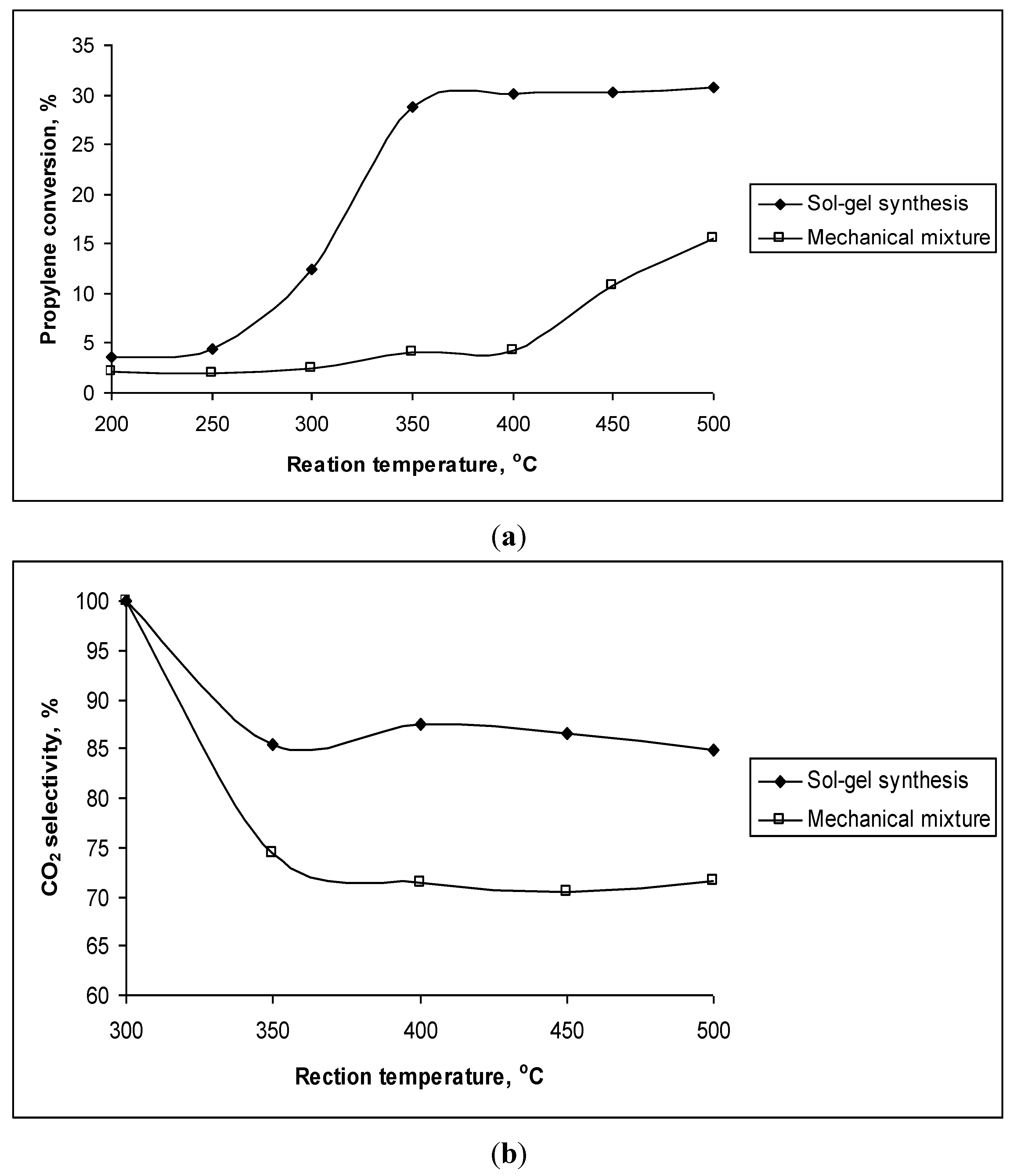
2. To prove this assumption, the CeO
2–ZrO
2 mechanical mixture containing 80% mol CeO
2 was also tested for the reaction (
Figure 3). This mechanical mixture showed the presence of only single CeO
2 and ZrO
2 phases but not solid solution. It can be observed that both propylene conversion and CO
2 selectivity of the mechanical sample are much lower than that of the sol-gel prepared sample, where the formation of a solid solution Ce
0.8Zr
0.2O
2 was detected.
Figure 3.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of the mixture containing 80% mol CeO2 synthesized by mechanical mixing and sol-gel method (Ce0.8Zr0.2O2) at different reaction temperatures.
Figure 3.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of the mixture containing 80% mol CeO2 synthesized by mechanical mixing and sol-gel method (Ce0.8Zr0.2O2) at different reaction temperatures.
However, the sol-gel samples with high content of ZrO
2 did not show improvement of activity. Thus, it may be assumed only a little change in the structure of Ce
1−xZr
xO
2 solid solution compared to that of CeO
2 helps to increase catalytic activity. For other Ce
1−xZr
xO
2 solid solution (
x > 0.5), where the structure shows a big shift of ceria reflections to higher values, the increase of catalytic activity will not happen. The formation of a solid solution with a little replacement of Zr ions to Ce ions may increase the catalytic activity since this replacement may result in appropriate vacancies inside the bulk structures of the catalysts, which increases mobility of oxygen transported inside the bulk structures,
i.e., also increases the OSC of the catalyst. An evidence of the increase of OSC of the Ce
1−xZr
xO
2 catalyst with low ZrO
2 content compared to pure oxides was estimated based on TPR-H
2 profiles of the pure oxides and chemically mixed samples (
Table 3). The consumed H
2 quantities on the solid solutions of CeO
2 and ZrO
2 (Ce
0.9Zr
0.1O
2 and Ce
0.8Zr
0.2O
2) were much higher than those on pure oxides (CeO
2 and especially ZrO
2). At the same time, the temperature of hydrogen reduction decreased significantly on the Ce
0.9Zr
0.1O
2 catalyst (the lowest reduction temperature of Ce
0.9Zr
0.1O
2 sample is only 316 °C while that of CeO
2 is 474 °C and of ZrO
2 is 623 °C), therefore, the catalyst reached to high activity at lower temperature. However, the fact that the catalytic activity of Ce
0.9Zr
0.1O
2 was a little lower than that of Ce
0.8Zr
0.2O
2 although the consumed hydrogen amount of Ce
0.9Zr
0.1O
2 was a little higher than that of Ce
0.8Zr
0.2O
2 is a bit non logical. Here, the influence of surface area might be a reason as surface area of Ce
0.8Zr
0.2O
2 was higher than that of Ce
0.9Zr
0.1O
2, which may help to expose more active sites.
Figure 4 shows the morphology change of the Ce
0.8Zr
0.2O
2 sol-gel sample before and after reaction (24 h on stream). Before the reaction, the sample possesses many pores. After reaction, almost all pores are encapsulated. The reason may be the exothermicity of complete oxidation of C
3H
6 and the formation of coke.
Figure 4.
SEM images of Ce0.8Zr0.2O2 sol-gel sample (a) before and (b) after reaction.
Figure 4.
SEM images of Ce0.8Zr0.2O2 sol-gel sample (a) before and (b) after reaction.
Chemical mixtures of CeO
2 and Co
3O
4 were also studied since the results in
Section 3.2 shows that Co
3O
4 exhibits high propylene conversion at low temperature although it did not exhibit high CO
2 selectivity at high temperatures. Meanwhile, CeO
2 exhibits high CO
2 selectivity at high temperatures although its propylene conversion is not as high as that of Co
3O
4 at low temperatures. Therefore, when CeO
2 and Co
3O
4 are mixed together, the obtained catalysts may exhibit high conversion of propylene at low temperatures and high CO
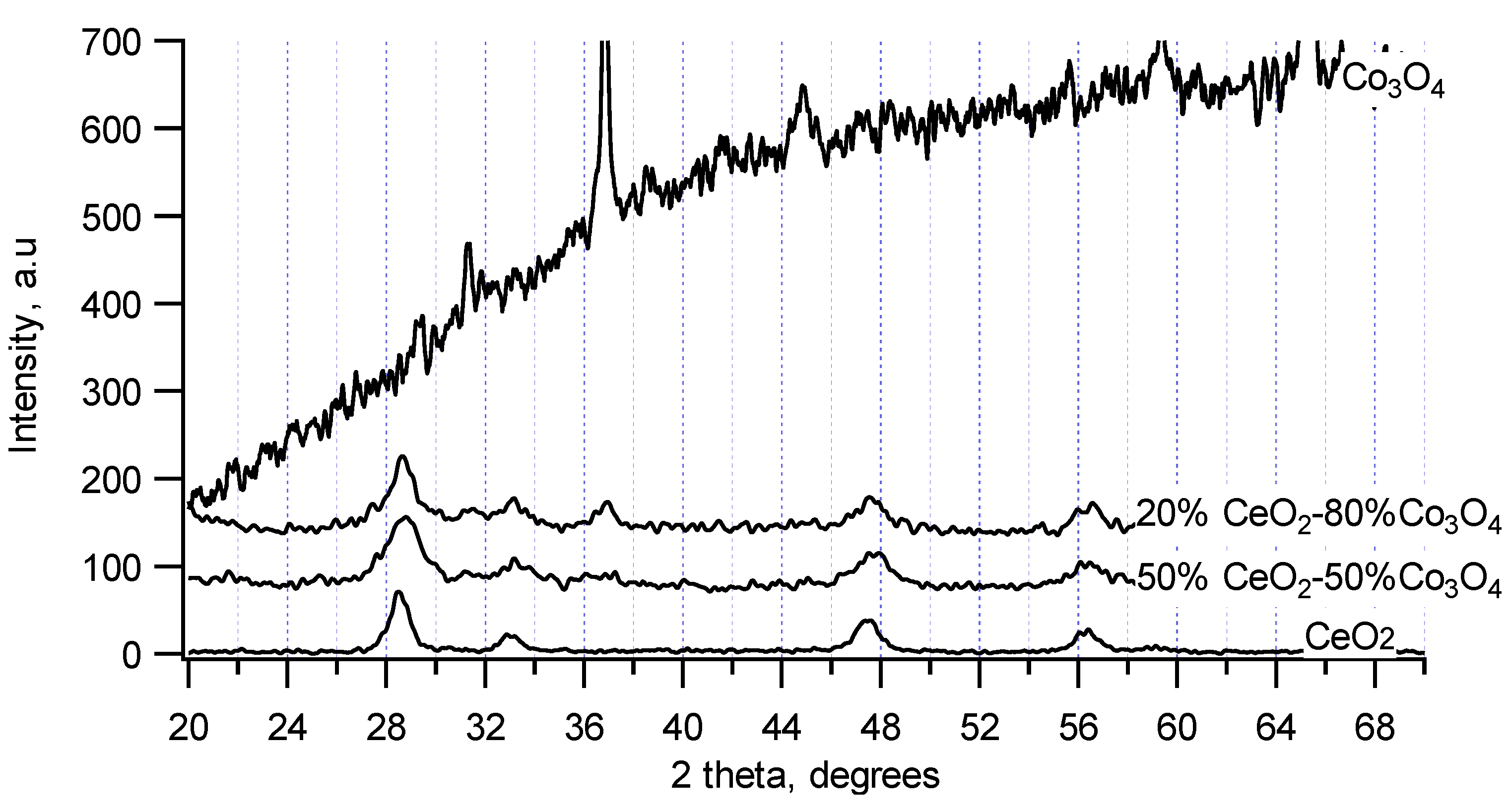
2 selectivity at high temperatures. XRD patterns of some CeO
2–Co
3O
4 chemical mixtures (20% and 50% mol of CeO
2) in the comparison with XRD patterns of pure CeO
2 and Co
3O
4 are presented in
Figure 5. Pure Co
3O
4 synthesized at 550 °C exhibited a strong amorphous nature with a high and rough baseline but the strongest XRD reflections of a cubic Co
3O
4 structure (a, b, c parameters of 8.1 nm) were still detected at 2θ = 31°, 37°, 45°, 59° and 65°. Meanwhile, XRD patterns of CeO
2–Co
3O
4 chemical mixtures (even up to 80% Co
3O
4) show the presence of mainly CeO
2-like structure. However, there are shifts of ceria reflections to higher 2θ values and rougher baselines than that of pure CeO
2. Thus, like the chemical mixtures of CeO
2 and ZrO
2, there may be also a formation of a solid solution in CeO
2–Co
3O
4 chemical mixtures with the replacement of Co (atomic radius is 125 pm) for Ce (atomic radius is 181.8 pm) in the structure of CeO
2. Because the content of Co
3O
4 was higher, Co
3O
4 phase may still be existed but as small or amorphous particles surround the solid solution of CeO
2–Co
3O
4, leading to the rougher baselines than that of pure CeO
2. The CeO
2–Co
3O
4 chemical mixtures possess surface areas around 45 m
2/g, which are higher than those of pure CeO
2 (33 m
2/g) and pure Co
3O
4 (11 m
2/g). These may be reasons for the higher activity of the mixtures compared to pure components as described below.
Figure 5.
X-ray patterns of CeO2-Co3O4 chemical mixtures.
Figure 5.
X-ray patterns of CeO2-Co3O4 chemical mixtures.
Propylene conversions and CO
2 selectivity of CeO
2–Co
3O
4 chemical mixtures are presented in
Figure 6. Compared to the single oxides, CeO
2–Co
3O
4 chemically mixed catalysts show high propylene conversions at lower temperature (200 °C). These chemical mixtures also possess as high CO
2 selectivity as that of pure CeO
2 at all reaction temperatures up to 400 °C. Under the oxygen sufficient condition (molar ratio of C
3H
6/O
2 was 1/4), CeO
2–Co
3O
4 chemical mixtures converted about 87% propylene since 250 °C with CO
2 selectivity of about 98%. Under oxygen excess condition (molar ratio of C
3H
6/O
2 was 1/5.5), CeO
2–Co
3O
4 chemical mixtures converted 100% propylene since 250 °C with CO
2 selectivity of 100%. Thus, the combination of Co
3O
4 with CeO
2 improves propylene conversion and CO
2 selectivity. Especially, this combination lowers the temperature of the maximum activity to 200 °C, which is very important for the treatment of hydrocarbon during the starting operation of the engines. However, the mechanical stability of Co
3O
4–CeO
2 chemical mixtures is low. The materials were broken-up at temperatures higher than 400 °C. Therefore, Co
3O
4–CeO
2 catalysts should be supported on high thermal resistant supports.
Figure 6.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of CeO2–Co3O4 chemical mixtures at different reaction temperatures.
Figure 6.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of CeO2–Co3O4 chemical mixtures at different reaction temperatures.
The reason for the enhancement in catalytic activity of CeO
2–Co
3O
4 chemical mixture samples may be the formation of the solid solution in the chemical mixtures of CeO
2–Co
3O
4 resulted by the replacement of Co atoms for Ce atoms as seen from XRD patterns (
Figure 5). To prove this assumption, the CeO
2–Co
3O
4 mechanical mixture containing 50 mol% CeO
2 was also tested for the reaction (
Figure 7). It can be observed that both propylene conversion and CO
2 selectivity of the mechanical sample are lower than that of the sol-gel prepared sample, where the formation of a solid solution was detected. Especially, like pure CeO
2 and Co
3O
4, the mechanical mixture exhibited very low propylene conversion at low temperature (200 °C) while much higher propylene conversion had already been obtained on the chemical mixture at this temperature.
Figure 7.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of the mixture containing 50 mol% CeO2 and 50 mol% Co3O4 synthesized by mechanical mixing and sol-gel method at different reaction temperatures.
Figure 7.
(a) Propylene conversion (%) and (b) CO2 selectivity (%) of the mixture containing 50 mol% CeO2 and 50 mol% Co3O4 synthesized by mechanical mixing and sol-gel method at different reaction temperatures.
TPR–H
2 data of pure CeO
2, Co
3O
4 and the chemical mixture of 20% CeO
2–80% Co
3O
4 in
Table 3 helped to explain the activity of the mixtures and the pure oxides. TPR–H
2 shows that Co
3O
4 exhibited an excellent mobility of oxygen as its consumed H
2 quantity was highest amongst the investigated catalysts. Co
3O
4 was also reduced at lower temperatures than CeO
2, which explains for the fact that Co
3O
4 exhibited good activity at lower temperature than CeO
2. The chemical mixture of 20% CeO
2–80% Co
3O
4 did not possess a larger quantity of mobile oxygen than pure Co
3O
4 but was reduced at lower temperature (since 279 °C); therefore, the chemical mixture of 20% CeO
2–80% Co
3O
4 was able to convert propylene at lower temperature than Co
3O
4. For the chemical mixture of 20% CeO
2–80% Co
3O
4, a solid solution was also formed as seen from XRD pattern of the sample, but the amount of consumed H
2 of the mixture was not higher than that of pure Co
3O
4. Therefore, this mixture did not result in higher conversion of propylene than those of pure oxides; the advantage of this catalyst was combining well activity of both CeO
2 and Co
3O
4 resulting in a catalyst with good activity at a wider temperature range. To explain for the fact that although the chemical mixture of 20% CeO
2–80% Co
3O
4 possesses lower quantity of mobile oxygen than pure Co
3O
4 but there was no decrease in catalytic activity, a careful look on both TPR-H
2 results and catalytic activities of good chemical mixtures of CeO
2 with ZrO
2 and Co
3O
4 also means something. It was seen that the chemical mixtures of CeO
2 with ZrO
2 (Ce
0.8Zr
0.2O
2, Ce
0.9Zr
0.1O
2) also exhibited good activity but the amount of consumed H
2 was only about 30 mL/g while that of CeO
2–Co
3O
4 chemical mixture was higher than 100 mL/g. Thus, it may be assumed that to ensure good oxidation of propylene, amount of mobile oxygen may be a certain value. If a catalyst possesses the amount of mobile oxygen higher than that necessary value, the activity may not increase significantly any more.
In order to explore the influences of co-existing gases and oxygen concentrations on catalytic performances, potential chemical mixtures of CeO
2 with ZrO
2 and Co
3O
4 (Ce
0.8Zr
0.2O
2 and 20% CeO
2 + 80% Co
3O
4) were studied in more details (
Table 4). It is clear from
Table 4 that these mixtures exhibited good activity for the oxidation of propylene not only under oxygen deficient condition but also under oxygen sufficient condition. Especially, under oxygen excess condition (condition 3 and 4), the chemical mixture of 20% CeO
2 and 80% Co
3O
4 was able to convert 100% propylene and CO since 200 °C. A presence of 2% H
2O did not influence significantly on the activity of this catalyst except that the minimum active temperature increases from 200 to 250 °C. The influence of CO and H
2O was also not significant for the catalyst Ce
0.8Zr
0.2O
2, proving that Ce
0.8Zr
0.2O
2 and 20% CeO
2–80% Co
3O
4 catalysts are stable catalyst for the oxidation of propylene under different reaction conditions. Compare to 20%CeO
2-80% Co
3O
4 catalyst, Ce
0.2Zr
0.2O
2 catalyst exhibited less activity under oxygen sufficient and excess conditions as conversion of propylene was less than and the temperature of the maximum activity was higher than those of 20% CeO
2–80% Co
3O
4 catalyst. Catalytic activities of these catalysts are comparable to those of noble catalysts under the same deficient and sufficient conditions (at the same air to fuel ratios of 10 and 14) [
27]. The catalyst mixtures of CeO
2 and Co
3O
4 in this work even exhibited an advantage of having maximum activity at lower reaction temperature. The catalysts were stable during different catalytic cycles; the conversion and selectivity were almost unchanged during at least three catalytic cycles.
Table 4.
The influences of co-existing gases (CO, H2O) and oxygen concentrations on catalytic activities (propylene conversion, %) of some potential chemical mixtures of CeO2 catalysts.
Table 4.
The influences of co-existing gases (CO, H2O) and oxygen concentrations on catalytic activities (propylene conversion, %) of some potential chemical mixtures of CeO2 catalysts.
| Temp. (°C) | Ce0.8Zr0.2O2 | 20% CeO2–80% Co3O4 |
|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
|---|
| 200 | 3.52 | 1.47 | - | 3.02 | 26.58 | 7.2 | 100 | 1.4 |
| 250 | 4.43 | - | - | 4.05 | 27.16 | 86.87 | 100 | 99.80 |
| 300 | 12.39 | 4.12 | - | 6.02 | 28.03 | 87.27 | 100 | 100 |
| 350 | 28.79 | 11.27 | 6.99 | 15.91 | 28.28 | 87.83 | 100 | 100 |
| 400 | 30.07 | 25.97 | 19.69 | 51.74 | - | 86.95 | 100 | 100 |
| 450 | 30.18 | 59.52 | 49.91 | 81.27 | - | 86.73 | 100 | 100 |
| 500 | 30.80 | 85.42 | 90.67 | 89.08 | - | 87.08 | 100 | 100 |
Mixtures of CeO
2 and MnO
2 were also expected to exhibit good activities since MnO
2 was one of the most active catalysts as investigated in
Section 3.2. However, this catalyst system requires a lot of detailed investigations in order to explain thoroughly their properties. Therefore, theses mixtures will be studied in a separate paper.