Flux-Aided Synthesis of Lu2O3 and Lu2O3:Eu—Single Crystal Structure, Morphology Control and Radioluminescence Efficiency
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis


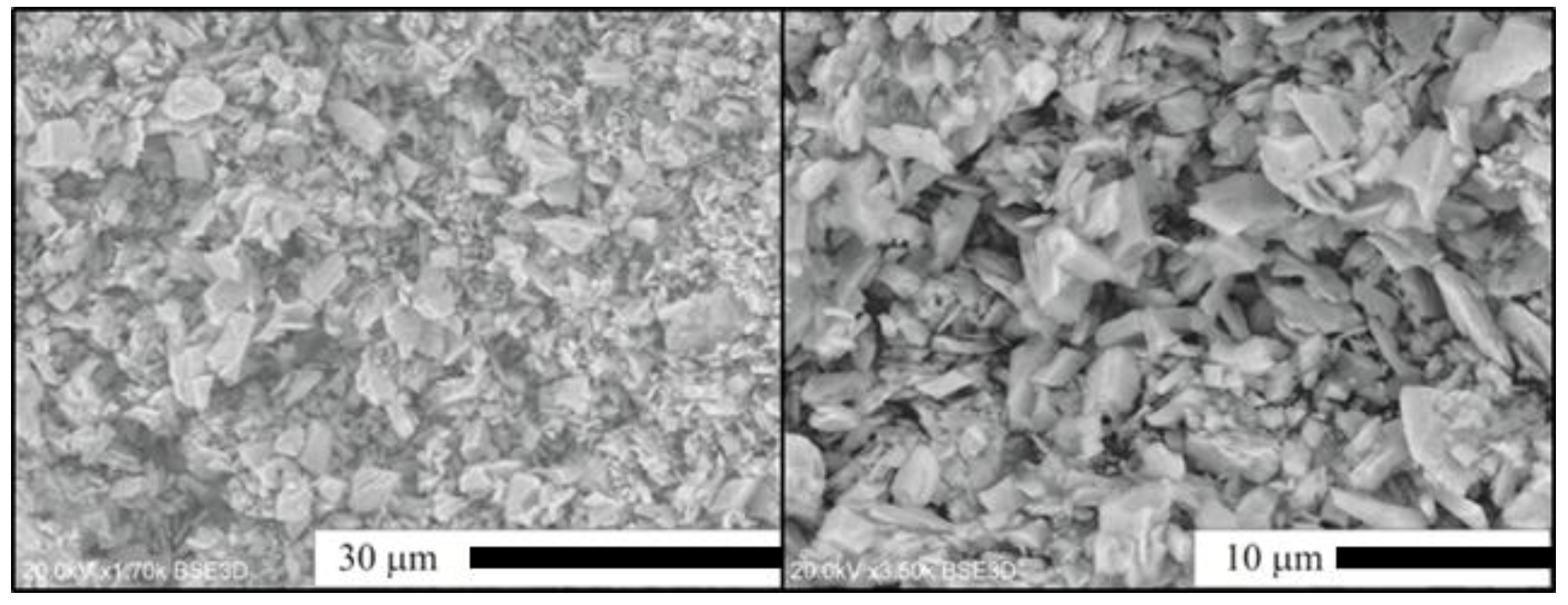
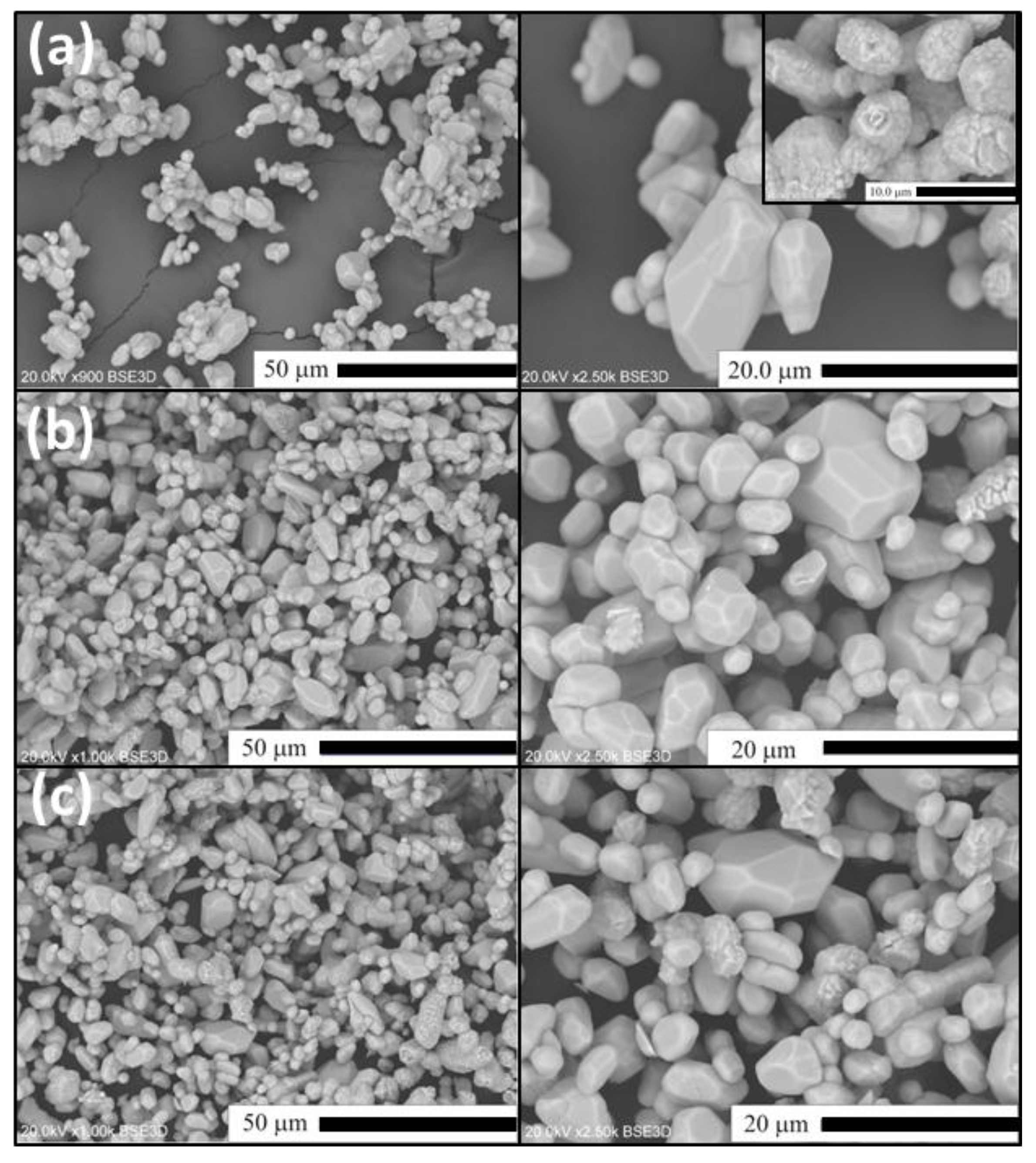
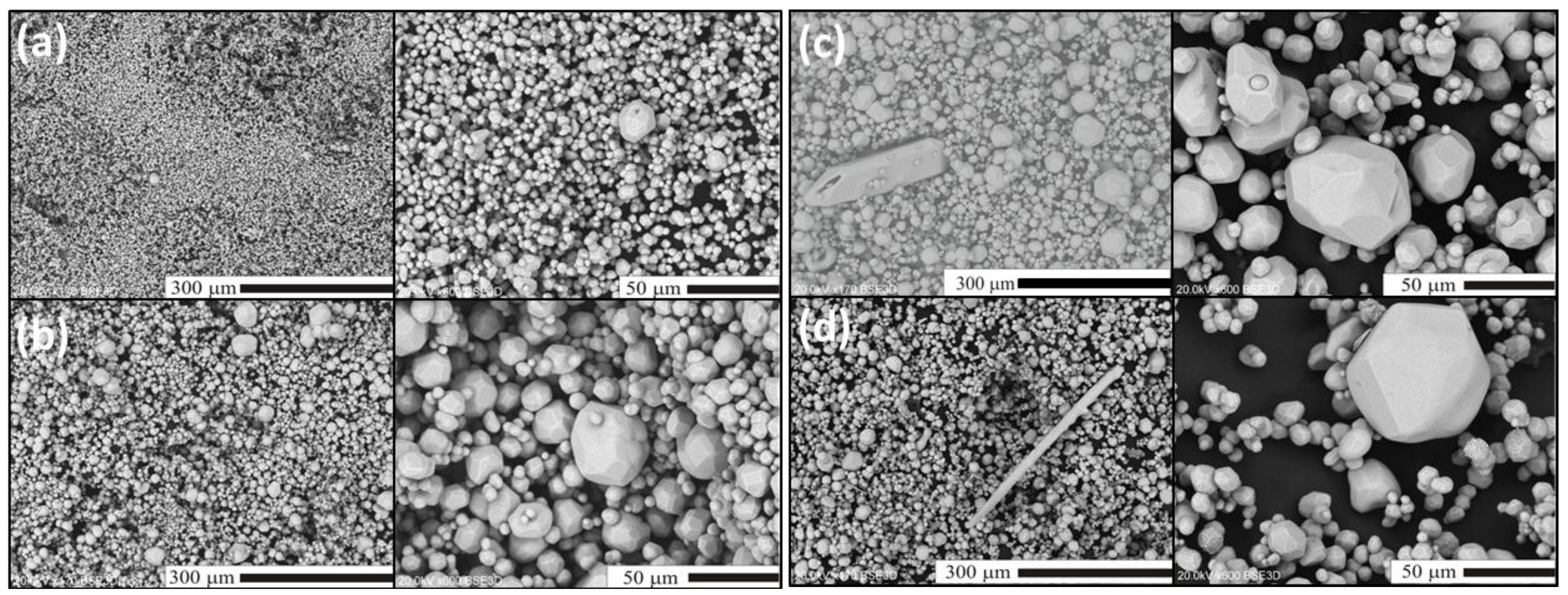
2.2. Flux-Stimulated Evolution of the Powders Morphology

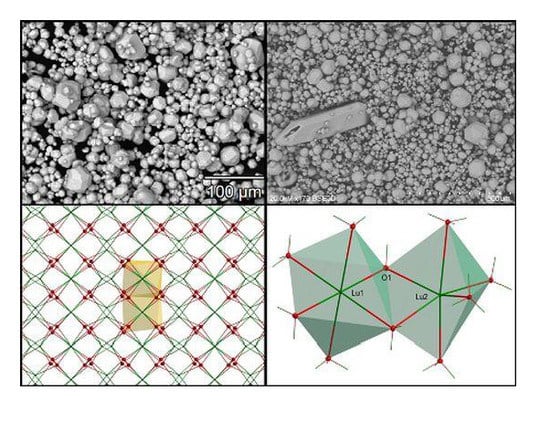
2.3. Single Crystal Structural Analysis
| Chemical Formula | Lu2O3 |
|---|---|
| Formula Mass | 397.94 |
| Crystal system | regular |
| a/Å | 10.393(2) |
| Unit cell volume/Å3 | 1122.6(6) |
| Temperature/K | 298(2) |
| Space group | Ia3 |
| No. of formula units per unit cell, Z | 16 |
| No. of reflections measured | 8753 |
| No. of independent reflections | 1789 |
| Rint | 0.0425 |
| Final R1 values (I > 2σ(I)) | 0.0287 |
| Final wR(F2) values (I > 2σ(I)) | 0.0555 |
| Final R1 values (all data) | 0.0307 |
| Final wR(F2) values (all data) | 0.0560 |

| Atoms involved | Distance (Å), Angle (°) |
|---|---|
| Lu1—O1 | 2.2392(18) |
| Lu1—Lu2 | 3.4395(7) |
| Lu2—O1 | 2.2277(19) |
| Lu2—O1i | 2.2945(19) |
| Lu2—O1x | 2.1954(18) |
| O1—Lu1—O1i | 80.10(6) |
| O1—Lu1—O1ii | 99.90(6) |
| O1—Lu1—O1iii | 180.0 |
| O1x—Lu2—O1xi | 87.14(10) |
| O1x—Lu2—O1 | 99.66(6) |
| O1xi—Lu2—O1 | 109.94(8) |
| O1—Lu2—O1xii | 138.89(9) |
| O1x—Lu2—O1i | 79.57(7) |
| O1xi—Lu2—O1i | 165.12(5) |
| O1—Lu2—O1i | 79.15(9) |
| O1x—Lu2—O1xiii | 165.12(5) |
| O1—Lu2—O1xiii | 78.91(6) |
| O1i—Lu2—O1xiii | 114.36(9) |
2.4. Radioluminescent Properties
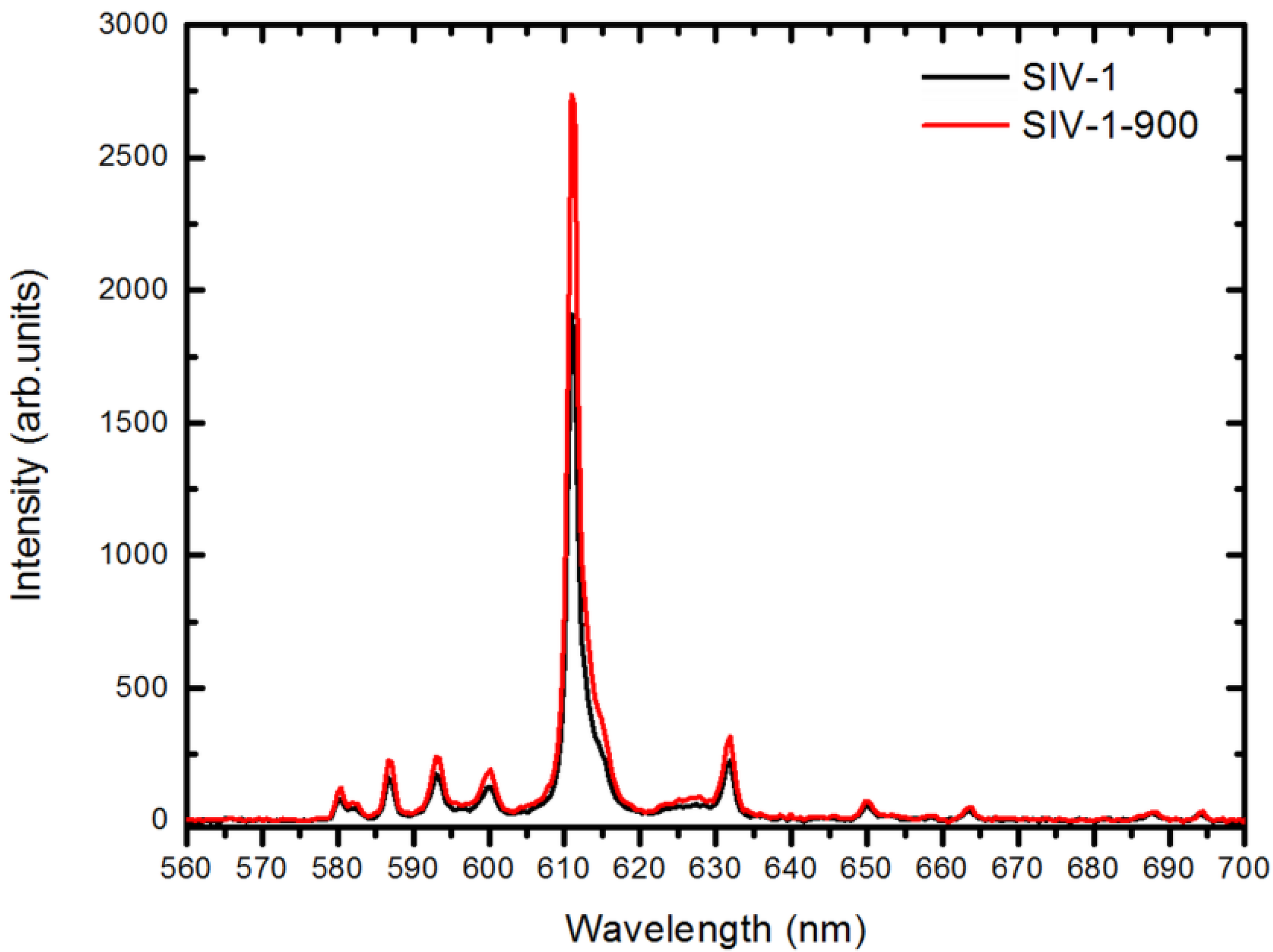
| Lu2O3:Eu sample # | XEL Efficiency (%) |
|---|---|
| SIV-1 | 72 |
| SIV-1-900 | 105 |
| SIV-1-1200 | 97 |
| SIV-2 | 66 |
| SIV-2-900 | 95 |
| SIV-3 | 80 |
| SIV-4 | 75 |
3. Experimental Section
| Series | Sample # | Batch Composition (g) | Process Parameters | Product Composition | |||
|---|---|---|---|---|---|---|---|
| Lu2O3 | Eu2O3 | Li2SO4 | SiO2 | Temperature (°C)/Time (h) | |||
| I | SI-1 | 1.7585 | – | 13.97 | 0.2661 | 1300/1 | Lu2SiO5, Lu2O3 |
| SI-2 | 1.7585 | – | 13.97 | 0.2661 | 1300/2 | Lu2SiO5, Lu2O3 | |
| SI-3 | 1.7585 | – | 13.97 | 0.2661 | 1300/20 | Lu2O3 | |
| II | SII-1 | 2 | – | 2 | – | 1400/50 | Lu2O3 |
| SII-2 | 2 | – | 4.77 | – | Lu2O3 | ||
| SII-3 | 2 | – | 9.54 | – | Lu2O3 | ||
| III | SIII-1 | 2 | – | 4.77 | 0.045 | Lu2O3 | |
| SIII-2 | 2 | – | 4.77 | 0.15 | Lu2O3 | ||
| SIII-3 | 2 | – | 4.77 | 0.3 | Lu2O3 | ||
| SIII-4 | 2 | – | 9.54 | 0.3 | Lu2O3 | ||
| IV | SIV-1 | 1.9 | 0.0884 | 4.77 | 0.045 | Lu2O3 | |
| SIV-2 | 1.9 | 0.0884 | 9.54 | 0.3 | Lu2O3 | ||
| SIV-3 | 1.9 | 0.0884 | 4.77 | 0.3 | Lu2O3 | ||
| SIV-4 | 1.84 | 0.1415 | 4.77 | 0.3 | Lu2O3 | ||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, S.R.; Nagarkar, V.V.; Tipnis, S.V.; Shestakova, I.; Brecher, C.; Lempicki, A.; Lingertat, H. Lu2O3:Eu scintillator screen for X-ray imaging. Proc. SPIE 2004, 5199, 167–172. [Google Scholar]
- Nagarkar, V.V.; Tipnis, S.V.; Miller, S.R.; Lempicki, A.; Brecher, C.; Szczupryczynski, P.; Lingertat, H. A new X-ray scintillator for digital radiography. IEEE Trans. Nucl. Sci. 2003, 50, 297–300. [Google Scholar] [CrossRef]
- Nagarkar, V.V.; Miller, S.R.; Tipnis, S.V.; Lempicki, A.; Brecher, C.; Lingertat, H. A new large area scintillator screen for X-ray imaging. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2004, 213, 250–254. [Google Scholar] [CrossRef]
- Marton, Z.; Bhandari, H.; Brecher, C.; Miller, S.R.; Singh, B.; Nagarkar, V.V. High performance microstructured Lu2O3:Eu thin film scintillator for X-ray computed tomography. Proc. SPIE 2013, 8668. [Google Scholar] [CrossRef]
- Garcia, A.; Le Luyer, C.; Pedrini, C.; Mugnier, J. Synthesis and properties of Lu2O3 sol-gel films. J. Alloy. Compd. 2001, 323, 4–77. [Google Scholar]
- Garcia, A.; Le Luyer, C.; Dujardin, C.; Martin, T.; Garapon, C.; Pédrini, C.; Mugnier, J. Elaboration and scintillation properties of Eu3+-doped Gd2O3 and Lu2O3 sol-gel films. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. 2002, 486, 181–185. [Google Scholar] [CrossRef]
- Daldosso, M.; Sokolnicki, J.; Kepinski, L.; Legendziewicz, J.; Speghini, A.; Bettinelli, M. Preparation and optical properties of nanocrystalline Lu2O3:Eu3+ phosphors. J. Lumin. 2007, 122–123, 858–861. [Google Scholar]
- Sokolnicki, J. Photoluminescence and structural characteristics of Lu2O3:Eu3+ nanocrystallites in silica matrix. J. Solid State Chem. 2007, 180, 2400–2408. [Google Scholar] [CrossRef]
- Yermolayeva, Y.V.; Tolmachev, A.V.; Dobrotvorskaya, M.V.; Vovk, O.M. Preparation and structural properties of Lu2O3:Eu3+ submicrometer spherical phosphors. J. Alloy. Compd. 2011, 509, 5320–5325. [Google Scholar] [CrossRef]
- Dulina, N.A.; Baumer, V.N.; Danylenko, M.I.; Mateychenko, P.V.; Tolmachev, A.V.; Vovk, O.M.; Yavetskiy, R.P. Effects of phase and chemical composition of precursor on structural and morphological properties of (Lu0.95Eu0.05)2O3 nanopowders. Ceram. Int. 2013, 39, 2397–2404. [Google Scholar] [CrossRef]
- Antic, Z.; Krsmanovic, R.; Wojtowicz, M.; Zych, E.; Bartova, B.; Dramicanin, M.D. Preparation, structural and spectroscopic studies of (YxLu1−x)2O3:Eu3+ nanopowders. Opt. Mater. 2010, 32, 1612–1617. [Google Scholar] [CrossRef]
- Trojan-Piegza, J.; Zych, E. Preparation of nanocrystalline Lu2O3:Eu phosphor via a molten salts route. J. Alloy. Compd. 2004, 380, 118–122. [Google Scholar] [CrossRef]
- Trojan-Piegza, J.; Zych, E.; Hreniak, D.; Strek, W. Comparison of spectroscopic properties of nanoparticulate Lu2O3:Eu synthesized using different techniques. J. Alloy. Compd. 2004, 380, 123–129. [Google Scholar] [CrossRef]
- Li, R.; Gai, S.; Wang, L.; Wang, J.; Yang, P. Facile synthesis and multicolor luminescent properties of uniform Lu2O3:Ln (Ln = Eu3+, Tb3+, Yb3+/Er3+, Yb3+/Tm3+, and Yb3+/Ho3+) nanospheres. J. Colloid Interface Sci. 2012, 368, 165–171. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Dong, N.; Jiang, Y.; Tao, Y.; Yin, M. Preparation and characterization of optical spectroscopy of Lu2O3:Eu nanocrystals. J. Solid State Chem. 2005, 178, 477–482. [Google Scholar] [CrossRef]
- Guo, H.; Yin, M.; Dong, N.; Xu, M.; Lou, L.; Zhang, W. Effect of heat-treatment temperature on the luminescent properties of Lu2O3:Eu film prepared by Pechini sol–gel method. Appl. Surf. Sci. 2005, 243, 245–250. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Lin, L.; You, B.; Fu, Y.; Yin, M. Preparation and spectroscopic characterization of Lu2O3:Eu3+ nanopowders and ceramics. Opt. Mater. 2008, 30, 1484–1488. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Y.; An, L.; Wang, S.; Chen, J.; Shi, J. A novel co-precipitation synthesis of a new phosphor Lu2O3:Eu3+. J. Eur. Ceram. Soc. 2007, 27, 191–197. [Google Scholar] [CrossRef]
- Liu, X.-J.; Li, H.-L.; Xie, R.-J.; Hirosaki, N.; Xu, X.; Huang, L.-P. Synthesis, characterization, and luminescent properties of Lu2O3:Eu phosphors. J. Lumin. 2007, 127, 469–473. [Google Scholar] [CrossRef]
- Sanghera, J.; Kim, W.; Villalobos, G.; Shaw, B.; Baker, C.; Frantz, J.; Sadowski, B.; Aggarwal, I. Ceramic laser materials. Materials 2012, 5, 258–277. [Google Scholar] [CrossRef]
- Kim, W.; Baker, C.; Bowman, S.; Florea, C.; Villalobos, G.; Shaw, B.; Sadowski, B.; Hunt, M.; Aggarwal, I.; Sanghera, J. Laser oscillation from Ho3+ doped Lu2O3 ceramics. Opt. Mater. Express 2013, 3. [Google Scholar] [CrossRef]
- Lempicki, A.; Brecher, C.; Szczupryczynski, P.; Lingertat, H.; Nagarkar, V.V.; Tipnis, S.V.; Miller, S.R. A new lutetia-based ceramic scintillator for X-ray imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2003, 488, 579–570. [Google Scholar] [CrossRef]
- Zych, E.; Trojan-Piegza, J.; Kepinski, L. Homogeneously precipitated Lu2O3:Eu nanocrystalline phosphor for X-ray detection. Sens. Actuators B Chem. 2005, 109, 112–118. [Google Scholar] [CrossRef]
- Farman, T.T.; Vandre, R.H.; Pajak, J.C.; Miller, S.R.; Lempicki, A.; Farman, A.G. Effects of scintillator on the detective quantum efficiency (DQE) of a digital imaging system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 219–223. [Google Scholar] [CrossRef]
- Zych, E. Spectroscopy of Eu-activated Lu2O3 X-ray phosphors. In Frontal Semiconductor Research; Nova Science Publishers: Hauppauge, NY, USA, 2006; pp. 1–24. [Google Scholar]
- Lempicki, A.; Wojtowicz, A.J.; Brecher, C. Wide-Gap Luminescent Materials: Theory and Applilcations; Kluwer AcademicPublishers: Dordrecht, The Netherlands, 1966; pp. 235–301. [Google Scholar]
- Zych, E. Luminescence and Scintillation of Inorganic Phosphor Materials. In Handbook of Luminescence, Display Materials, and Devices. Inorganic Display Materials; Vollume 2, Academic Scientific: San Francisco, CA, USA, 2003; pp. 251–300. [Google Scholar]
- Seeley, Z.; Cherepy, N.; Payne, S. Two-step sintering of Gd0.3Lu1.6Eu0.1O3 transparent ceramic scintillator. Opt. Mater. Express 2013, 3. [Google Scholar] [CrossRef]
- Seeley, Z.M.; Dai, Z.R.; Kuntz, J.D.; Cherepy, N.J.; Payne, S.A. Phase stabilization in transparent Lu2O3:Eu ceramics by lattice expansion. Opt. Mater. 2012, 35, 74–78. [Google Scholar] [CrossRef]
- Seeley, Z.M.; Kuntz, J.D.; Cherepy, N.J.; Payne, S.A. Transparent Lu2O3:Eu ceramics by sinter and HIP optimization. Opt. Mater. 2011, 33, 1721–1726. [Google Scholar] [CrossRef]
- Kopylov, Y.L.; Kravchenko, V.B.; Dulina, N.A.; Lopin, A.V.; Parkhomenko, S.V.; Tolmachev, A.V.; Yavetskiy, R.P.; Zelenskaya, O.V. Fabrication and characterization of Eu3+-doped Lu2O3 scintillation ceramics. Opt. Mater. 2013, 35, 812–816. [Google Scholar] [CrossRef]
- Zych, E.; Hreniak, D.; Strek, W.; Kepinski, L.; Domagala, K. Sintering properties of urea-derived Lu2O3-based phosphors. J. Alloy. Compd. 2002, 341, 391–394. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Liu, Q. Controlled synthesis of europium-doped lutetium compounds: Nanoflakes, nanoquadrels, and nanorods. J. Mater. Chem. 2005, 15, 4141–4146. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, N.; Jia, Y.; Lv, W.; Shao, B.; Jiao, M.; You, H. Facile surfactant-free synthesis and luminescent properties of hierarchical europium-doped lutetium oxide phosphors. J. Colloid Interface Sci. 2013, 394, 216–222. [Google Scholar] [CrossRef]
- Cascales, C.; Zaldo, C.; Esteban-Betegon, F.; Calderon-Villajos, R. From porous to dense Tm3+-Lu2O3 micro-and nanosized crystalline morphologies designed through hydrothermal precursors: Assessment on infrared emission properties. CrystEngComm 2012, 14, 3577–3585. [Google Scholar] [CrossRef]
- Dulina, N.A.; Yermolayeva, Y.V.; Tolmachev, A.V.; Sergienko, Z.P.; Vovk, O.M.; Vovk, E.A.; Matveevskaya, N.A.; Mateychenko, P.V. Synthesis and characterization of the crystalline powders on the basis of Lu2O3:Eu3+spherical submicron-sized particles. J. Eur. Ceram. Soc. 2010, 30, 1717–1724. [Google Scholar] [CrossRef]
- Guzik, M.; Pejchal, J.; Yoshikawa, A.; Ito, A.; Goto, T.; Siczek, M.; Lis, T.; Boulon, G. Structural investigations of Lu2O3 as single crystal and polycrystalline transparent ceramic. Cryst. Growth Des. 2014, 14, 3327–3334. [Google Scholar] [CrossRef]
- FIZ Karlsruhe. Available online: http://www.fiz-karlsruhe.de/request_for_deposited_data.html (accessed on 26 September 2014).
- Agilent, CrysAlis PRO; Agilent Technologies: Santa Clara, CA, USA, 2011.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr 2008, A64, 112–122. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeler, J.; Jerzykiewicz, L.B.; Zych, E. Flux-Aided Synthesis of Lu2O3 and Lu2O3:Eu—Single Crystal Structure, Morphology Control and Radioluminescence Efficiency. Materials 2014, 7, 7059-7072. https://doi.org/10.3390/ma7107059
Zeler J, Jerzykiewicz LB, Zych E. Flux-Aided Synthesis of Lu2O3 and Lu2O3:Eu—Single Crystal Structure, Morphology Control and Radioluminescence Efficiency. Materials. 2014; 7(10):7059-7072. https://doi.org/10.3390/ma7107059
Chicago/Turabian StyleZeler, Justyna, Lucjan B. Jerzykiewicz, and Eugeniusz Zych. 2014. "Flux-Aided Synthesis of Lu2O3 and Lu2O3:Eu—Single Crystal Structure, Morphology Control and Radioluminescence Efficiency" Materials 7, no. 10: 7059-7072. https://doi.org/10.3390/ma7107059