Effect of Isomorphous Substitution on the Thermal Decomposition Mechanism of Hydrotalcites
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction
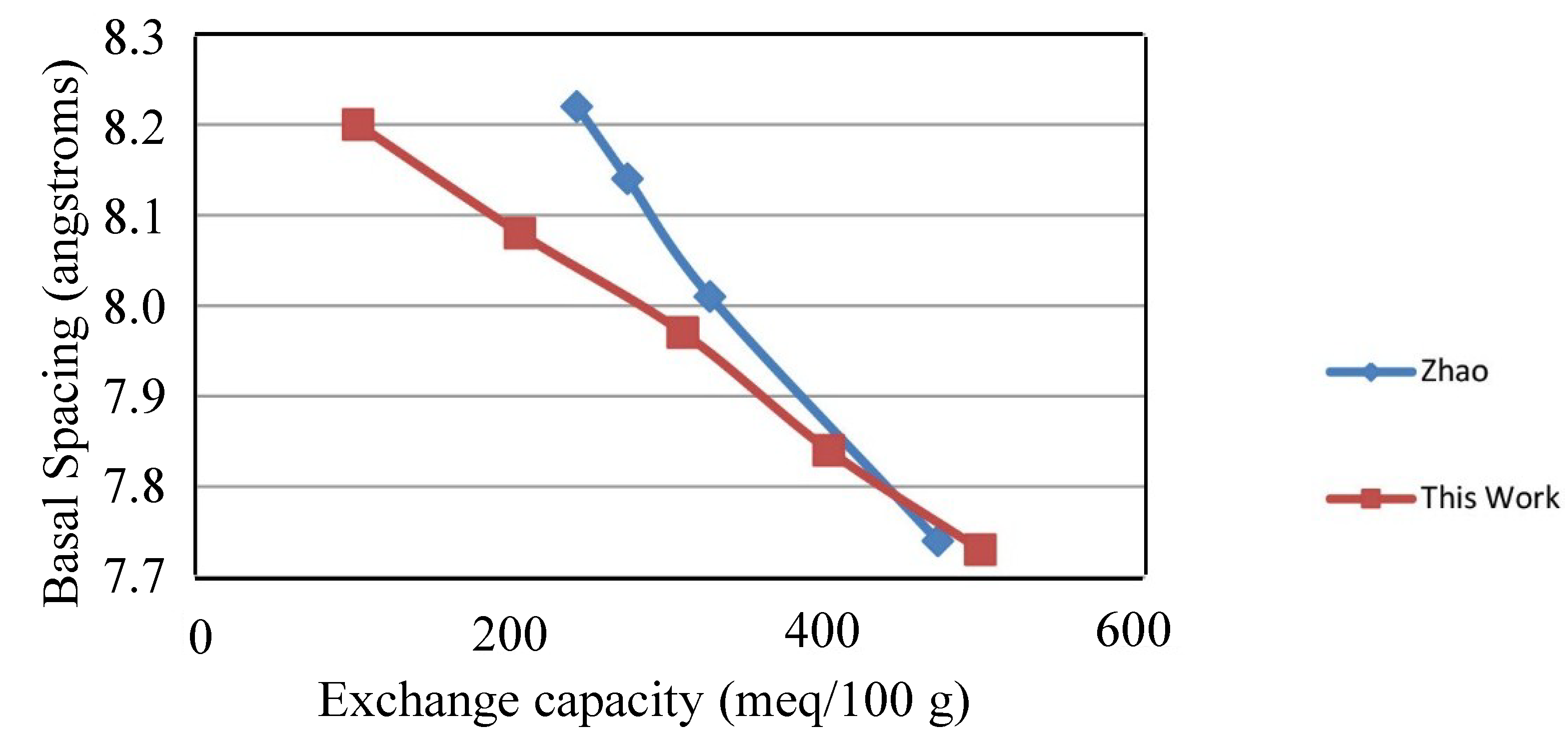
2.2. Thermal Decomposition
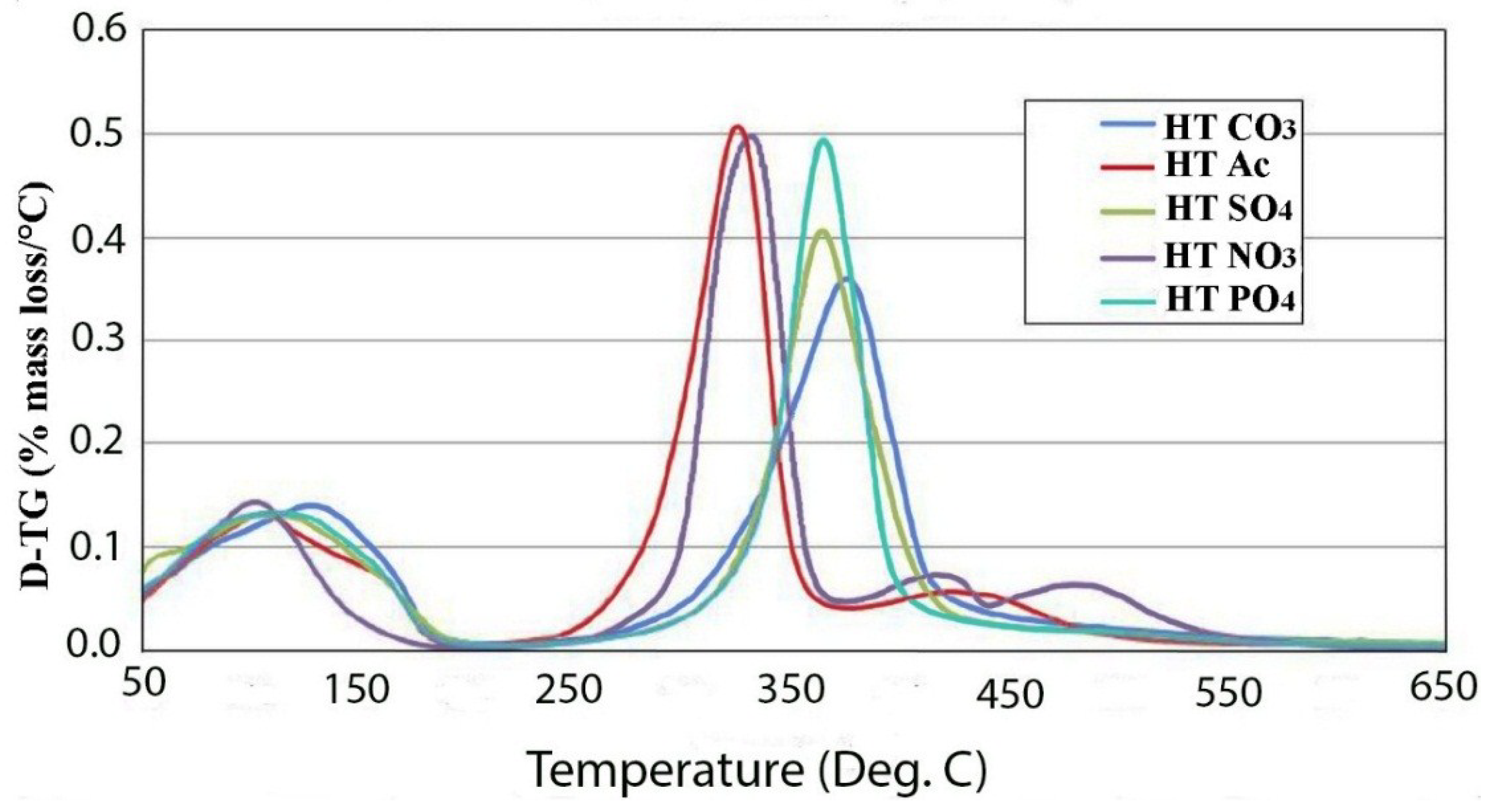
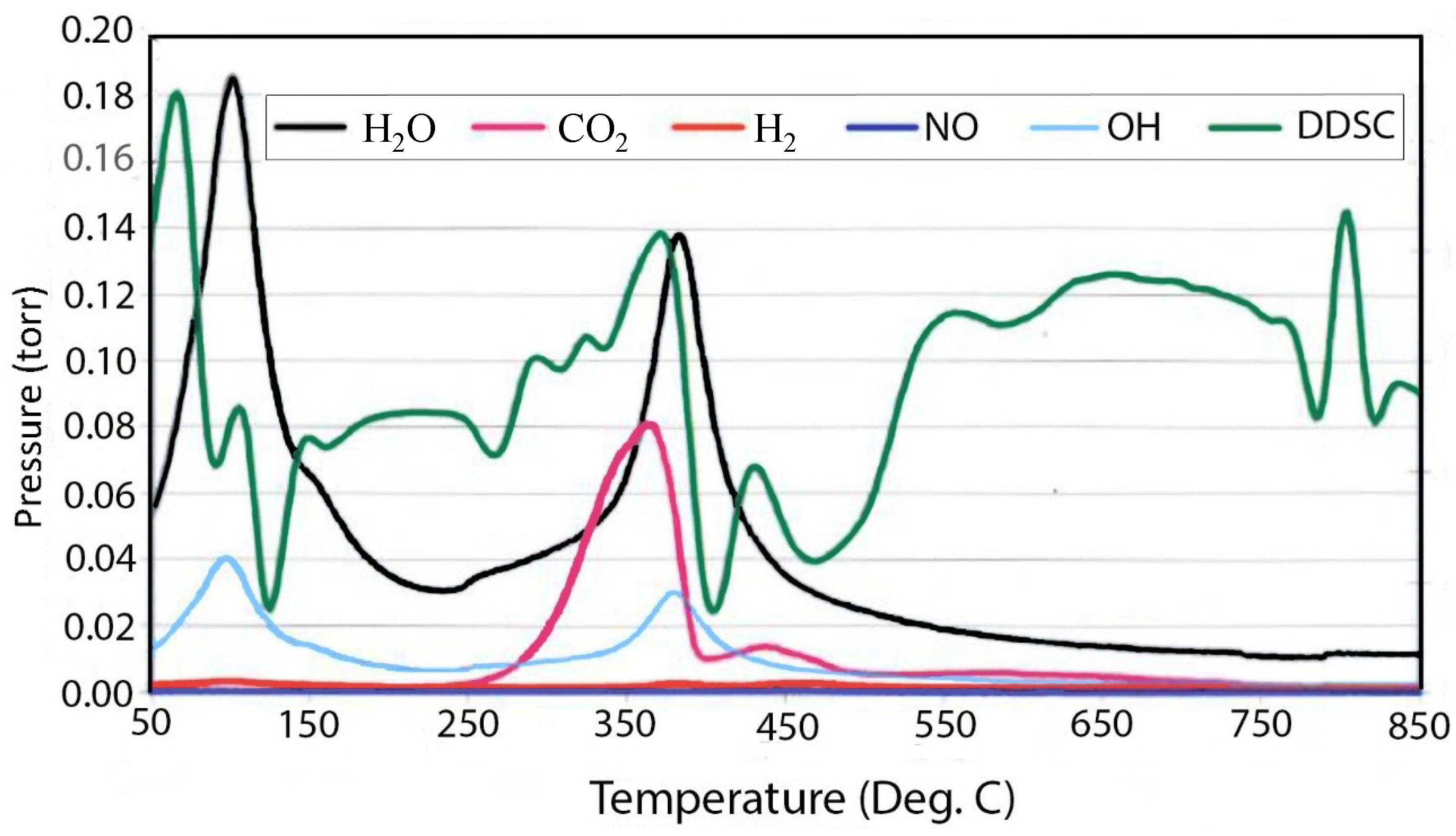
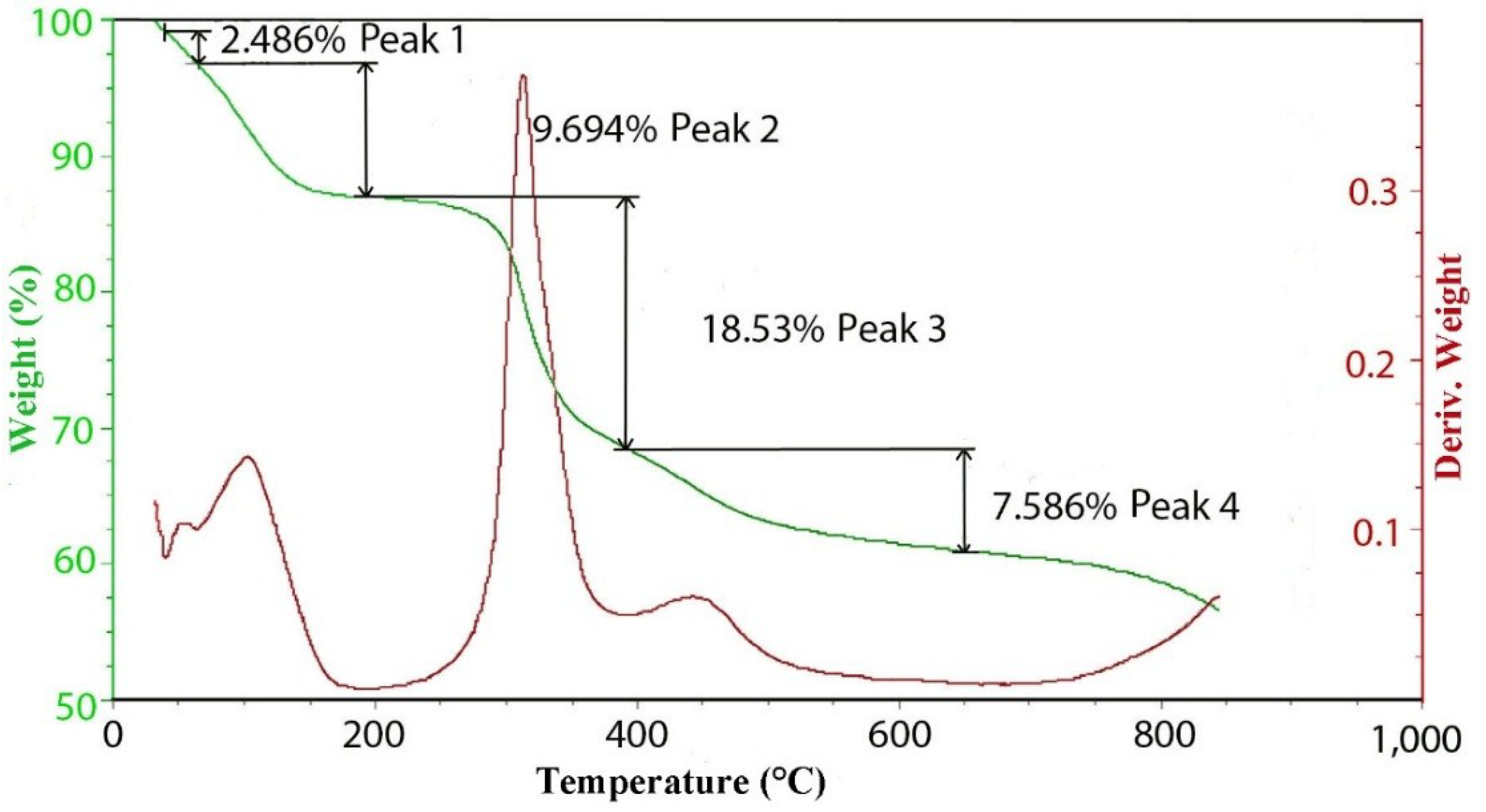
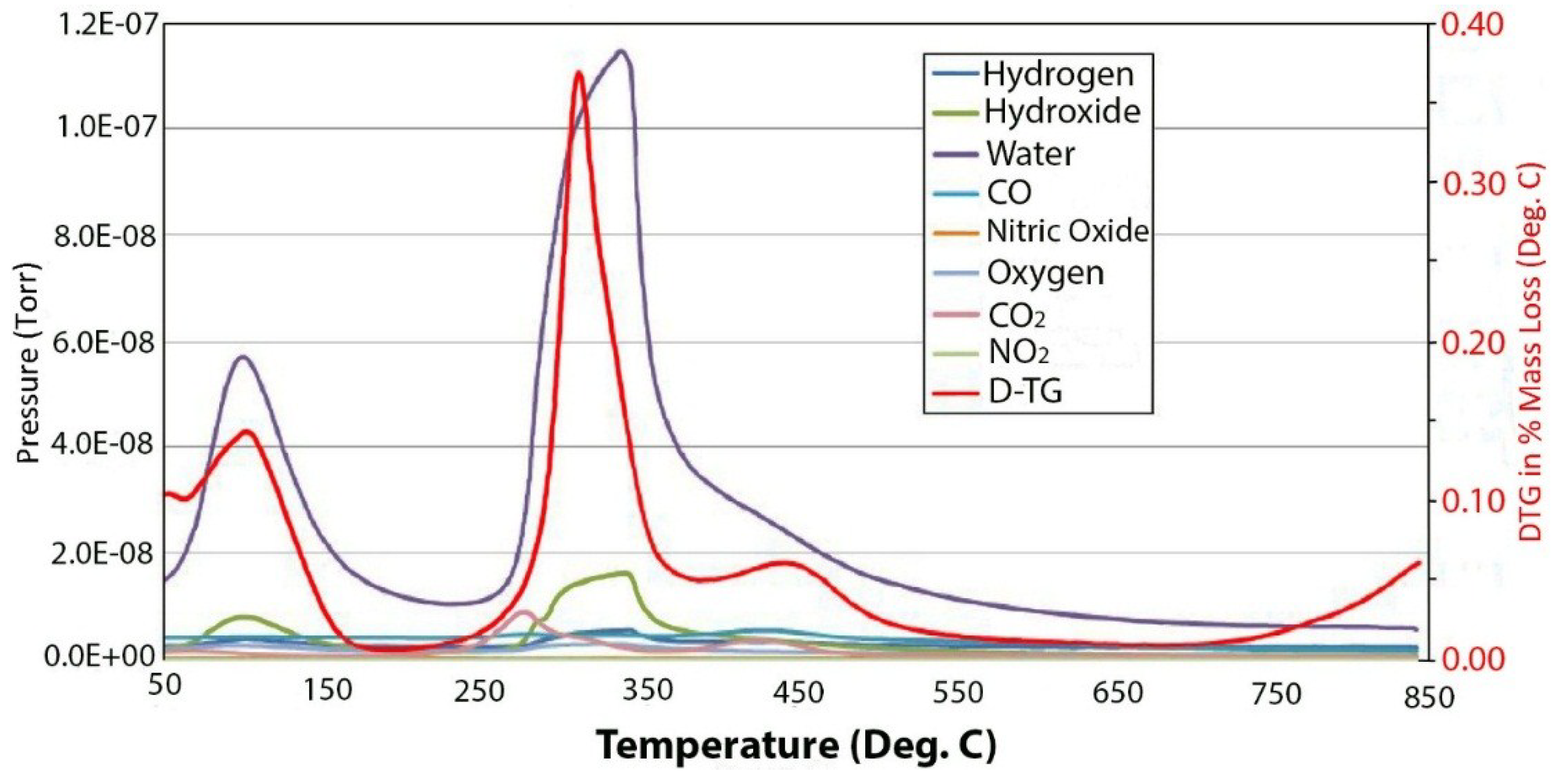
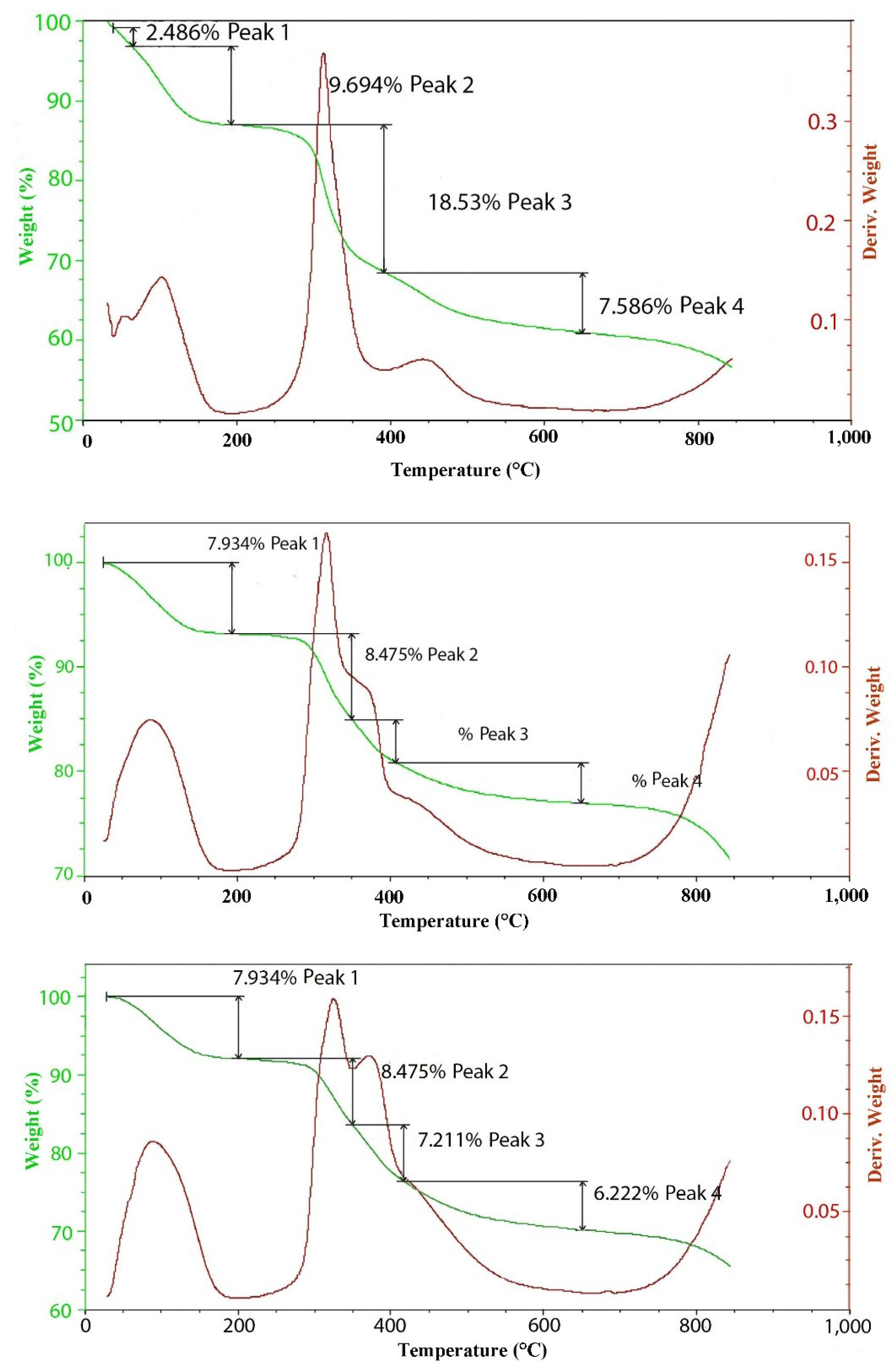
3. Experimental Section
3.1. Hydrotalcite Preparation
| Exchange capacity meq/100 g | Formula Mg = 2+, Al = 3+, Cl = 1− | X Mg(1−X)AlX(OH)2Cl−X |
|---|---|---|
| 100 | [Mg.94Al.06(OH)2]Cl.06 | 0.06 |
| 200 | [Mg.88Al.12(OH)2]Cl.12 | 0.12 |
| 300 | [Mg.82Al.18(OH)2]Cl.18 | 0.18 |
| 400 | [Mg.77Al.23(OH)2]Cl.23 | 0.23 |
| 500 | [Mg.71Al.29(OH)2]Cl.06 | 0.29 |
3.2. Anion Exchange
3.3. TGA/EGA
3.4. X-ray Diffraction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silva, C.G.; Bouizi, Y.; Fornes, V.; Garca, H. Layered double hydroxides as highly efficient photocatalysts for visible light oxygen generation from water. J. Am. Chem. Soc. 2009, 131, 13833–13839. [Google Scholar] [CrossRef] [PubMed]
- Leroux, F.; Besse, J.P. Delamination and re-stacking of layered double hydroxides. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, D.; Duan, X. Synthesis, characterization, and magnetically controlled release behavior of novel core-shell structural magnetic Ibuprofin-Intercalated LDH nanohybrid. J. Phys. Chem. C 2009, 113, 12140–12148. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z.L. Enhanced arsenic removal by hydrothermally treated nanocrystalline Mg/Al layered double hydroxides with nitrate intercalated. Environ. Sci. Technol. 2009, 43, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Nagy, K.L. Dodecyl sulfate-hydrotalcite nanocomposites for trapping chlorinated organic pollutants in water. J. Colloid Interfacial Sci. 2004, 274, 613–624. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, G.Q. Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar] [CrossRef]
- Johnsen, R.E.; Norby, P. A structural study of stacking disorder in the decomposition oxide of MgAl layered double hydroxide: A DIFFaX+ analysis. J. Phys. Chem. C 2009, 113, 19061–19066. [Google Scholar] [CrossRef]
- Kanezaki, E. Effects of atomic ratio Mg/Al in layers of Mg and Al layered double hydroxides on thermal stability of hydrotalcite-like layered structure by means of in situ high temperature powder diffraction. Mat. Res. Bull. 1998, 33, 773–778. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaim, E. Hydrotalcite decomposition mechanism: A clue to the structure of spinel like mixed oxides. J. Phys. Chem. 1996, 100, 8535–8542. [Google Scholar] [CrossRef]
- Vagvolgyi, V.; Palmer, S.J.; Kristaf, J.; Frost, R.L.; Horvath, E. Mechanism for hydrotalcite decomposition: A controlled rate thermal analysis study. J. Coll. Inter. Sci. 2008, 318, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Rey, F.; Fornes, V.; Rojo, J.M. Thermal decomposition of hydrotalcite: An infrared and nuclear magnetic resonance spectroscopy study. J. Chem. Soc. Faraday Trans. 1992, 88, 2233–2238. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Kristof, J.; Frost, R.L. FT-Raman spectroscopy and SEM of gibbsite, bayerite, boehmite and diaspora in relation to the characterization of bauxite. In Proceedings of the 12th International Clay Conference, Bahai-Blanca, Argentina, 22–28 July 2001; Elsevier Science: Amsterdam, The Nederland, 2001; pp. 451–465. [Google Scholar]
- Frost, R.L.; Musumeci, A.W.; Bostrom, T.; Adebajo, M.O.; Weier, M.L.; Martens, W. Thermal decomposition of hydrotalcite with chromate, molybdenate or sulfatein the interlayer. Thermochem. Acta 2005, 429, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.A.; Novaro, O.; Bokhimi, X.; Lopez, T.; Gomez, R.; Navarette, J.; Llanos, M.E.; Lopez-Salinas, E. Characterization of the thermal decomposition of brucite prepared by sol-gel technique for synthesis of nanocrystalline MgO. Mater. Lett. 1998, 35, 317–323. [Google Scholar] [CrossRef]
- Digne, M.; Saute, P.; Raybaud, P.; Toulhaut, H.; Artacho, E. Structure and stability of aluminum hydroxides: A theoretical study. J. Phys. Chem. B 2002, 106, 5155–5162. [Google Scholar] [CrossRef]
- Sanderson, B.A.; Sowersby, D.S.; Crosby, S.; Goss, M.; Lewis, L.K.; Beall, G.W. Charge density and particle size effects on oligonucleotide and plasmid DNA binding to nanosized hydrotalcite. Biointerphases 2013, 8, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.K.; Pinnavaia, T.J. Water content and particle texture of synthetic hydrotalcite-like layered double hydroxides. Chem. Mater. 1995, 7, 348–354. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crosby, S.; Tran, D.; Cocke, D.; Duraia, E.-S.M.; Beall, G.W. Effect of Isomorphous Substitution on the Thermal Decomposition Mechanism of Hydrotalcites. Materials 2014, 7, 7048-7058. https://doi.org/10.3390/ma7107048
Crosby S, Tran D, Cocke D, Duraia E-SM, Beall GW. Effect of Isomorphous Substitution on the Thermal Decomposition Mechanism of Hydrotalcites. Materials. 2014; 7(10):7048-7058. https://doi.org/10.3390/ma7107048
Chicago/Turabian StyleCrosby, Sergio, Doanh Tran, David Cocke, El-Shazly M. Duraia, and Gary W. Beall. 2014. "Effect of Isomorphous Substitution on the Thermal Decomposition Mechanism of Hydrotalcites" Materials 7, no. 10: 7048-7058. https://doi.org/10.3390/ma7107048




