Waste Minimization Protocols for the Process of Synthesizing Zeolites from South African Coal Fly Ash
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fly Ash Characterization

| Major oxides (mean wt%) | Trace elemental concentrations (ppm) | ||
|---|---|---|---|
| SiO2 | 55.44 | Ba | 486 |
| Al2O3 | 31.51 | Ce | 254 |
| Fe2O3 | 4.94 | Co | 30 |
| MnO | 0.03 | Cu | 110 |
| MgO | 1.18 | Nb | 37 |
| CaO | 3.76 | Ni | 125 |
| Na2O | 0.04 | Pb | 90 |
| K2O | 0.47 | Rb | 56 |
| TiO2 | 1.11 | Sr | 989 |
| P2O5 | 0.30 | V | 79 |
| SO3 | 0.06 | Y | 94 |
| Loss On Ignition | 1.22 | Zn | 135 |
| SiO2/Al2O3 | 1.76 | - | - |
2.2. Waste Recycle without a Prior pH Adjustment
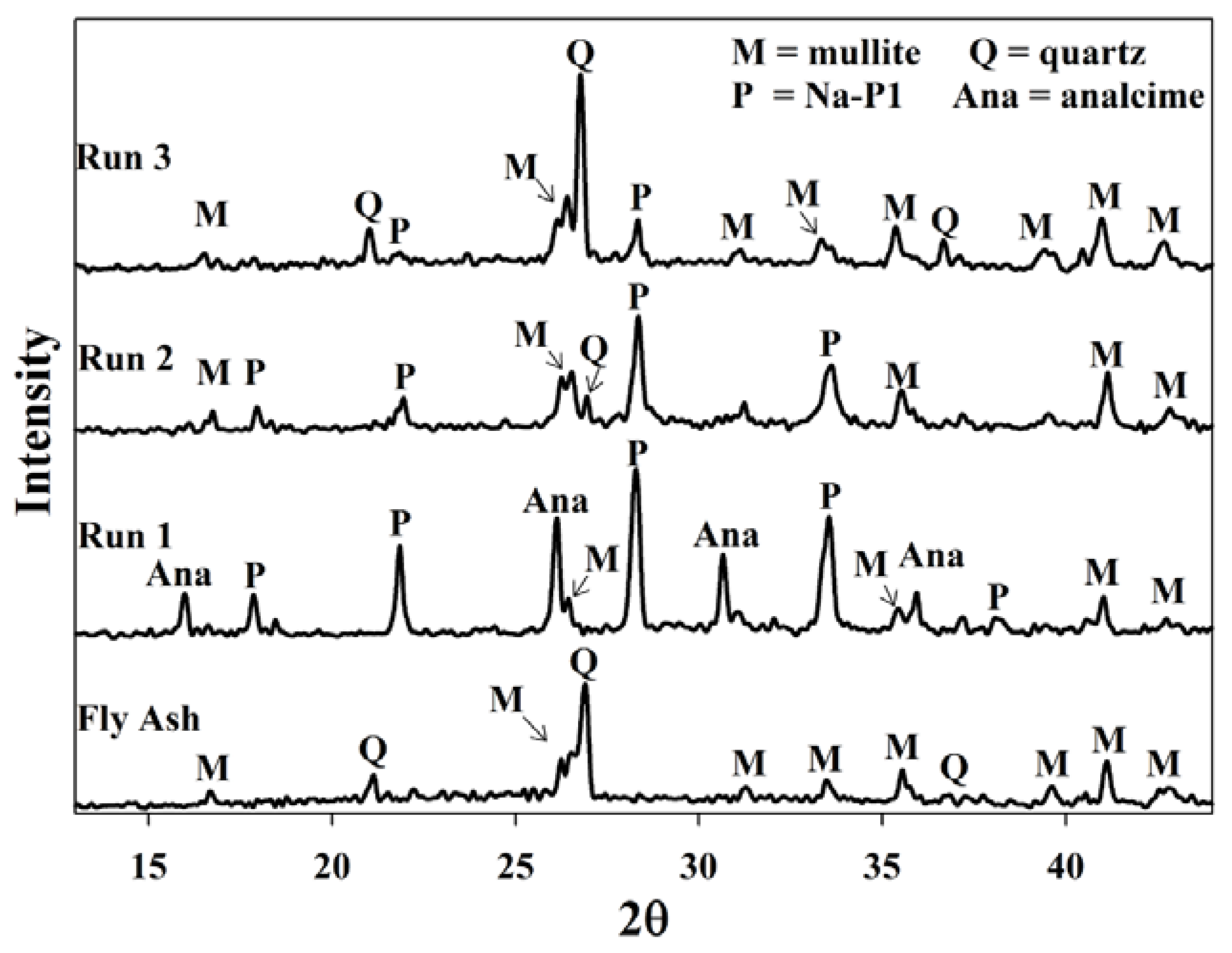

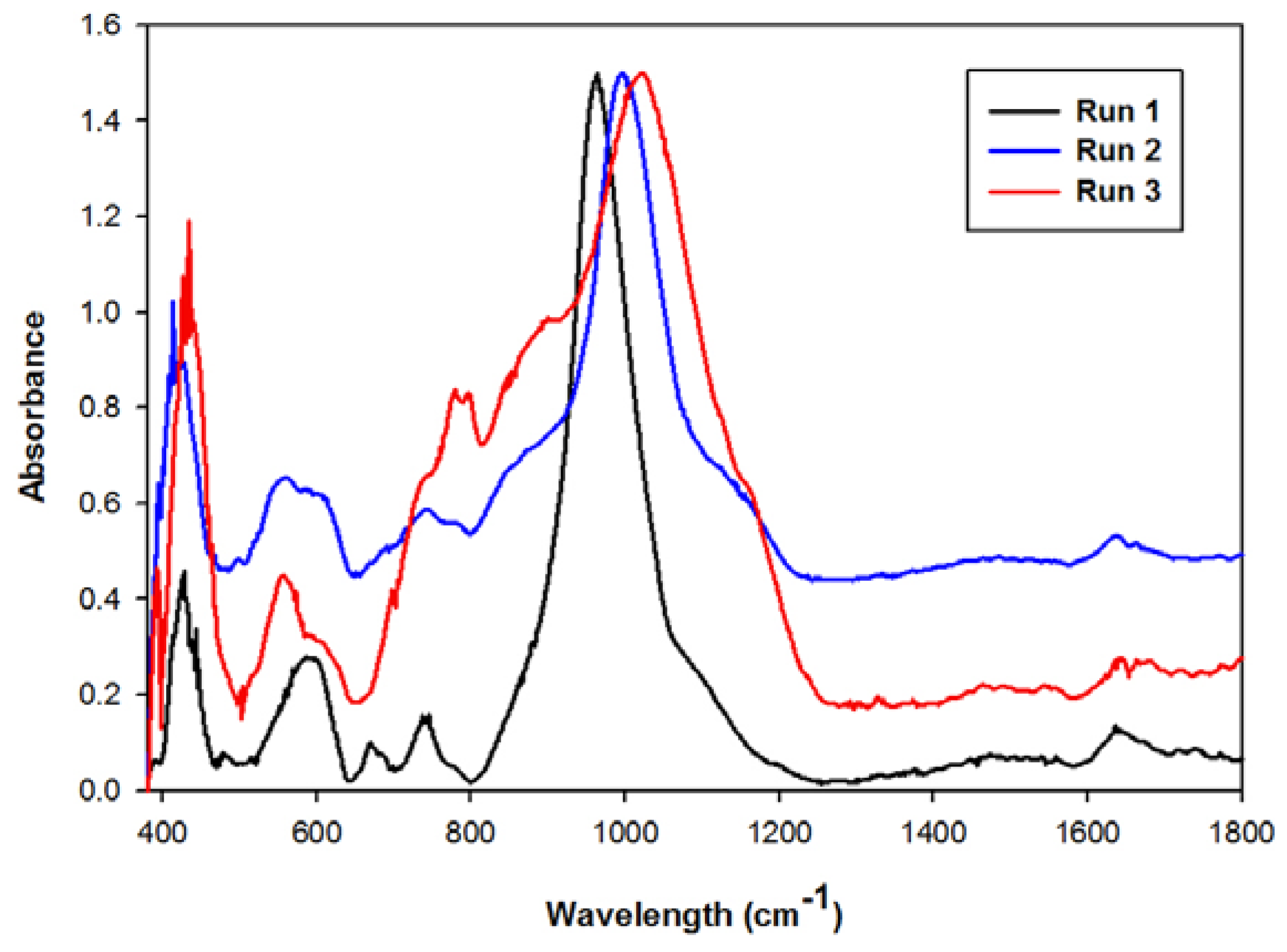
2.3. Waste Recycles with Prior pH Adjustment
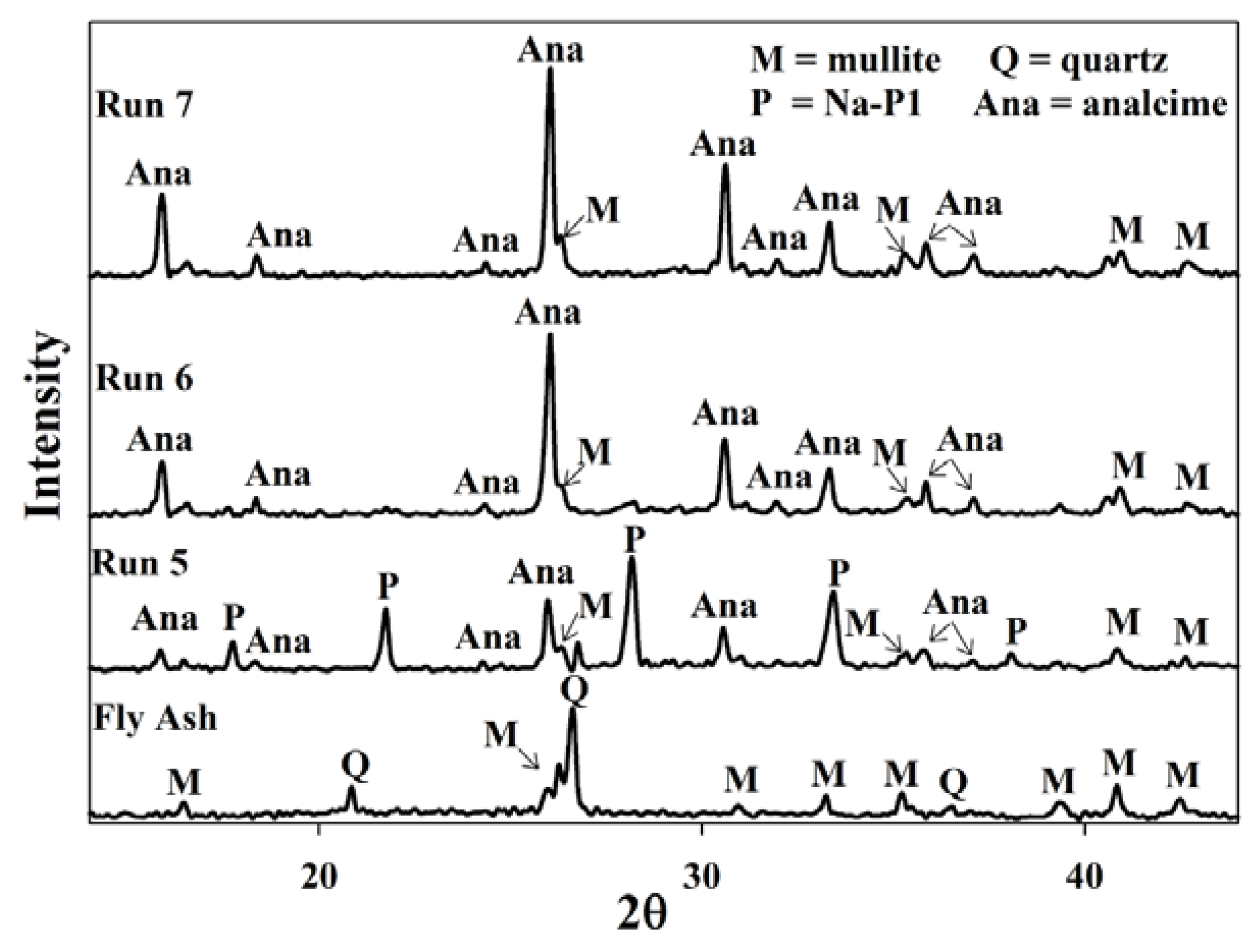


| Mean elemental concentration (ppm) | |||
|---|---|---|---|
| Element | Run 5 | Run 6 | Run 7 |
| Al | 35.5 | 34.0 | 31.3 |
| Fe | 5.3 | 6.9 | 7.4 |
| K | 215.1 | 434.6 | 497.2 |
| Na | 20971.3 | 25348.0 | 26101.4 |
| P | 80.6 | 163.5 | 196.8 |
| Si | 7198.7 | 15124.7 | 16624.1 |
| Ti | 4.9 | 11.3 | 12.8 |
| V | 5.1 | 9.8 | 11.3 |
2.4. Recycling 100% Waste Supernatant
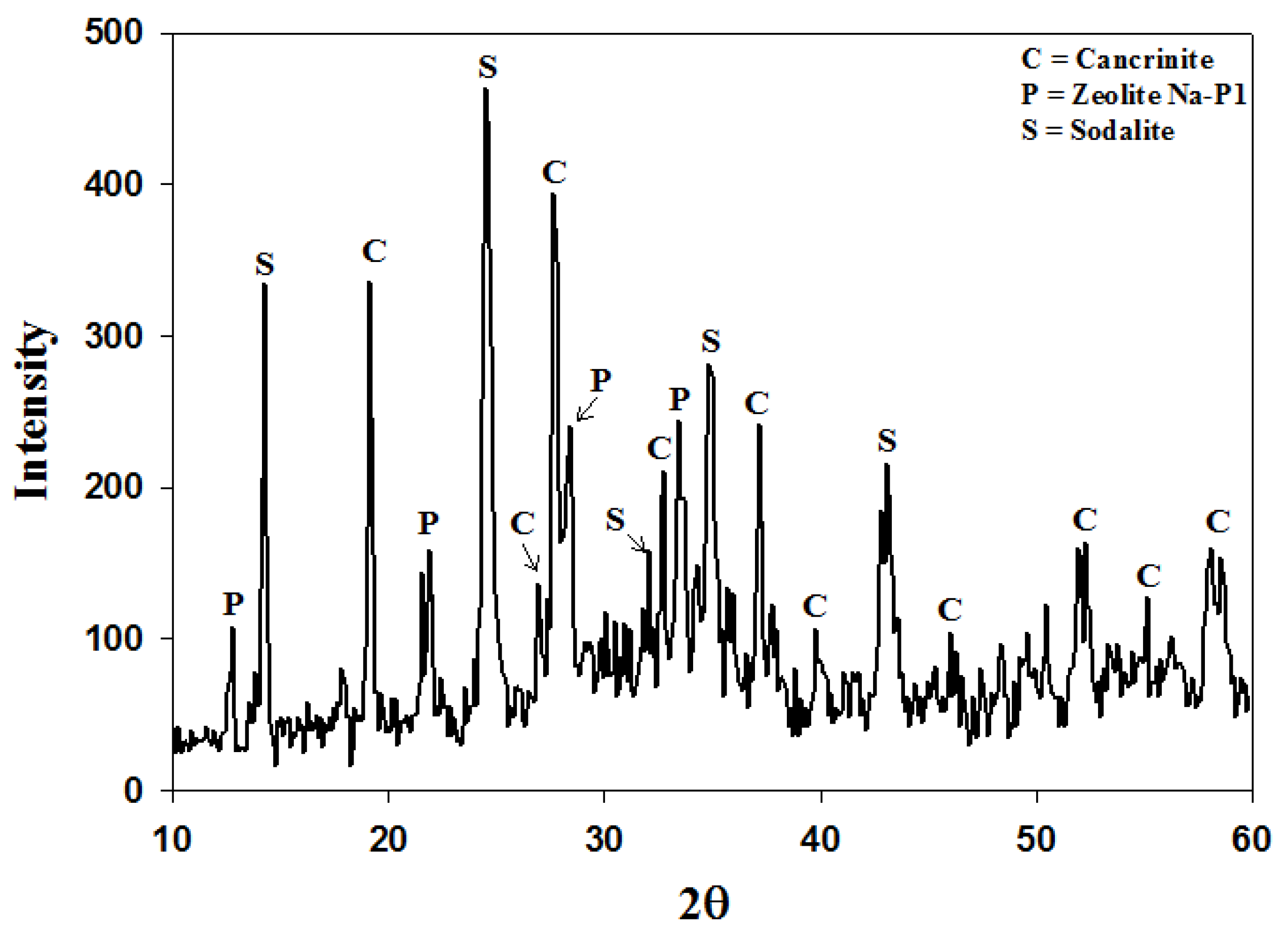
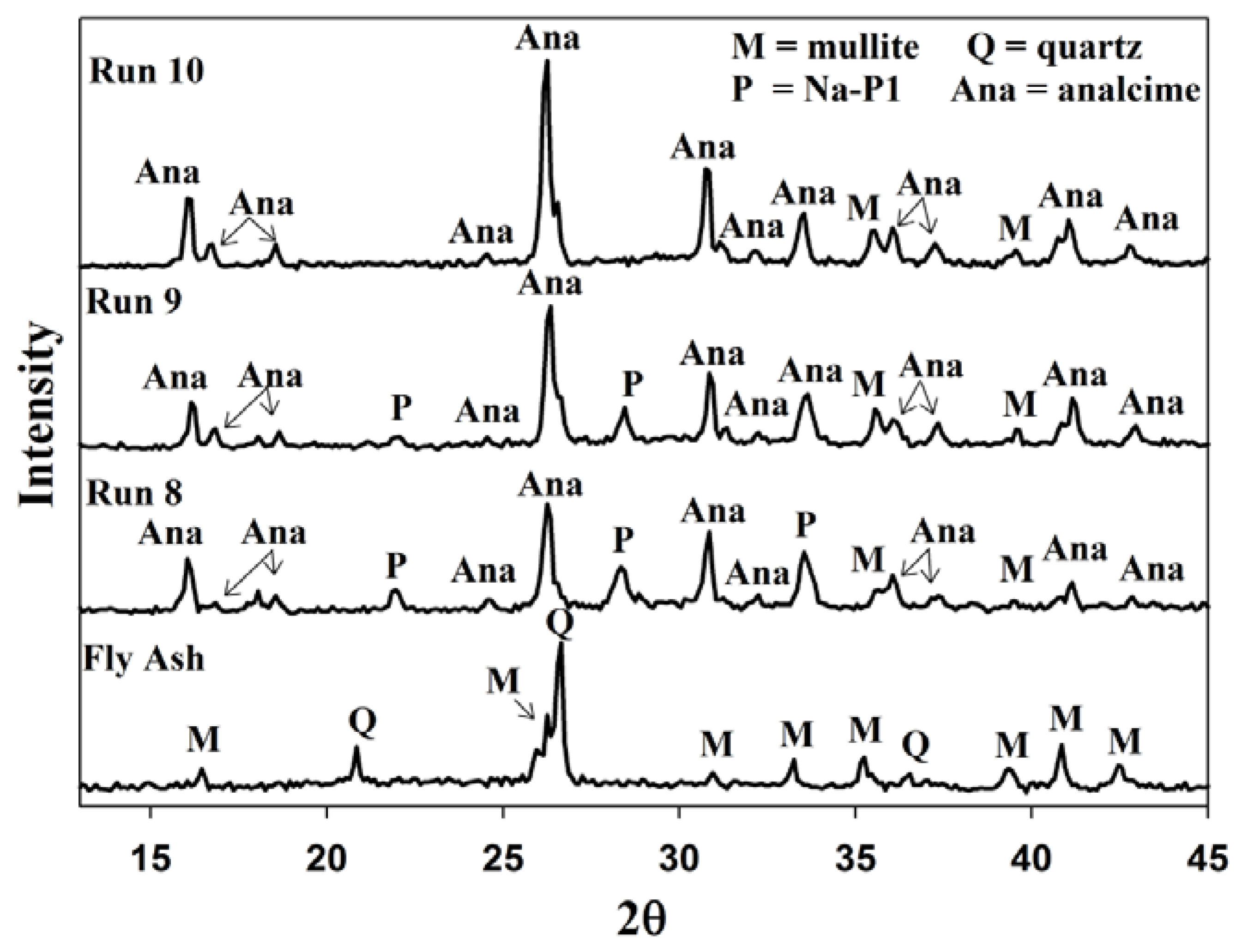

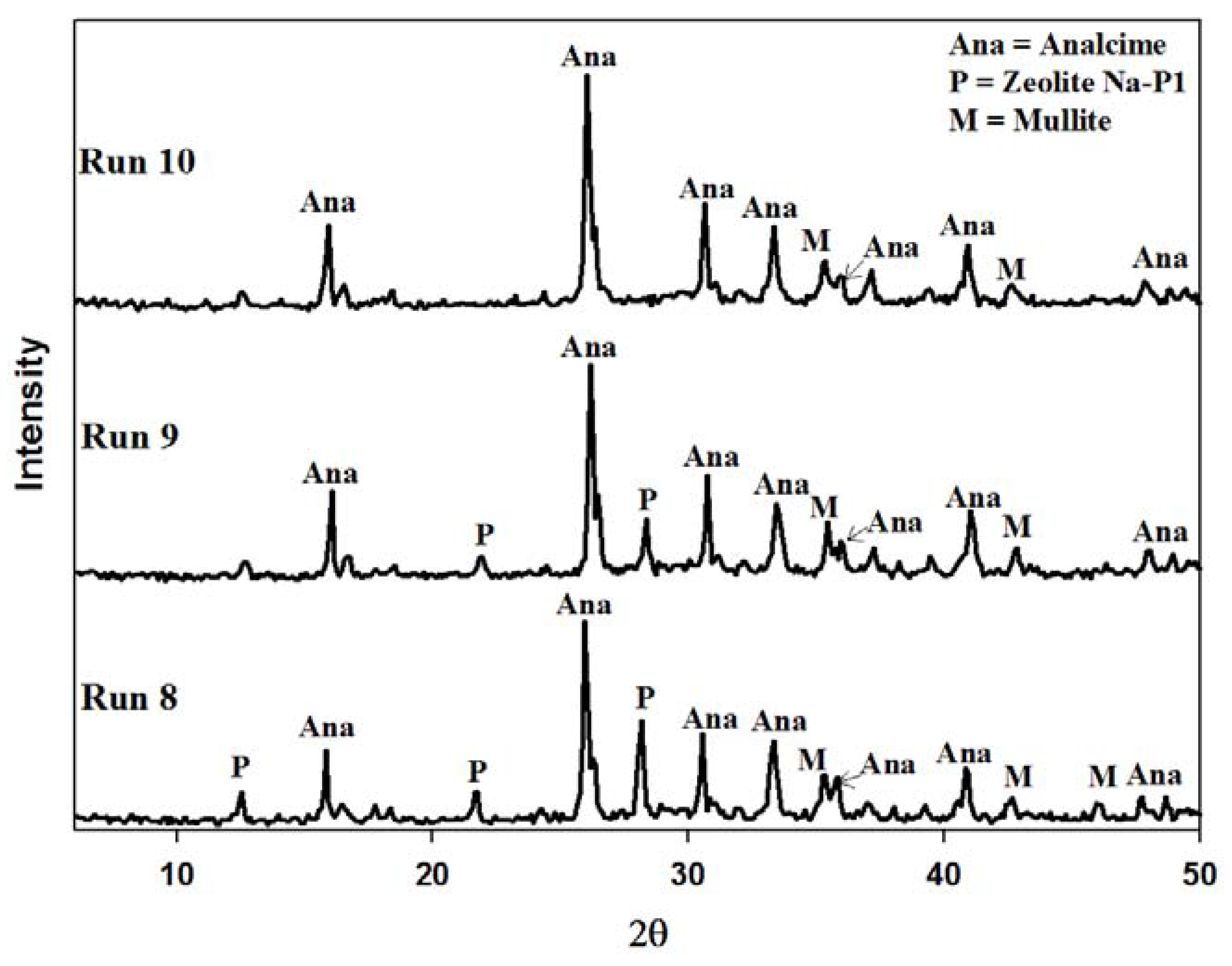
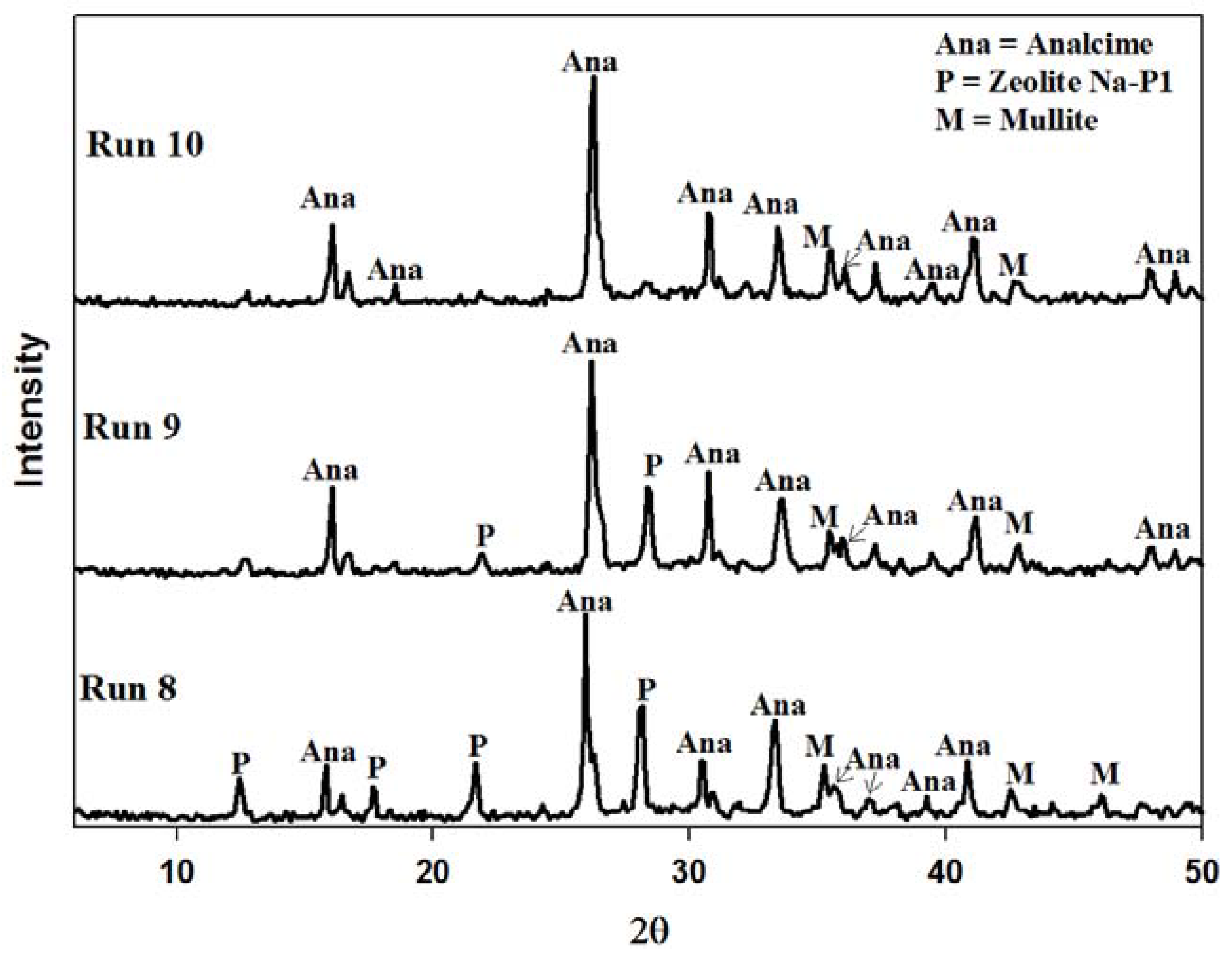
3. Experimental Section
3.1. Materials and Characterization
3.2. Synthesis of Zeolites from Coal Fly Ash
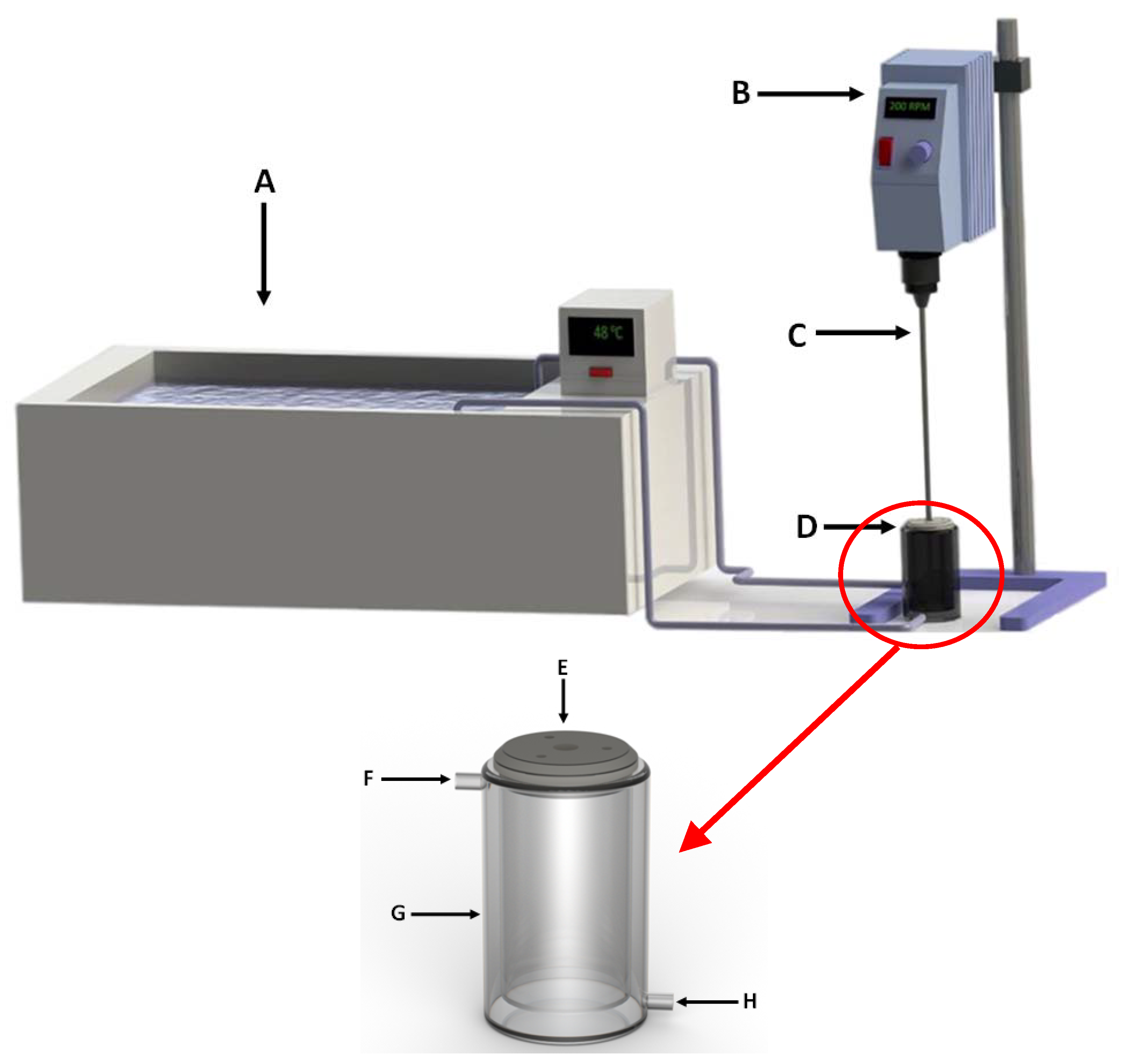
3.3. Research Structure
| Run | Process description | Adjustments to alkali source |
|---|---|---|
| 1 | Synthesis of zeolites using 50 mL of a fresh 5 M NaOH batch as alkali source | - |
| 2 | Utilizing the supernatant waste generated from run 1 as alkali source | None |
| 3 | Utilizing the supernatant waste generated from run 2 as alkali source | None |
| 4 | Repetition of reference run (Run 1) to generate supernatant for titration purposes | - |
| 5 | Synthesis of zeolites using 50 mL of a fresh 5 M NaOH batch as alkali source | - |
| 6 | Utilizing the supernatant waste generated from run 5 as alkali source | pH adjusted |
| 7 | Utilizing the supernatant waste generated from run 6 as alkali source | pH adjusted |
| 8 | Synthesis of zeolites using 125 mL of a fresh 2 M NaOH batch as alkali source | - |
| 9 | Utilizing the supernatant waste generated from run 8 as alkali source | None |
| 10 | Utilizing the supernatant waste generated from run 9 as alkali source | None |
4. Conclusions
Acknowledgments
References
- Eskom Integrated Report 2011. Available online: www.eskom.co.za/annreport11/ (accessed on 18 July 2011).
- Hower, J.C.; Robertson, J.D.; Thomas, G.A.; Wong, A.S.; Schram, W.H.; Graham, U.M.; Rathbone, R.F.; Robl, T.L. Characterization of fly ash from Kentucky power stations. Fuel 1996, 75, 403–411. [Google Scholar] [CrossRef]
- Koukouzas, N.; Hamalainen, J.; Papanikolaou, D.; Tourunen, A.; Jantti, T. Mineralogical and elemental composition of fly ash from pilot scale fluidized bed combustion of lignite, bituminous coal, wood chips and their blends. Fuel 2007, 86, 2186–2193. [Google Scholar] [CrossRef]
- Lyer, R.S.; Scott, J.A. Power station fly ash—A review of value-added utilization outside of the construction industry. Resour. Conserv. Recycl. 2001, 31, 217–228. [Google Scholar] [CrossRef]
- Scheetz, B.E.; Earle, R. Utilization of fly ash. Curr. Opin. Solid State Mater. Sci. 1998, 3, 510–520. [Google Scholar] [CrossRef]
- Madzivire, G.; Gitari, W.M.; Vadapalli, V.R.K.; Ojumu, T.V.; Petrik, L.F. Fate of sulphate removed during the treatment of circumneutral mine water and acid mine drainage with coal fly ash: Modelling and experimental approach. Miner. Eng. 2011, 24, 1467–1477. [Google Scholar] [CrossRef]
- Madzivire, G.; Petrik, L.F.; Gitari, W.M.; Ojumu, T.V.; Balfour, G. Application of coal fly ash to circumneutral mine waters for the removal of sulphates as gypsum and ettringite. Miner. Eng. 2010, 23, 252–257. [Google Scholar] [CrossRef]
- Höller, H.; Wirsching, U. Zeolite formation from fly ash. Fortschr. Mineral. 1985, 63, 21–43. [Google Scholar]
- Hollman, G.; Steenbruggen, G.; Janssen-Jurkovičová, M. A two-step process for the synthesis of zeolites from coal fly ash. Fuel 1999, 78, 1225–1230. [Google Scholar] [CrossRef]
- Molina, A.; Poole, C. A comparative study using two methods to produce zeolites from fly ash. Miner. Eng. 2004, 17, 167–173. [Google Scholar] [CrossRef]
- Musyoka, N.M. Hydrothermal Synthesis and Optimisation of Zeolite Na-P1 from South African Coal Fly Ash. Unpublished MSc Thesis, University of the Western Cape, Cape Town, South Africa, 2009; pp. 1–196. [Google Scholar]
- Musyoka, N.M.; Petrik, L.F.; Gitari, W.M.; Balfour, G.; Hums, E. Optimization of hydrothermal synthesis of pure phase zeolite Na-P1 from South African coal fly ashes. J. Environ. Sci. Health. Part A 2012, 47, 337–350. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Alastuey, A.; Juan, R.; Andres, J.M.; Lopez-Soler, A.; Ayora, C.; Medinaceli, A.; Valero, A. Synthesis of high ion exchange zeolites from coal fly ash. Geol. Acta 2007, 5, 49–57. [Google Scholar]
- Querol, X.; Umana, J.C.; Plana, F.; Alastuey, A.; Lopez-Soler, A.; Medinaceli, A.; Valero, A.; Domingo, M.J.; Garcia-Rojo, E. Synthesis of zeolites from fly ash at pilot plant scale. Examples of potential applications. Fuel 2001, 80, 857–865. [Google Scholar] [CrossRef]
- Mainganye, D.; du Plessis, P.W.; Ojumu, T.V.; Petrik, L.F. Synthesis of zeolites Na-P1 from South African coal fly ash: effect of impeller design and agitation. Materials 2013, in press. [Google Scholar]
- Wu, D.; Zhang, B.; Yan, L.; Kong, H.; Wang, X. Effect of some additives on synthesis of zeolite from coal fly ash. Int. J. Miner. Process. 2006, 80, 266–272. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Querol, X.; Plana, F.; Alastuey, A.; Lopez-Soler, A. Synthesis of Na-zeolites from fly ash. Fuel 1997, 76, 793–799. [Google Scholar] [CrossRef]
- Cao, G.; Shah, M.J. In situ monitoring of zeolite crystallization by electrical conductivity measurement: New insight into zeolite crystallization mechanism. Microporous Mesoporous Mater. 2007, 101, 19–23. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Rios, C.A.; Williams, G.D.; Roberts, C.L. A comparative study of two methods for the synthesis of fly ash-based sodium and potassium type zeolites. Fuel 2009, 88, 1403–1416. [Google Scholar] [CrossRef]
- Criado, M.; Fernandez-Jimenez, A.; Palomo, A. Alkali activation of fly ash. Part III: Effect of curing conditions on reaction and its graphical description. Fuel 2010, 89, 3185–3192. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Zeolite Science and Technology; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Azizi, S.N.; Yousefpour, M. Synthesis of aluminum—Rich analcime using an ethylene diamine derivative as template. J. Inorg. Gen. Chem. 2009, 635, 1654–1658. [Google Scholar]
- Tatlier, M.; Barış Cigizoglu, K.; Tokay, B.; Erdem-Şenatalar, A. Microwave vs. conventional synthesis of analcime from clear solutions. J. Cryst. Growth 2007, 306, 146–151. [Google Scholar] [CrossRef]
- Akar, G.; Polat, M.; Galecki, G.; Ipekoglu, U. Leaching behavior of selected trace elements in coal fly ash samples from Yenikoy coal-fired power plants. Fuel Process. Technol. 2012, 104, 50–56. [Google Scholar] [CrossRef]
- Iwasaki, A.; Kudo, I.; Sano, T. Three-dimensional real-time observation of growth and dissolution of silicalite crystal. Stud. Surf. Sci. Catal. 1997, 105, 317–324. [Google Scholar]
- Querol, X.; Alastuey, A.; Fernandez-Turiel, J.L.; Lopez-Soler, A. Synthesis of zeolites by alkaline activation of ferro-aluminous fly ash. Fuel 1995, 74, 1226–1231. [Google Scholar] [CrossRef]
- Choi, J.; Kimoto, K.; Ichikawa, Y. Quartz dissolution experiments at various pH, temperature and stress conditions: CLSM and ICP-AES investigations. Environ. Earth. Sci. 2012, 66, 2431–2440. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, H.; Li, L. The composition of fly ash glass phase and its dissolution properties applying to geopolymeric materials. J. Am. Ceram. Soc. 2011, 94, 1773–1778. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Plessis, P.W.D.; Ojumu, T.V.; Petrik, L.F. Waste Minimization Protocols for the Process of Synthesizing Zeolites from South African Coal Fly Ash. Materials 2013, 6, 1688-1703. https://doi.org/10.3390/ma6051688
Plessis PWD, Ojumu TV, Petrik LF. Waste Minimization Protocols for the Process of Synthesizing Zeolites from South African Coal Fly Ash. Materials. 2013; 6(5):1688-1703. https://doi.org/10.3390/ma6051688
Chicago/Turabian StylePlessis, Pieter W. Du, Tunde V. Ojumu, and Leslie F. Petrik. 2013. "Waste Minimization Protocols for the Process of Synthesizing Zeolites from South African Coal Fly Ash" Materials 6, no. 5: 1688-1703. https://doi.org/10.3390/ma6051688





