Ba-Doped Iron Oxide as a New Material for NO2 Detection
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
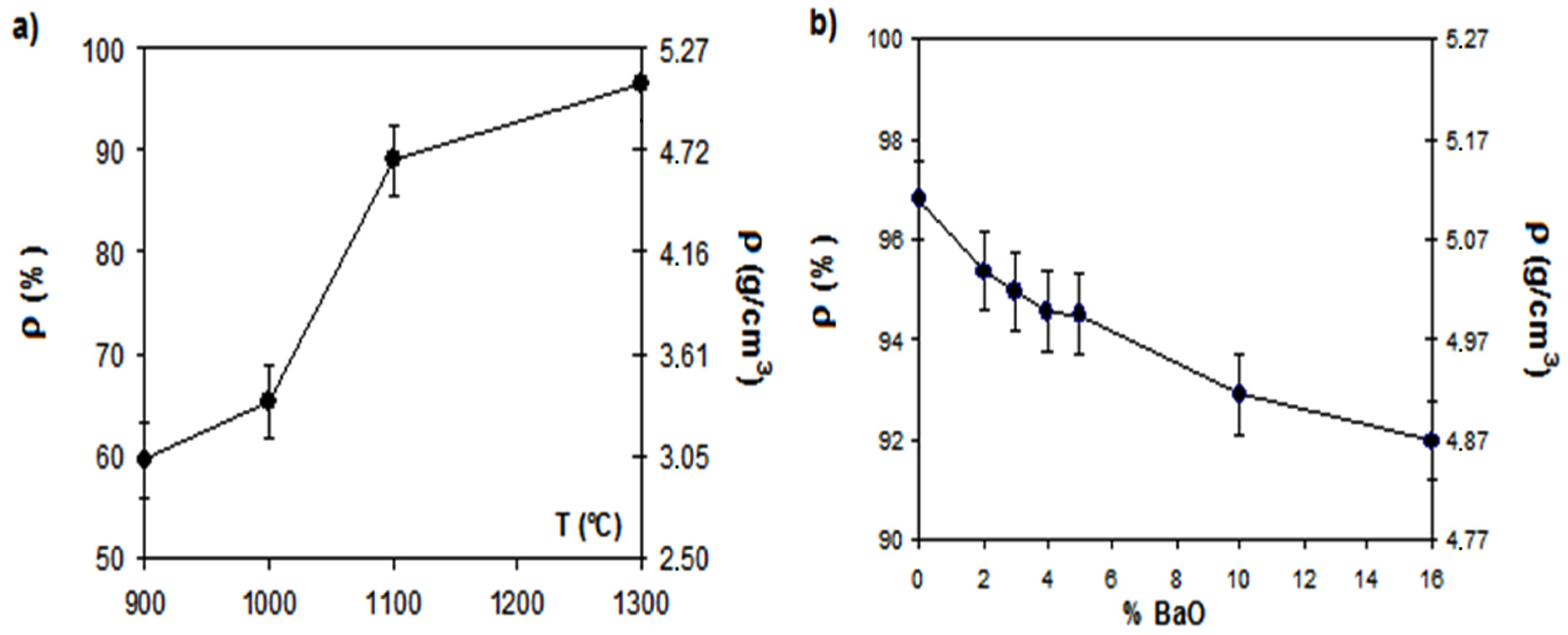
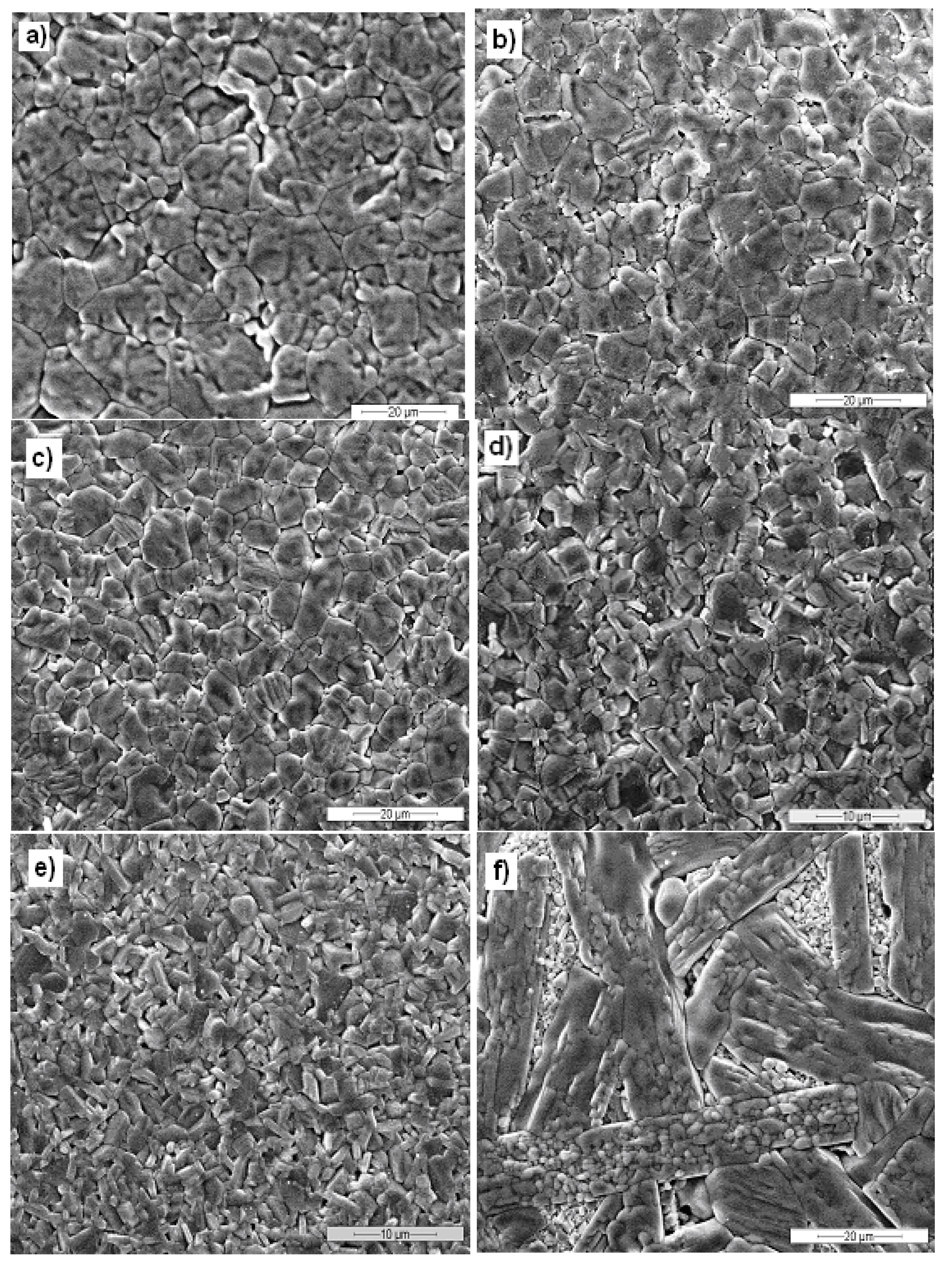
3.1. Microstructure
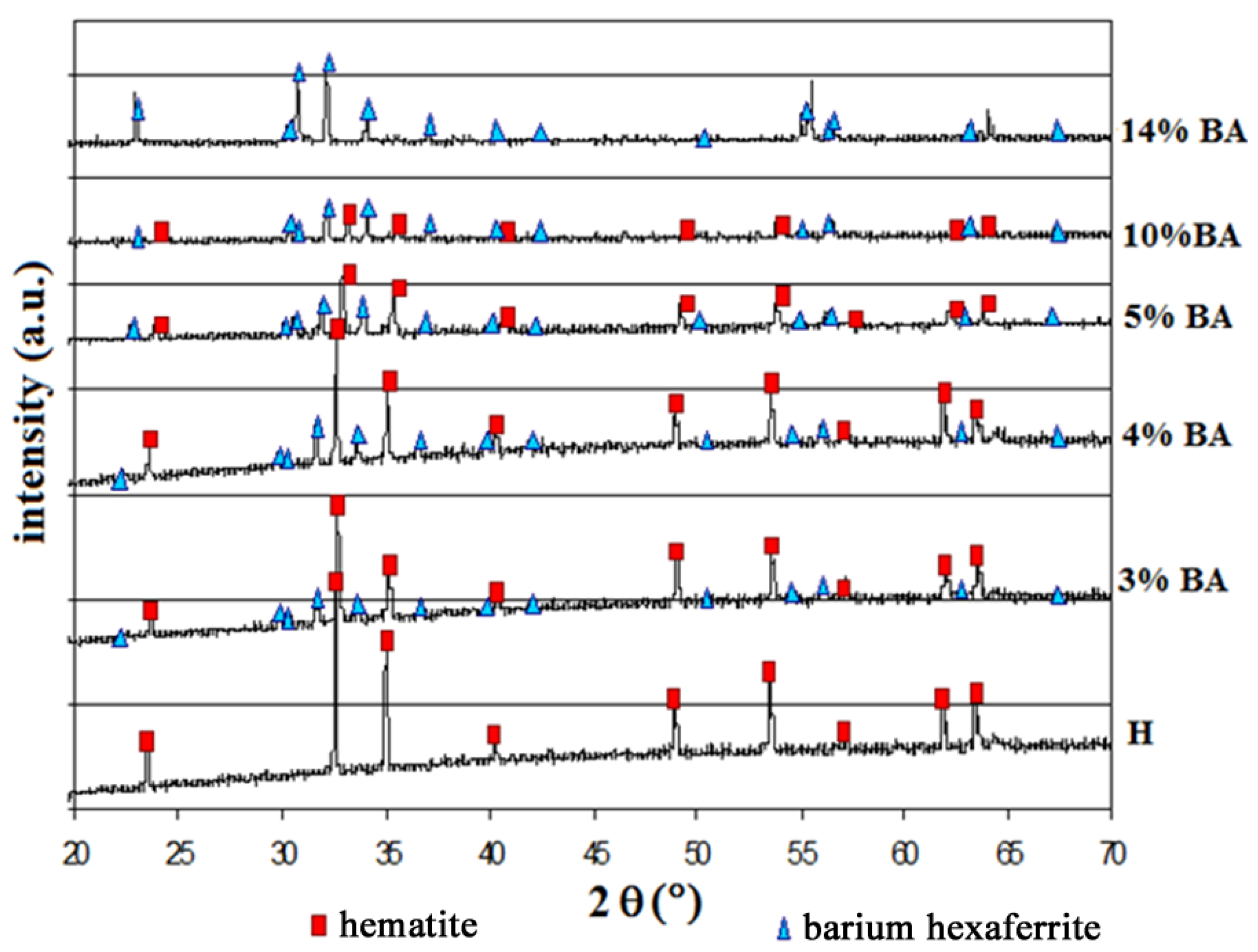
| Label | Sample | Expected BaFe12O19 (wt %) | Relative density (%) |
|---|---|---|---|
| H | α-Fe2O3 | 0 | 96.9 |
| 3% BA | α-Fe2O3 + 3 wt % BaO fired at 1300 °C | 18.12 | 95.2 |
| 4% BA | α-Fe2O3 + 4 wt % BaO fired at 1300 °C | 21.75 | 94.8 |
| 5% BA | α-Fe2O3 + 5 wt % BaO fired at 1300 °C | 36.24 | 94.8 |
| 10% BA | α-Fe2O3 + 10 wt % BaO fired at 1300 °C | 72.48 | 93.1 |
| 14% BA | α-Fe2O3 + 14 wt % BaO fired at 1300 °C | 100.00 | 92.0 |
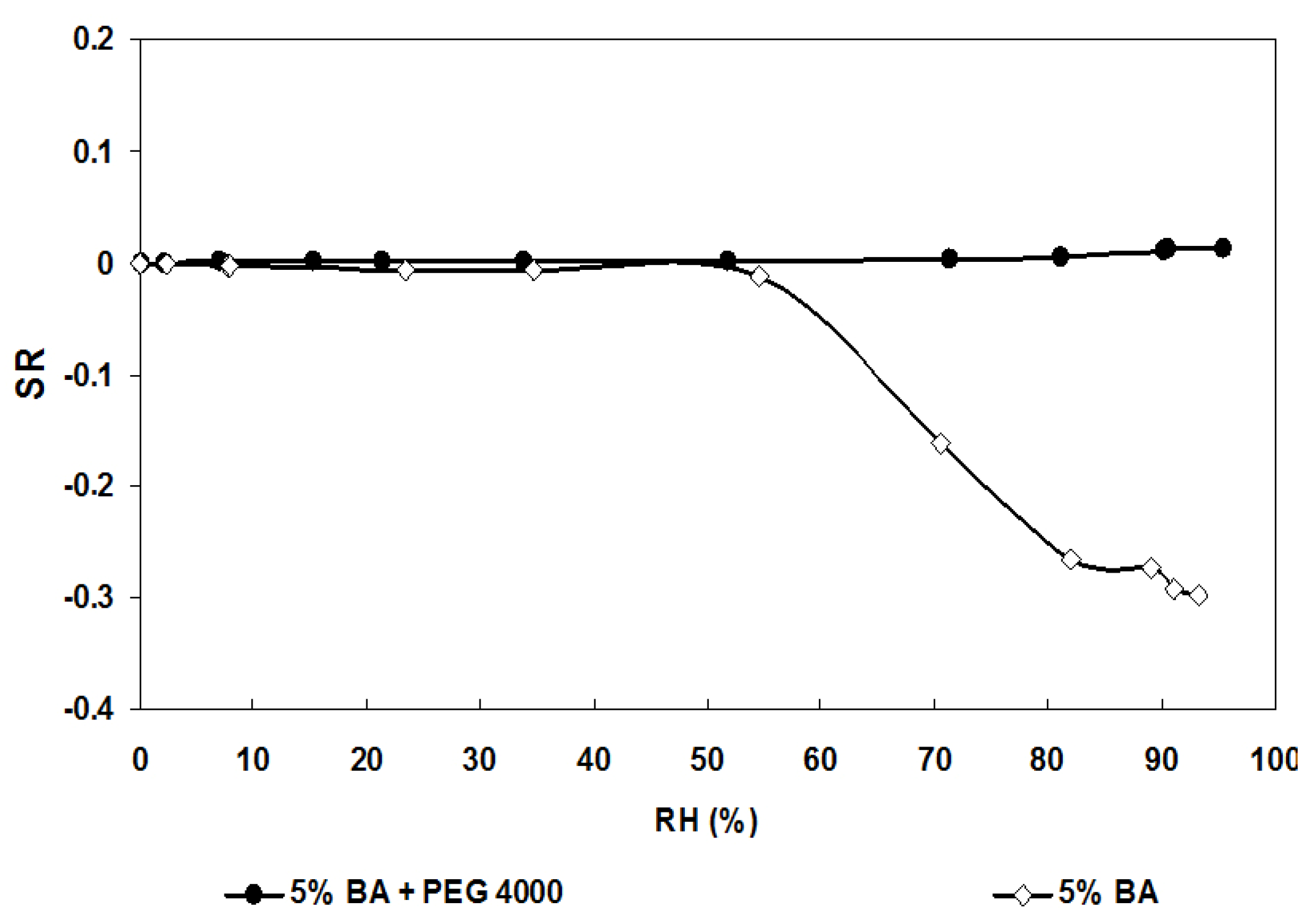
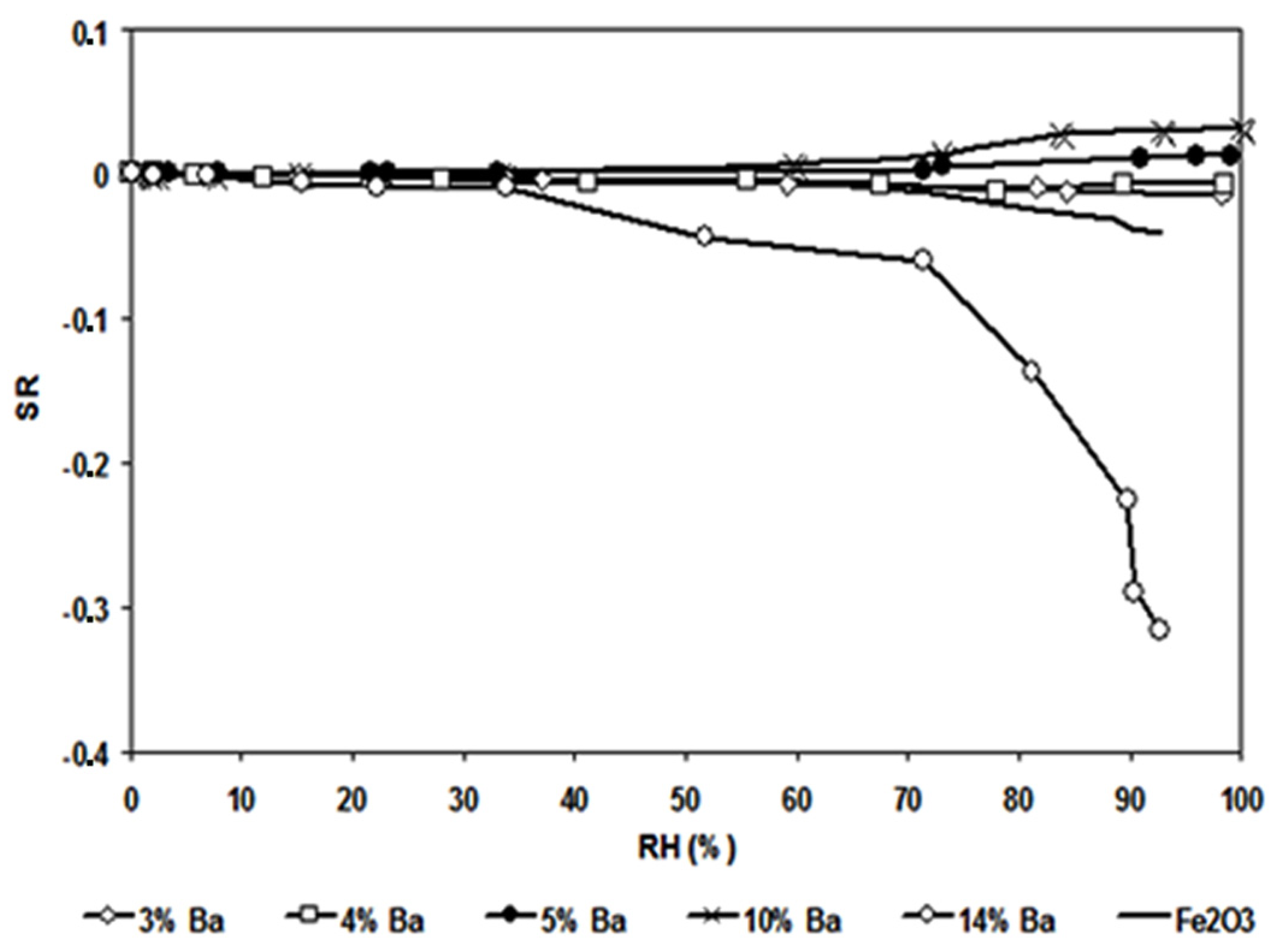
3.2. Humidity Sensitivity
| Sample | Geometrical density (g/cm3) | Relative density (%) |
|---|---|---|
| 5% BA | 4.32 ± 0.05 | 83.0 |
| 5% BA + PEG 4000 | 5.08 ± 0.05 | 95.0 |
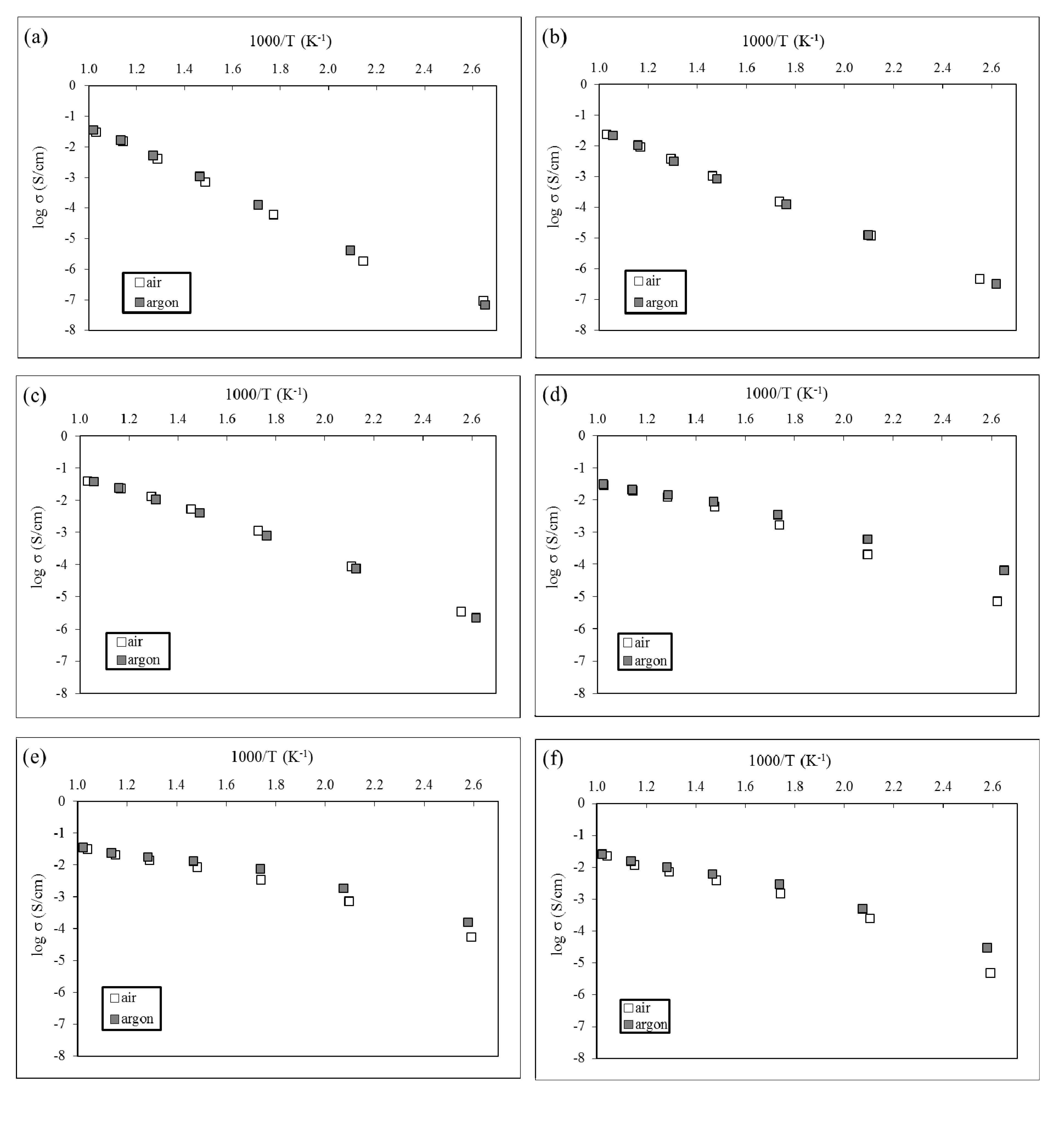
3.3. Electrical Study
| Sample | Activation Energy (eV) | |||
|---|---|---|---|---|
| Below 500 °C | Above 500 °C | |||
| Argon | Air | Argon | Air | |
| H | 0.71 | |||
| 3% BA | 0.61 | |||
| 4% BA | 0.54 | |||
| 5% BA | 0.36 | 0.51 | 0.27 | |
| 10% BA | 0.35 | 0.40 | 0.26 | |
| 14% BA | 0.43 | 0.52 | 0.34 | |
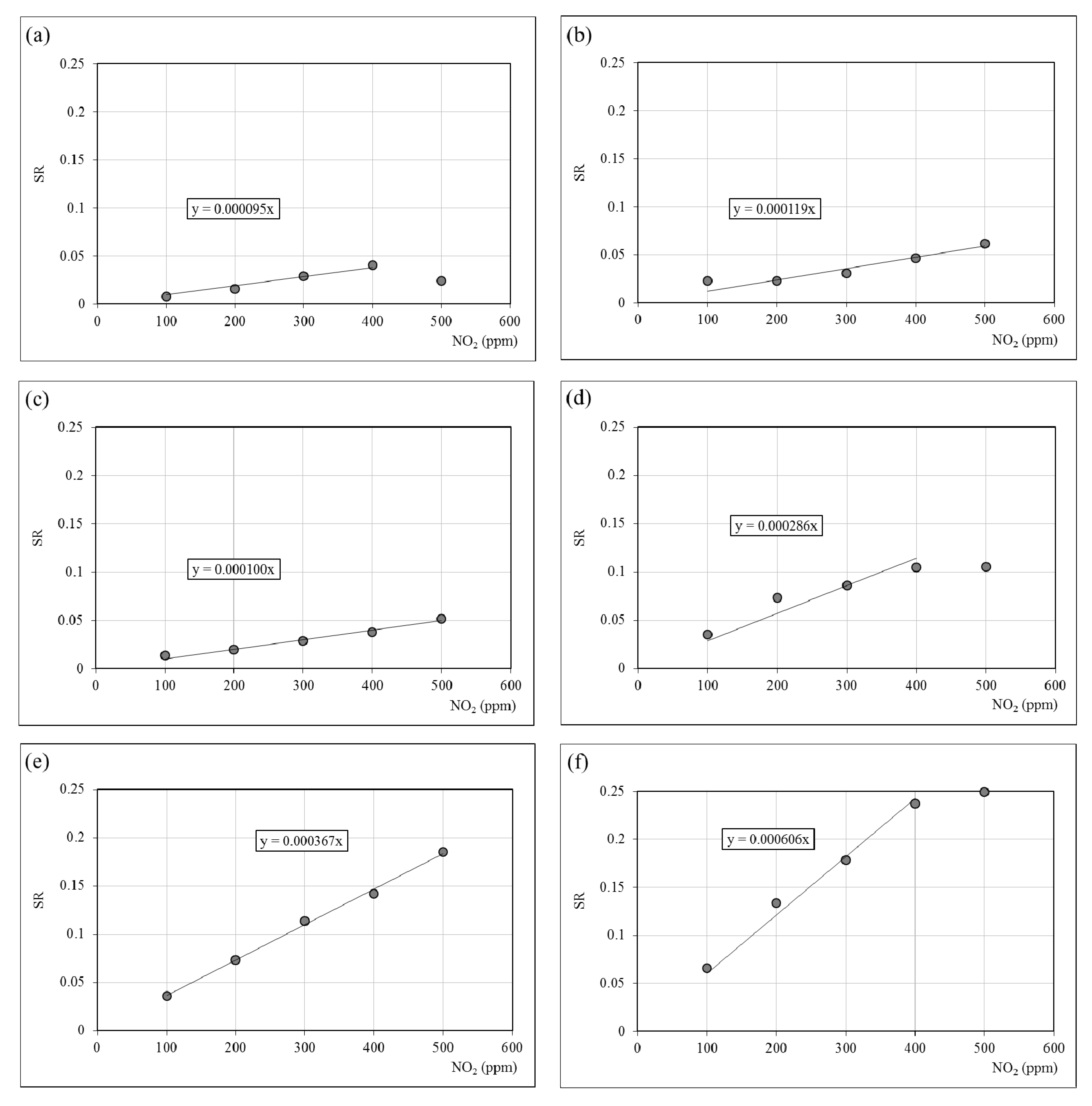
3.4. NO2 Sensitivity
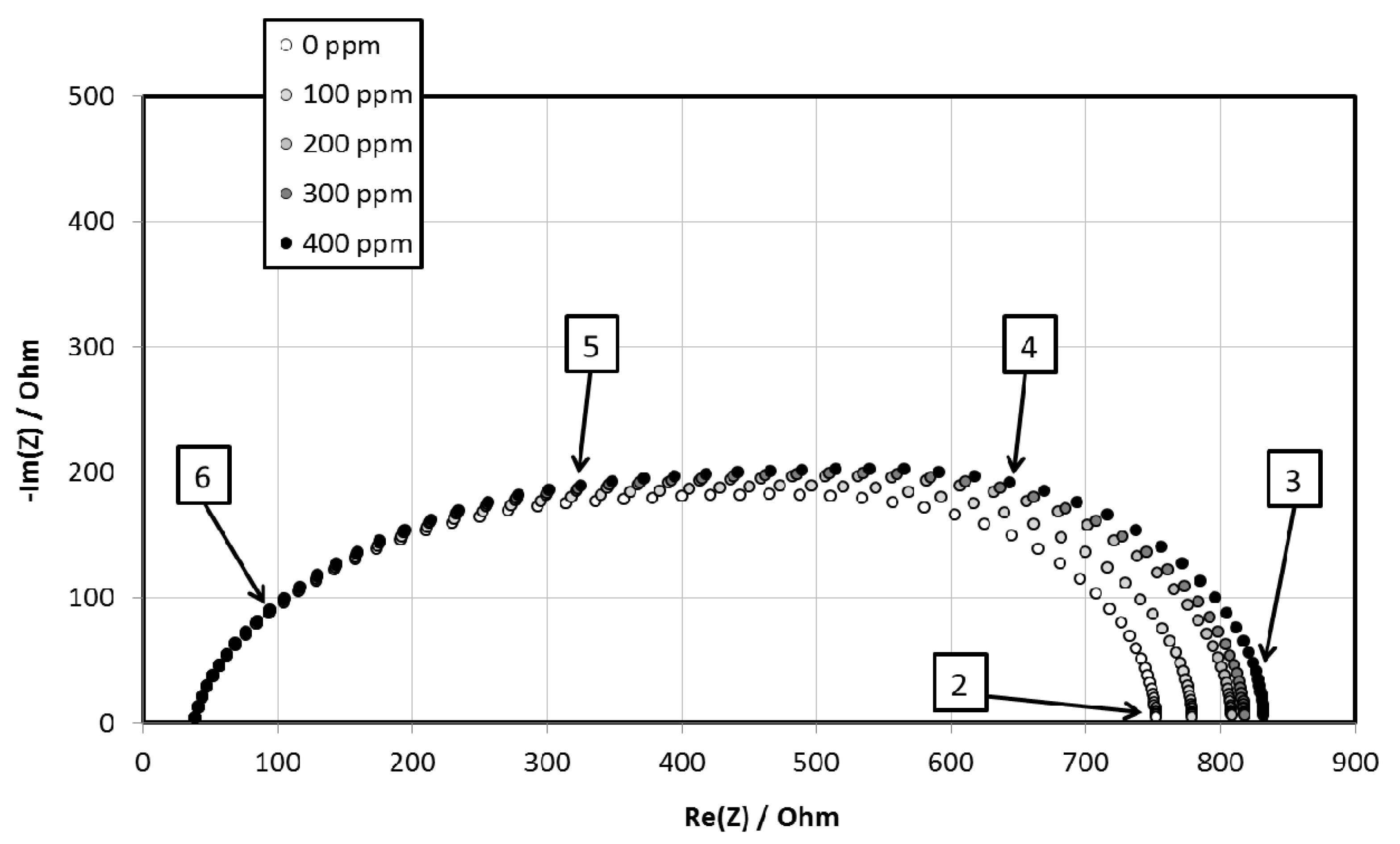
| Sample | S (ppm−1) | R(PNO2→0) (Ohm) |
|---|---|---|
| H | 0.95 × 10−4 | 115,200 |
| 3% BA | 1.19 × 10−4 | 11,485 |
| 4% BA | 1.00 × 10−4 | 3943 |
| 5% BA | 2.86 × 10−4 | 747 |
| 10% BA | 3.67 × 10−5 | 695 |
| 14% BA | 6.06 × 10−4 | 380 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- The European Parliament and the Council of the European Union. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:152:0001:0044:EN:PDF (accessed on 22 September 2013).
- The European Commission. Laying down rules for Directives 2004/107/EC and 2008/50/EC of the European Parliament and of the Council as regards the reciprocal exchange of information and reporting on ambient air quality. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:335:0086:0106:EN:PDF (accessed on 22 September 2013).
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; Hubalek, J.; Malysz, K.; Prášek, J.; Vilanova, X.; Llobet, E.; Correig, X. A route toward more selective and less humidity sensitive screen-printed SnO2 and WO3 gas sensitive layers. Sens. Actuators Chem. 2004, 100, 221–227. [Google Scholar] [CrossRef]
- Chung, Y.K.; Kim, M.H.; Um, W.S.; Lee, H.S.; Song, J.K.; Choi, S.C.; Yi, K.M.; Lee, M.J.; Chung, K.W. Gas sensing properties of WO3 thick film for NO2 gas dependent on process condition. Sens. Actuators Chem. 1999, 60, 49–56. [Google Scholar] [CrossRef]
- Lee, D.S.; Han, S.D.; Huh, J.S.; Lee, D.D. Nitrogen oxides-sensing characteristics of WO3-based nanocrystalline thick film gas sensor. Sens. Actuators Chem. 1999, 60, 57–63. [Google Scholar] [CrossRef]
- Ismail, B.; Abaab, M.; Rezig, B. Structural and electrical properties of ZnO films prepared by screen printing technique. Thin Solid Film. 2001, 383, 92–94. [Google Scholar] [CrossRef]
- Yasushi, Y.; Katsuji, Y.; Yumi, M.; Masami, O. A Thick-film NO2 sensor fabricated using Zn–Sn–Sb–O composite material. Jpn. J. Appl. Phys. 2003, 42, 7594–7598. [Google Scholar] [CrossRef]
- Coppedè, N.; Villani, M.; Mosca, R.; Iannotta, S.; Zappettini, A.; Calestani, D. Low temperature sensing properties of a nano hybrid material based on ZnO nanotetrapods and titanyl phthalocyanine. Sensors 2013, 13, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Tonezzer, M.; Hieu, N.V. Size-dependent response of single-nanowire gas sensors. Sens. Actuators Chem. 2012, 163, 146–152. [Google Scholar] [CrossRef]
- Ando, M.; Steffes, H.; Chabicovsky, R.; Haruta, M.; Stangl, G. Optical and electrical H2- and NO2-sensing properties of Au/InxOyNz films. IEEE Sens. J. 2004, 4, 232–236. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Benussi, P.; Coccoli, G.; Groppelli, S.; Nelli, P. Reactively sputtered indium tin oxide polycrystalline thin films as NO and NO2 gas sensors. Thin Solid Film. 1990, 186, 349–360. [Google Scholar] [CrossRef]
- Siciliano, T.; Giulio, M.D.; Tepore, M.; Filippo, E.; Micocci, G.; Tepore, A. Room temperature NO2 sensing properties of reactivity sputtered TeO2 thin films. Sens. Actuators Chem. 2009, 137, 644–648. [Google Scholar]
- Suehiro, J.; Zhou, G.; Imakiire, H.; Ding, W.; Hara, M. Controlled fabrication of carbon nanotube NO2 gas sensor using dielectrophoretic impedance measurement. Sens. Actuators Chem. 2005, 108, 398–403. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Ono, T.; Yamazoe, N.; Miura, N. High-temperature NOx sensors using zirconia solid electrolyte and zinc-family oxide sensing electrode. Solid State Ion. 2002, 152–153, 801–807. [Google Scholar] [CrossRef]
- Sun, H.T.; Cantalini, C.; Faccio, M.; Pelino, M. NO2 gas sensitivity of sol-gel-derived α-Fe2O3 thin films. Thin Solid Film. 1995, 269, 97–101. [Google Scholar] [CrossRef]
- Cantalini, C.; Sun, H.T.; Faccio, M.; Ferri, G.; Pelino, M. Niobium-doped α-Fe2O3 semiconductor ceramic sensors for the measurement of nitric oxide gases. Sens. Actuators Chem. 1995, 25, 673–677. [Google Scholar] [CrossRef]
- Tulliani, J.-M.; Baroni, C.; Lopez, C.; Dessemond, L. New NOx sensors based on hematite doped with alkaline and alkaline-earth elements. J. Eur. Ceram. Soc. 2011, 31, 2357–2364. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, R813–R839. [Google Scholar] [CrossRef]
- Tulliani, J.M.; Bonville, P. Influence of the dopants on the electrical resistance of hematite-based humidity sensors. Ceram. Int. 2005, 31, 507–514. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Tulliani, J.M.; Pecharromán, C.; Moya, J.S. Iron-oxide nanoparticles supported on sepiolite as a novel humidity sensor. J. Eur. Ceram. Soc. 2007, 27, 1983–1989. [Google Scholar] [CrossRef]
- Nenov, T.G.; Yordanov, S.P. Ceramic Sensor: Technology and Applications; CRC PressTechnomic Publishing Company, Inc.: Lancaster, PA, USA, 1996. [Google Scholar]
- Traversa, E. Ceramic sensors for humidity detection: the state-of-the-art and future developments. Sens. Actuators Chem. 1995, 23, 135–156. [Google Scholar]
- Traversa, E.; Bearzotti, A. Humidity sensitive electrical properties of dense ZnO with non-ohmic electrode. J. Ceram. Soc. Jpn. 1995, 103, 11–15. [Google Scholar] [CrossRef]
- Santilli, C.V.; Bonnet, J.P.; Dordor, P.; Onillion, M.; Hagenmuller, P. Influence of structural defects on the electrical properties of α-Fe2O3 ceramics. Ceram. Int. 1990, 16, 25–32. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.H.; Choi, J.S. Electrical conductivity of pure and doped α-ferric oxides. J. Phys. Chem. Solids 1985, 46, 331–338. [Google Scholar] [CrossRef]
- Gardner, R.F.G.; Sweett, F.; Tanner, D.W. The electrical properties of alpha ferric oxide-II. Ferric oxide of high purity. J. Phys. Chem. Solids 1963, 24, 1183–1196. [Google Scholar] [CrossRef]
- Chang, R.H.; Wagner, J.B. Direct-current conductivity and iron tracer diffusion in hematite at high temperatures. J. Am. Ceram. Soc. 1972, 55, 211–213. [Google Scholar] [CrossRef]
- Stone, H.E.N. Electrical conductivity and sintering in iron oxides at high temperatures. J. Mater. Sci. 1968, 3, 321–325. [Google Scholar] [CrossRef]
- Han, J.S.; Davey, D.E.; Mulcahy, D.E.; Yu, A.B. Effect of pH value of the precipitation solution on the CO sensitivity of α-Fe2O3. Sens. Actuators Chem. 1999, 61, 83–91. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Gurlo, A.; Bogdanov, P. Mechanism of O3 and NO2 detection and selectivity of the In2O3 sensors. Sens. Actuators Chem. 2001, 77, 264–267. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, Y.; Tan, J.; He, C.; Shah, A.N.; Ding, Y.; Yu, L.; Zhao, W. Impacts of continuously regenerating trap and particle oxidation catalyst on the NO2 and particulate matter emissions emitted from diesel engine. J. Environ. Sci. 2012, 24, 624–631. [Google Scholar] [CrossRef]
- Liu, Z.; Shah, A.N.; Ge, D.Y.; Tan, J.; Jiang, L.; Yu, L.; Zhao, W.; Wang, C.; Zeng, T. Effects of continuously regenerating diesel particulate filters on regulated emissions and number-size distribution of particles emitted from a diesel engine. J. Environ. Sci. 2011, 23, 798–807. [Google Scholar] [CrossRef]
- Aono, H.; Hirazawa, H.; Naohara, T.; Maehara, T. Surface study of fine MgFe2O4 ferrite powder prepared by chemical methods. Appl. Surf. Sci. 2008, 254, 2319–2324. [Google Scholar] [CrossRef]
- Berger, O.; Hoffmann, T.; Fischer, W.-J.; Melev, V. Tungsten-oxide thin films as a novel materials with high sensitivity to NO2, O3, and H2S. Part II: Application as gas sensors. J. Mater. Sci. Mater. Electron. 2004, 15, 483–493. [Google Scholar] [CrossRef]
- Chiorino, A.; Ghiotti, G.; Prinetto, F.; Carotta, M.C.; Gnani, D.; Martinelli, G. Preparation and characterization of SnO2 and MoOx–SnO nanosized powders for thick film gas sensors. Sens. Actuators Chem. 1999, 58, 338–349. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lopez, C.; Baroni, C.; Tulliani, J.-M. Ba-Doped Iron Oxide as a New Material for NO2 Detection. Materials 2013, 6, 4801-4816. https://doi.org/10.3390/ma6104801
Lopez C, Baroni C, Tulliani J-M. Ba-Doped Iron Oxide as a New Material for NO2 Detection. Materials. 2013; 6(10):4801-4816. https://doi.org/10.3390/ma6104801
Chicago/Turabian StyleLopez, Christian, Chiara Baroni, and Jean-Marc Tulliani. 2013. "Ba-Doped Iron Oxide as a New Material for NO2 Detection" Materials 6, no. 10: 4801-4816. https://doi.org/10.3390/ma6104801





