Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts
Abstract
:1. Introduction
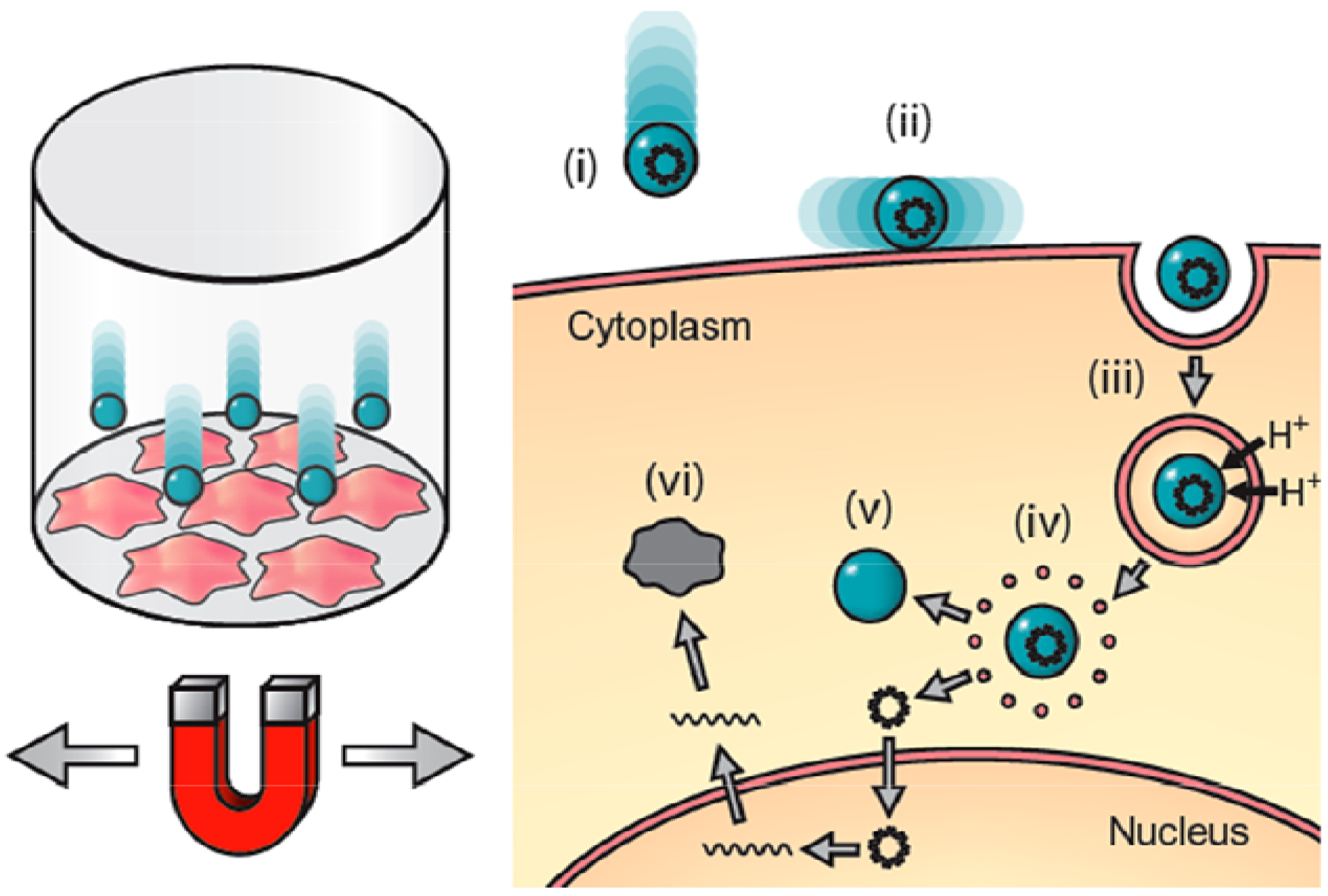
2. Materials and Methods
2.1. Cell Culture
2.2. Nanoparticle: DNA Optimization and Binding
2.3. Transfection of NIH3T3 Cells Using the Magnefect-Nano System
2.4. Transfection of NIH3T3 Cells with Lipofectamine 2000
2.5. Immunofluorescence & Fluorescence Activated Cell Sorting (FACS) Assays
2.6. Cell Viability
2.7. Statistical Analysis
3. Results
3.1. DNA Binding to MNPs and Transfection of NIH3T3 Cells

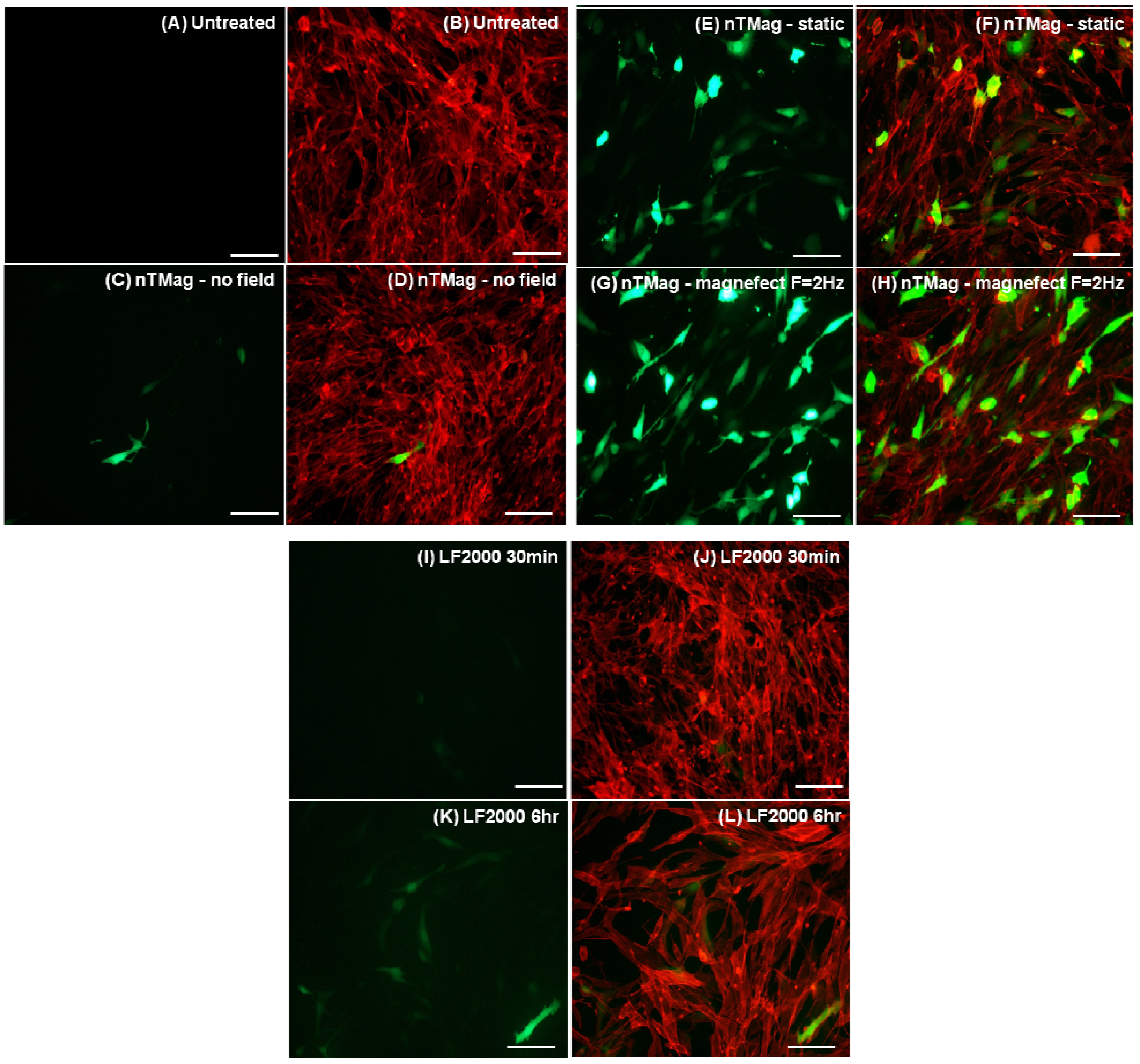
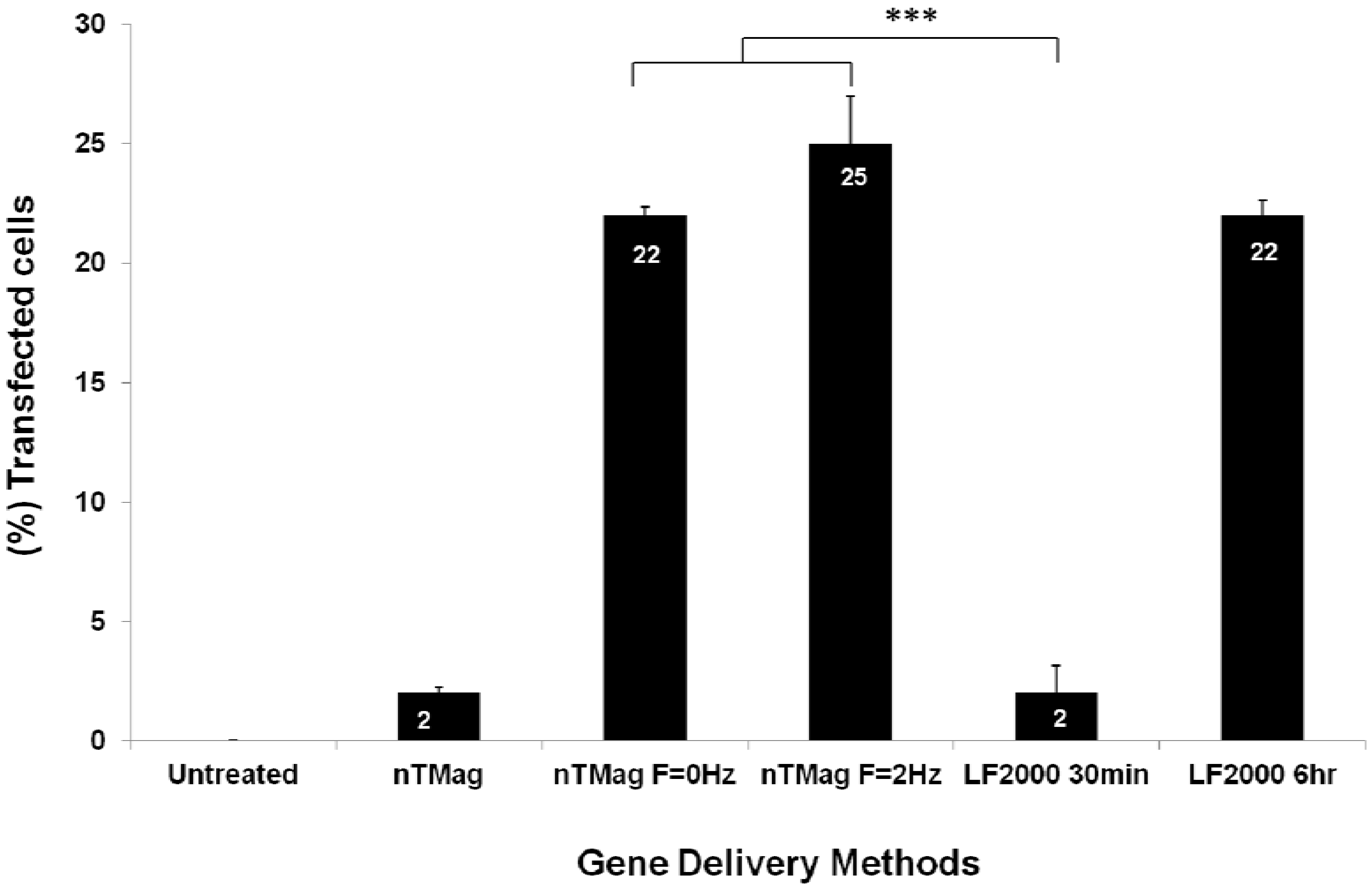
3.2. Evaluation of MNPs Toxicity and Determination of Transfected NIH3T3 Cells Viability
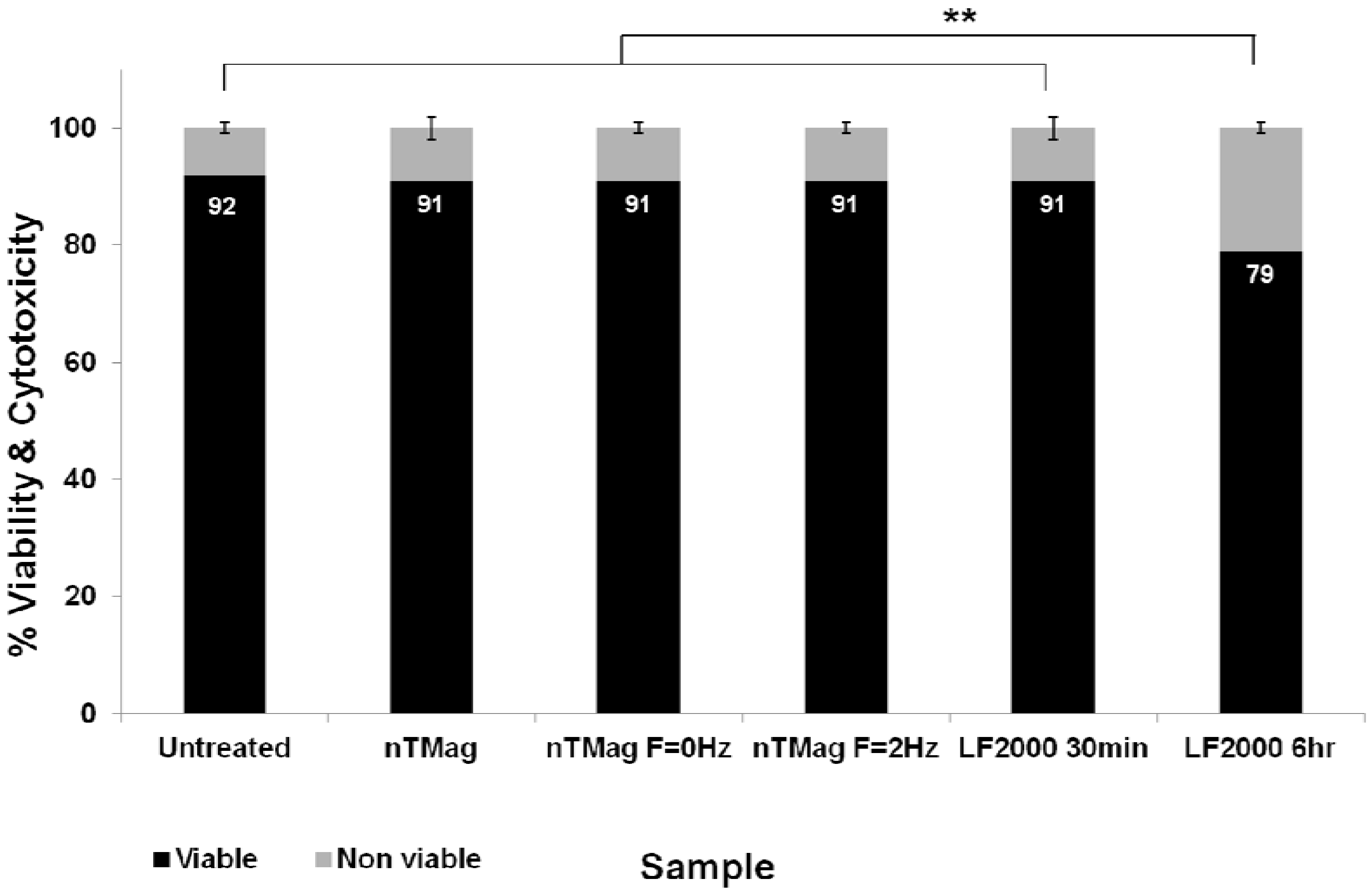
4. Discussion and Conclusions
Acknowledgements and Disclosure
References
- Cho, H.J.; Lee, T.S.; Park, J.B.; Park, K.K.; Choe, J.Y.; Sin, D.I.; Park, Y.Y.; Moon, Y.S.; Lee, K.G.; Yeo, J.H.; et al. Disulfiram suppresses invasive ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9 expression. J. Biochem. Mol. Biol. 2007, 40, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Kersting, C.; Gebert, C.; Agelopoulos, K.; Schmidt, H.; van Diest, P.J.; Juergens, H.; Winkelmann, W.; Kevric, M.; Gosheger, G.; Brandt, B.; et al. Epidermal growth factor receptor expression in high-grade osteosarcoma is associated with a good clinical outcome. Clin. Cancer Res. 2007, 13, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, F.; Richard, P.; Wittrant, Y.; Battaglia, S.; Pilet, P.; Trichet, V.; Blanchard, F.; Gouin, F.; Pitard, B.; Heymann, D.; Redini, F. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: Blockage of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007, 67, 7308–7318. [Google Scholar]
- Lu, Y. Viral based gene therapy for prostate cancer. Curr. Gene Ther. 2001, 1, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.R.; Kim, S.Y.; Jang, K.Y.; Lee, K.C.; Yi, H.K.; Lee, D.Y.; Kim, H.Y.; Hwang, P.H. Laboratory formulated magnetic nanoparticles for enhancement of viral gene expression in suspension cell line. J. Virol. Methods 2008, 147, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.M. A tumultuous year for gene therapy. Mol. Ther. 2000, 2, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Spack, E.G.; Corgi, F.L. Developing non-viral DNA delivery systems for cancer and infectious disease. Drug Discov. Today 2001, 6, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Wobus, A.M.; Boheler, K.R. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol. Rev. 2005, 85, 635–678. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Marini, R.; van Blitterswijk, C.A.; Mulligan, R.C.; D'Amore, P.A.; Langer, R. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.; Zolotukhin, I.; Fraites, T.J.; Dobson, J.; Batich, C.; Byrne, B.J. Microsphere-mediated delivery of recombinant AVV vectors in vitro and in vivo. Mol. Ther. 2000, 1, S239–S242. [Google Scholar] [CrossRef]
- Mah, C.; Fraites, T.J.; Zolotukhin, I.; Song, S.H.; Flotte, T.R.; Dobson, J.; Batich, C.; Byrne, B.J. Improved method of recombinant AAV2 delivery for systemic targeted gene delivery. Mol. Ther. 2002, 6, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Schillinger, U.; Scherer, F.; Bergemann, C.; Remy, J.S.; Krotz, F.; Anton, M.; Lausier, J.; Rosenecker, J. The magnetofection method: Using magnetic force to enhance gene delivery. Biol. Chem. 2003, 384, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Krotz, F.; Sohn, H.Y.; Gloe, T.; Plank, C.; Pohl, U. Magnetofection potentiates gene delivery to cultured endothelial cells. J. Vasc. Res. 2003, 40, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Griesenbach, U.; Xenariou, S.; Keramane, A.; Batich, C.D.; Alton, E.W.F.W.; Dobson, J. Magnetic nanoparticles as gene delivery agents: Enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology 2008, 19, 405102:1–405102:5. [Google Scholar] [CrossRef]
- Pickard, M.; Chari, D. Enhancement of magnetic nanoparticle-mediated gene transfer to astrocytes by “magnetofection”: Effects of static and oscillating fields. Nanomedicine 2010, 5, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Fouriki, A.; Clements, M.A.; Farrow, N.; Dobson, J. Efficient transfection of MG-63 osteoblasts using magnetic nanoparticles and oscillating magnetic fields. J. Tissue Eng. Regen. Med. 2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Kohler, N.; Zhang, M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Magnetic micro and nano particle based targeting for drug and gene delivery. Nanomedicine 2006, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yoon, T.J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Dobson, J.; Batich, C.D. Gene delivery. Eur. Pat. Appl. WO2006111770, 22 April 2006. [Google Scholar]
- Fouriki, A.; Farrow, N.; Clements, M.; Dobson, J. Evaluation of the magnetic field requirements for nanomagnetic gene transfection. Nano Rev. 2010, 1, 5167. [Google Scholar] [CrossRef]
- Lim, J.; Dobson, J. Delivery of short interfering ribonucleic acid-complexed magnetic nanoparticles in an oscillating field occurs via caveolae-mediated endocytosis. PLoS One 2012, 7, e51350. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fouriki, A.; Dobson, J. Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts. Materials 2013, 6, 255-264. https://doi.org/10.3390/ma6010255
Fouriki A, Dobson J. Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts. Materials. 2013; 6(1):255-264. https://doi.org/10.3390/ma6010255
Chicago/Turabian StyleFouriki, Angeliki, and Jon Dobson. 2013. "Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts" Materials 6, no. 1: 255-264. https://doi.org/10.3390/ma6010255



