Self-Assembly of Bi2Te3-Nanoplate/Graphene-Nanosheet Hybrid by One-Pot Route and Its Improved Li-Storage Properties
Abstract
:1. Introduction
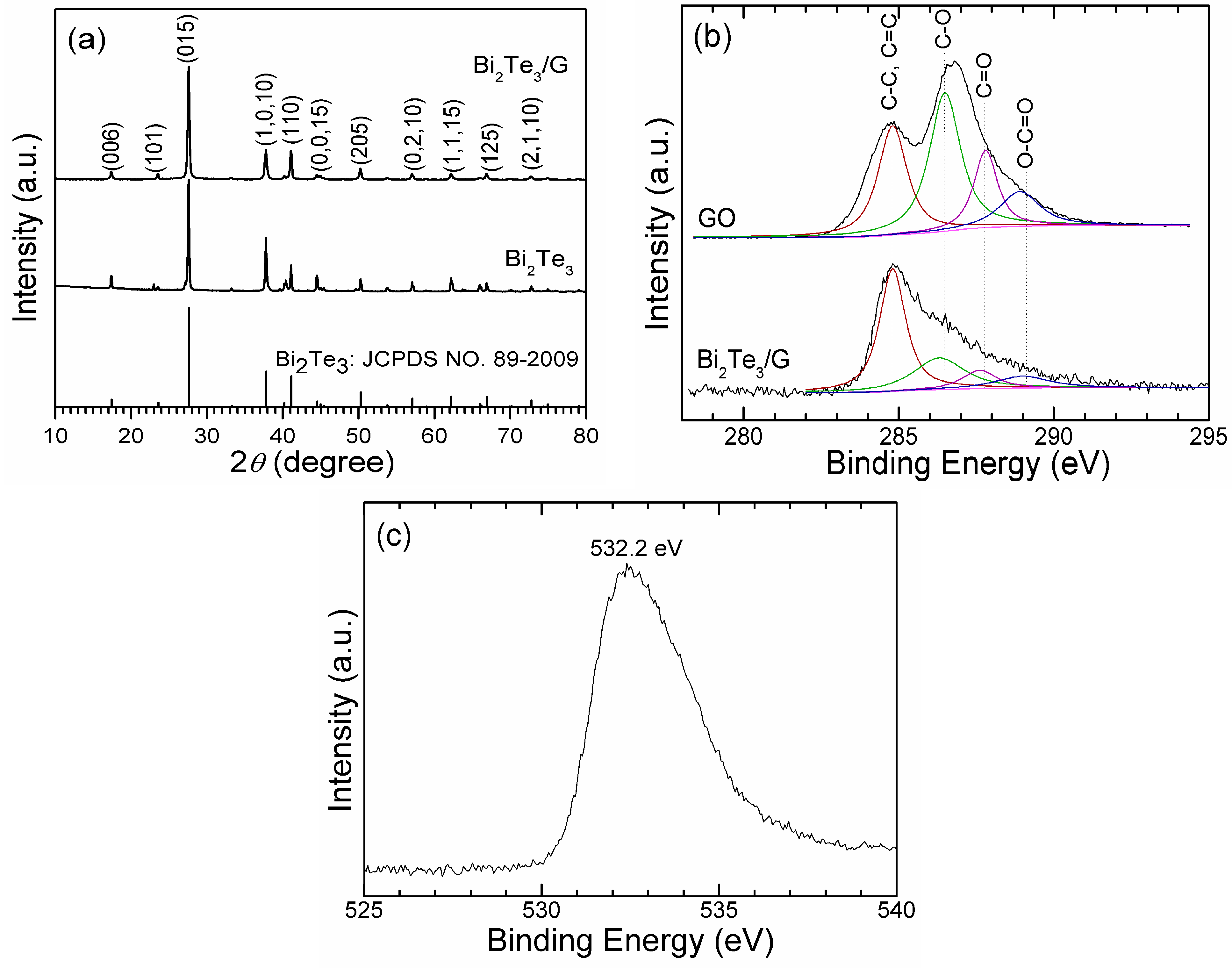
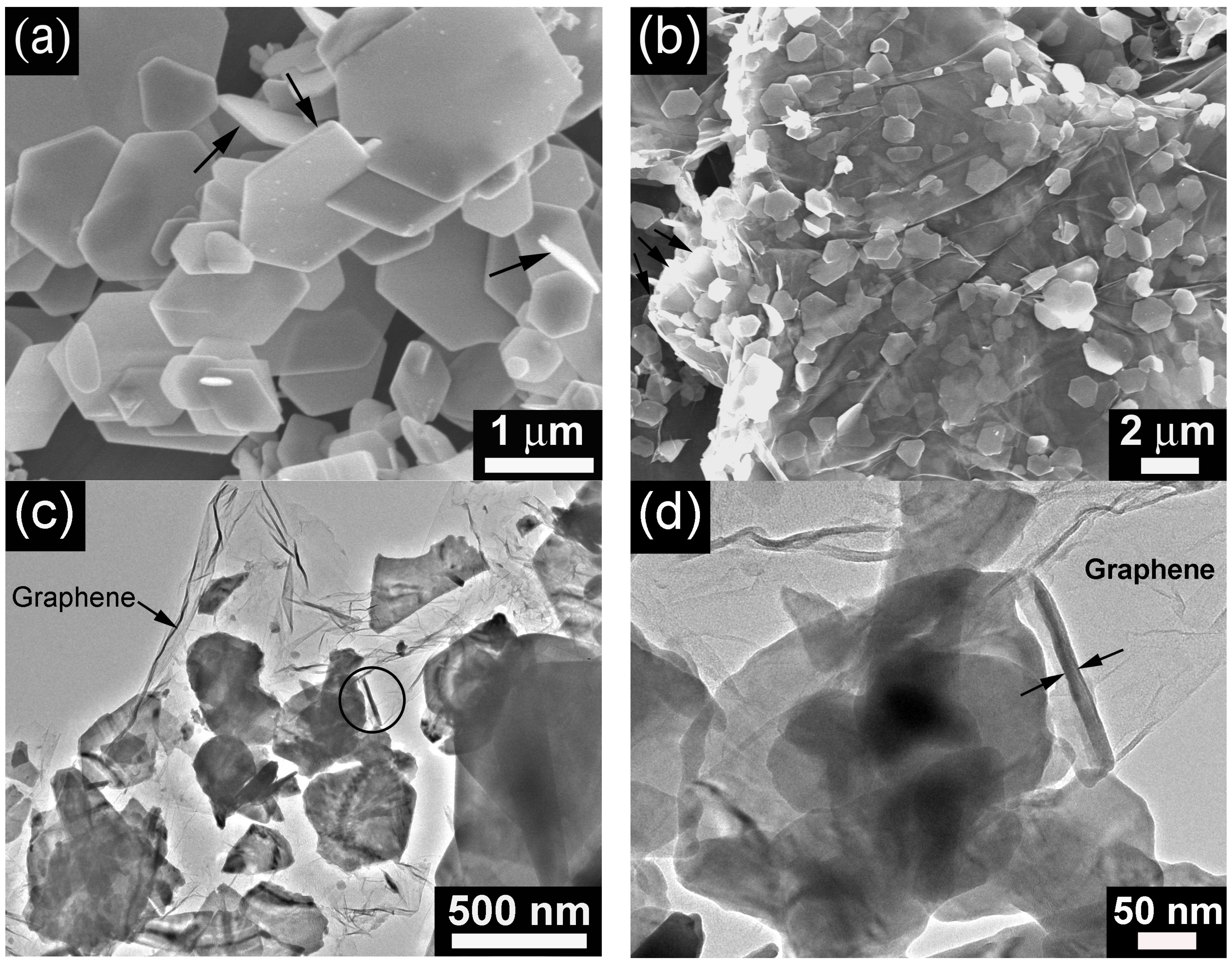
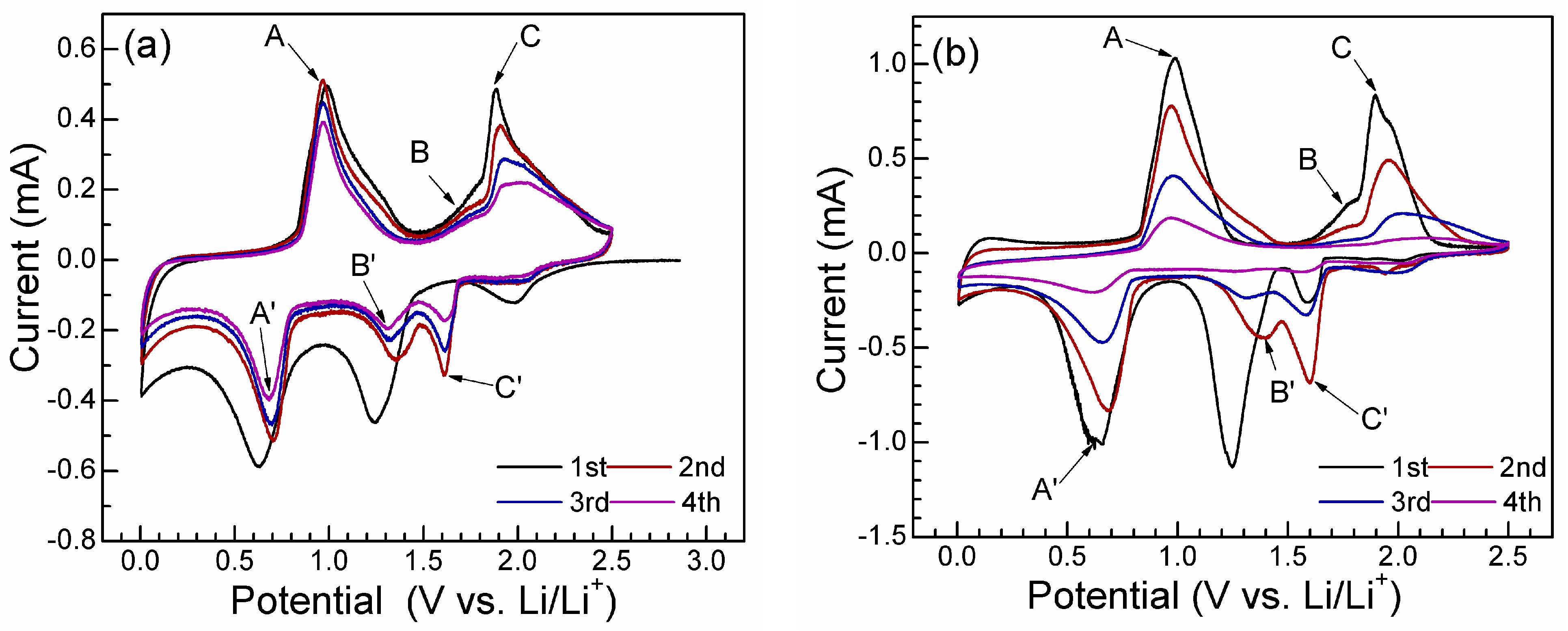
2. Results and Discussion

3. Experimental Section
3.1. Preparation of Bi2Te3-Nanoplate/Graphene-Nanosheet (Bi2Te3/G) Nanohybrid
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Tirado, J.L. Inorganic materials for the negative electrode of lithium ion batteries: State-of-the-art and future prospects. Mater. Sci. Eng. R 2003, 40, 103–136. [Google Scholar] [CrossRef]
- Zhang, W.J. Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 877–887. [Google Scholar] [CrossRef]
- Huggins, R.A. Lithium alloy negative electrodes. J. Power Sources 1999, 81–82, 13–19. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L.; Le, D.B.; Dahn, J.R. Alloy design for lithium-ion battery anodes. J. Electrochem. Soc. 2007, 154, A849–A855. [Google Scholar] [CrossRef]
- Alcántara, R.; Fernández-Madrigal, F.J.; Lavela, P.; Tirado, J.L.; Jumas, J.C.; Olivier-Fourcade, J. Electrochemical reaction of lithium with the CoSb3 skutterudite. J. Mater. Chem. 1999, 9, 2517–2521. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Antimonides (FeSb2, CrSb2) with orthorhombic structure and their nanocomposites for rechargeable Li-ion batteries. Electrochim. Acta 2010, 55, 4987–4994. [Google Scholar] [CrossRef]
- Villevieille, C.; Ionica-Bousquet, C.M.; Ducourant, B.; Jumas, J.C.; Monconduit, L. NiSb2 as negative electrode for Li-ion batteries: An original conversion reaction. J. Power Sources 2007, 172, 388–394. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Electrochemical characteristics of TiSb2 and Sb/TiC/C nanocomposites as anodes for rechargeable Li-ion batteries. J. Electrochem. Soc. 2010, 157, A46–A49. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.B.; Cao, G.S.; Zhao, M.J.; Su, S.F. Solvothermal synthesis and electrochemical performances of nanosized CoSb3 as anode materials for Li-ion batteries. J. Power Sources 2005, 140, 350–354. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.B.; Cao, G.S.; Zhong, Y.D.; Zhao, M.J.; Tu, J.P. Solvothermal synthesis of nanosized CoSb2 alloy anode for Li-ion batteries. Electrochim. Acta 2005, 50, 1903–1907. [Google Scholar] [CrossRef]
- Sarakonsri, T.; Johnson, C.S.; Hackney, S.A.; Thackeray, M.M. Solution route synthesis of InSb, Cu6Sn5 and Cu2Sb electrodes for lithium batteries. J. Power Sources 2006, 153, 319–327. [Google Scholar] [CrossRef]
- Kim, H.; Cho, J. Template synthesis of hollow Sb nanoparticles as a high-performance lithium battery anode material. Chem. Mater. 2008, 20, 1679–1681. [Google Scholar]
- Chen, W.X.; Lee, J.Y.; Liu, Z.L. The nanocomposites of carbon nanotube with Sb and SnSb0.5 as Li-ion battery anodes. Carbon 2003, 41, 959–966. [Google Scholar] [CrossRef]
- Hassoun, J.; Derrien, G.; Panero, S.; Scrosati, B. The role of the morphology in the response of Sb–C nanocomposite electrodes in lithium cells. J. Power Sources 2008, 183, 339–343. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Li, X.; Xiao, Y.; Xu, G.L.; Li, J.T.; Sun, S.G. Facile synthesis of hollow Cu2Sb@C core-shell nanoparticles as a superior anode material for lithium ion batteries. J. Mater. Chem. 2011, 21, 18517–18519. [Google Scholar] [CrossRef]
- Applestone, D.; Yoon, S.; Manthiram, A. Mo3Sb7-C composite anodes for Lithium-ion batteries. J. Phys. Chem. C 2011, 115, 18909–18915. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I. V.; Firsov, A.A. Electric filed effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, J.H.; Jung, I.W.; Piner, R.D.; An, S.J.; Li, X.S.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-based ultracapacitor. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.X.; Pan, R.J.; Liu, S.Y.; Song, W.T.; Cao, G.S.; Zhu, T.J.; Zhao, X.B. Sb-based alloy (NiSb, FeSb2) nanoparticles decorated graphene prepared by one-step solvothermal route as anode for Li-ion batteries. Int. J. Electrochem. Sci. 2011, 6, 4811–4821. [Google Scholar]
- Zheng, Y.X.; Xie, J.; Liu, S.Y.; Song, W.T.; Cao, G.S.; Zhu, T.J.; Zhao, X.B. Self-assembly of Co-Sb-nanocrystal/graphene hybrid nanostructure with improved Li-storage properties via a facile in situ solvothermal route. J. Power Sources 2012, 202, 276–283. [Google Scholar] [CrossRef]
- Crosnier, O.; Devaux, X.; Brousse, T.; Fragnaud, P.; Schleich, D.M. Influence of particle size and matrix in “metal” anodes for Li-ion cells. J. Power Sources 2001, 97–98, 188–190. [Google Scholar] [CrossRef]
- Pérez-Flores, J.C.; Kuhn, A.; García-Alvarado, F. Electrochemical performances of BiSbO4 as electrode material for lithium batteries. J. Power Sources 2008, 182, 365–369. [Google Scholar] [CrossRef]
- Zhao, X.B.; Cao, G.S.; Lv, C.P.; Zhang, L.J.; Hu, S.H.; Zhu, T.J.; Zhou, B.C. Electrochemical properties of some Sb or Te based alloys for candidate anode materials of lithium-ion batteries. J. Alloys Compd. 2001, 315, 265–269. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, K.K.; Benayad, A.; Yoon, S.M.; Park, H.K.; Jung, I.S.; Jin, M.H.; Jeong, H.K.; Kim, J.M.; Choi, J.Y.; Lee, Y.H. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.C.; Wen, L.; Gao, L.B.; Zhao, J.P.; Chen, Z.P.; Zhou, G.M.; Li, F.; Cheng, H.M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Robinson, J.T.; Diankov, G.; Dai, H.J. Nanocrystal growth on graphene with various degrees of oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Sun, Z.L.; Zhu, Y.J.; Chen, N.F.; Zhou, Y.F.; Ding, J.; Chen, X.H.; Chen, L.D. Top-down fabrication of nano-scaled Bi2Se0.3Te2.7 associated by electrochemical Li intercalation. Dalton Trans. 2011, 40, 340–343. [Google Scholar] [CrossRef]
- Cunningham, P.T.; Johnson, S.A.; Cairns, E.J. Phase equilibria in lithium-chalcogen systems. J. Electrochem. Soc. 1973, 120, 328–330. [Google Scholar] [CrossRef]
- Liu, S.Y.; Xie, J.; Zheng, Y.X.; Cao, G.S.; Zhu, T.J.; Zhao, X.B. Nanocrystal manganese oxide (Mn3O4, MnO) anchored on graphite nanosheet with improved electrochemical Li-storage properties. Electrochim. Acta 2012, 66, 271–278. [Google Scholar] [CrossRef]
- Pan, D.Y.; Wang, S.; Zhao, B.; Wu, M.H.; Zhang, H.J.; Wang, Y.; Jiao, Z. Li storage properties of disordered graphene nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tu, F.; Xie, J.; Cao, G.; Zhao, X. Self-Assembly of Bi2Te3-Nanoplate/Graphene-Nanosheet Hybrid by One-Pot Route and Its Improved Li-Storage Properties. Materials 2012, 5, 1275-1284. https://doi.org/10.3390/ma5071275
Tu F, Xie J, Cao G, Zhao X. Self-Assembly of Bi2Te3-Nanoplate/Graphene-Nanosheet Hybrid by One-Pot Route and Its Improved Li-Storage Properties. Materials. 2012; 5(7):1275-1284. https://doi.org/10.3390/ma5071275
Chicago/Turabian StyleTu, Fangfang, Jian Xie, Gaoshao Cao, and Xinbing Zhao. 2012. "Self-Assembly of Bi2Te3-Nanoplate/Graphene-Nanosheet Hybrid by One-Pot Route and Its Improved Li-Storage Properties" Materials 5, no. 7: 1275-1284. https://doi.org/10.3390/ma5071275



