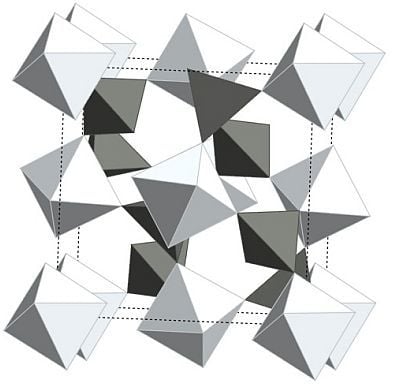
Two Decades of Negative Thermal Expansion Research: Where Do We Stand?
Abstract
:1. Introduction
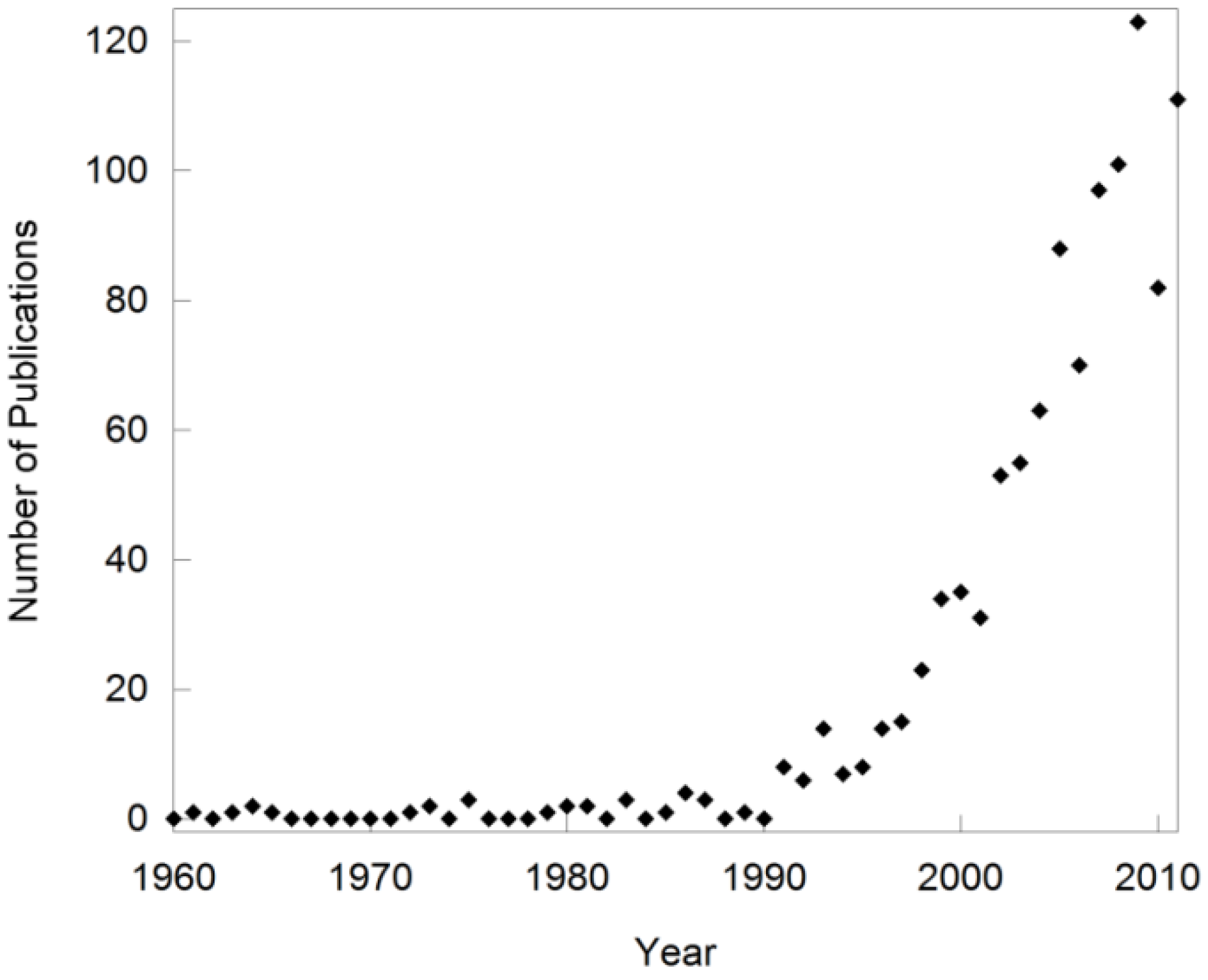
2. Negative Thermal Expansion Due to Transverse Vibrations
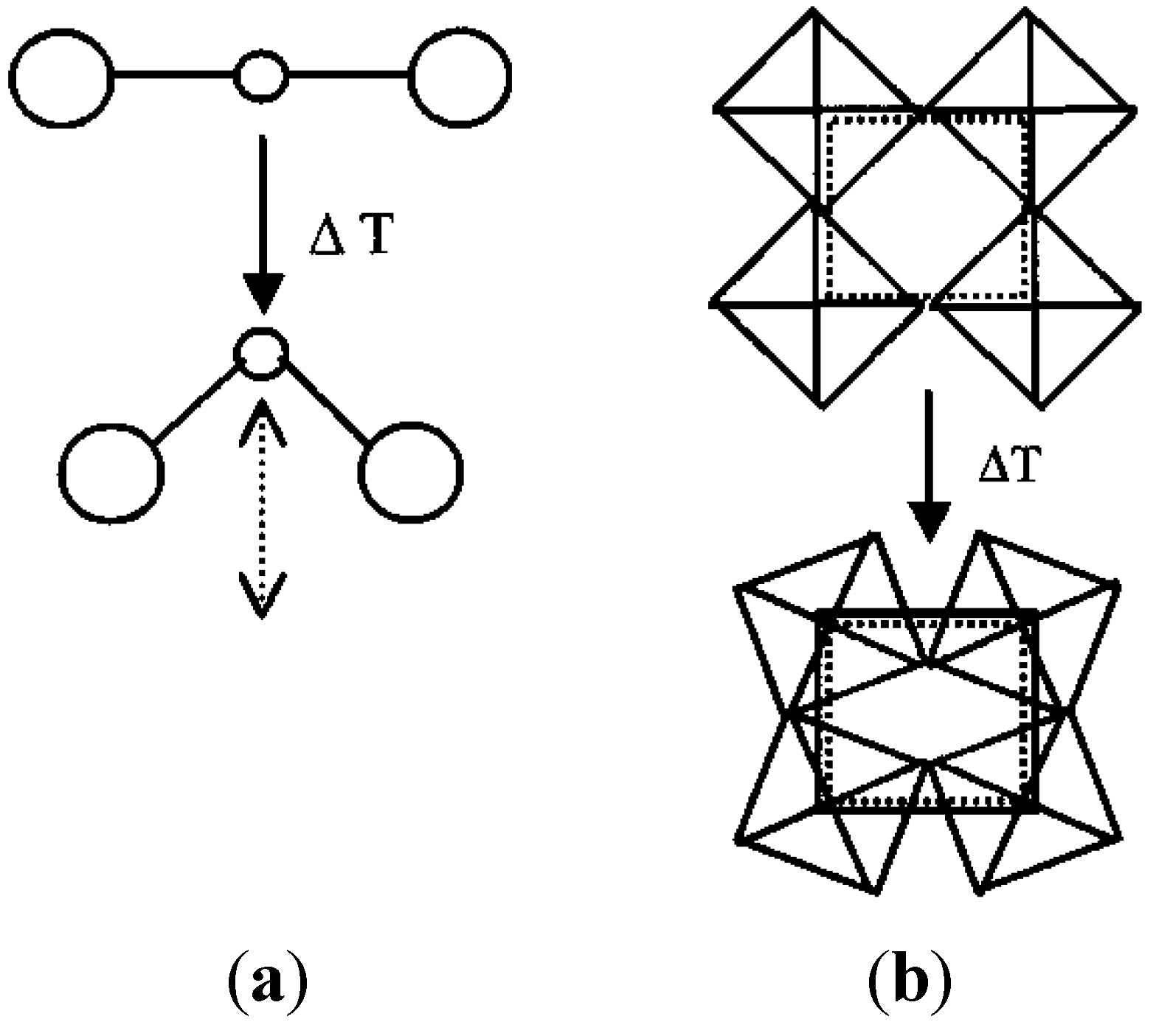
2.1. ZrW2O8 Family

2.1.1. Substitution of ZrW2O8
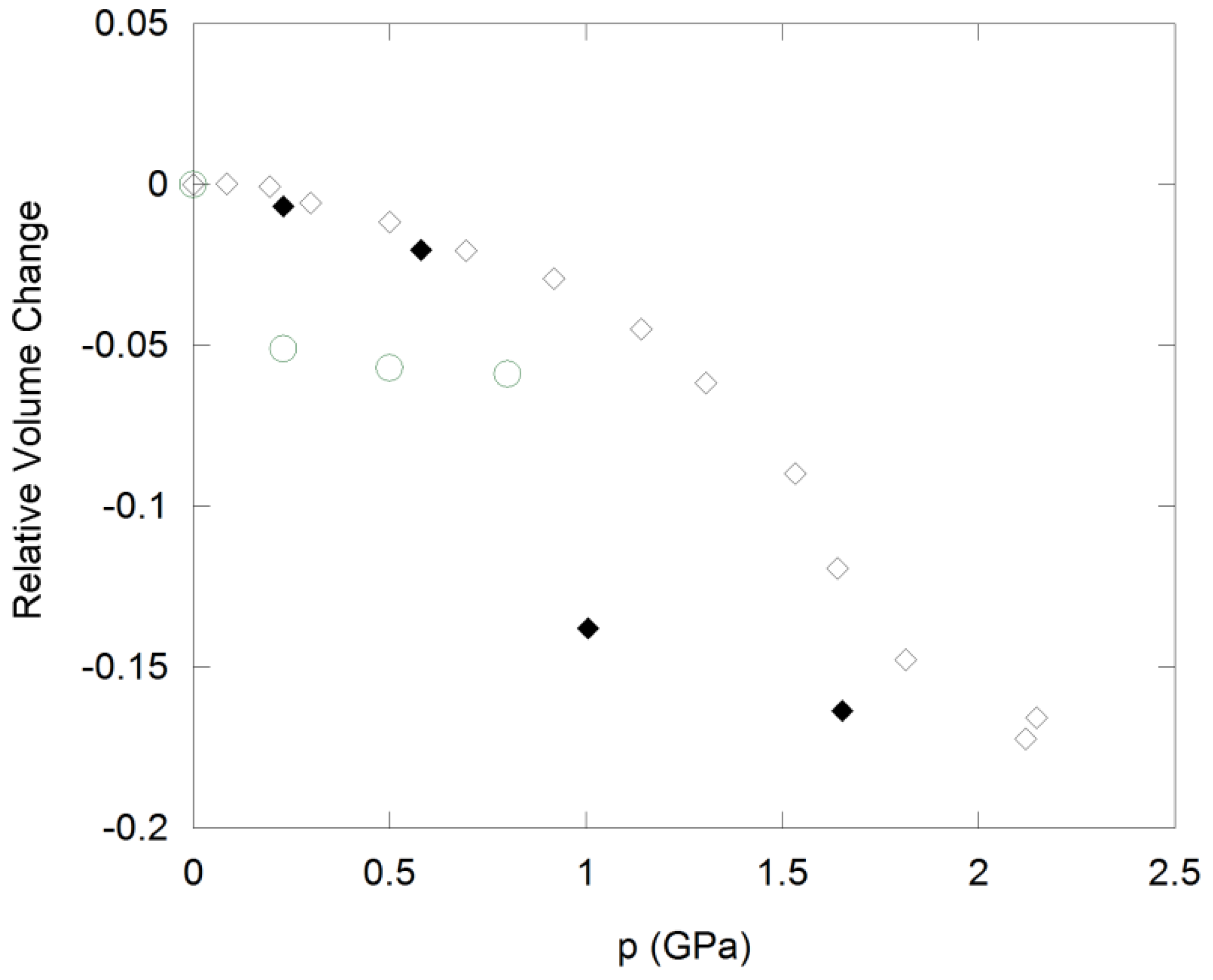
2.1.2. High Pressure Behavior
2.2. ZrV2O7 Family
2.2.1. Substitution of ZrV2O7
2.2.2. High Pressure Behavior
2.3. Sc2W3O12 Family
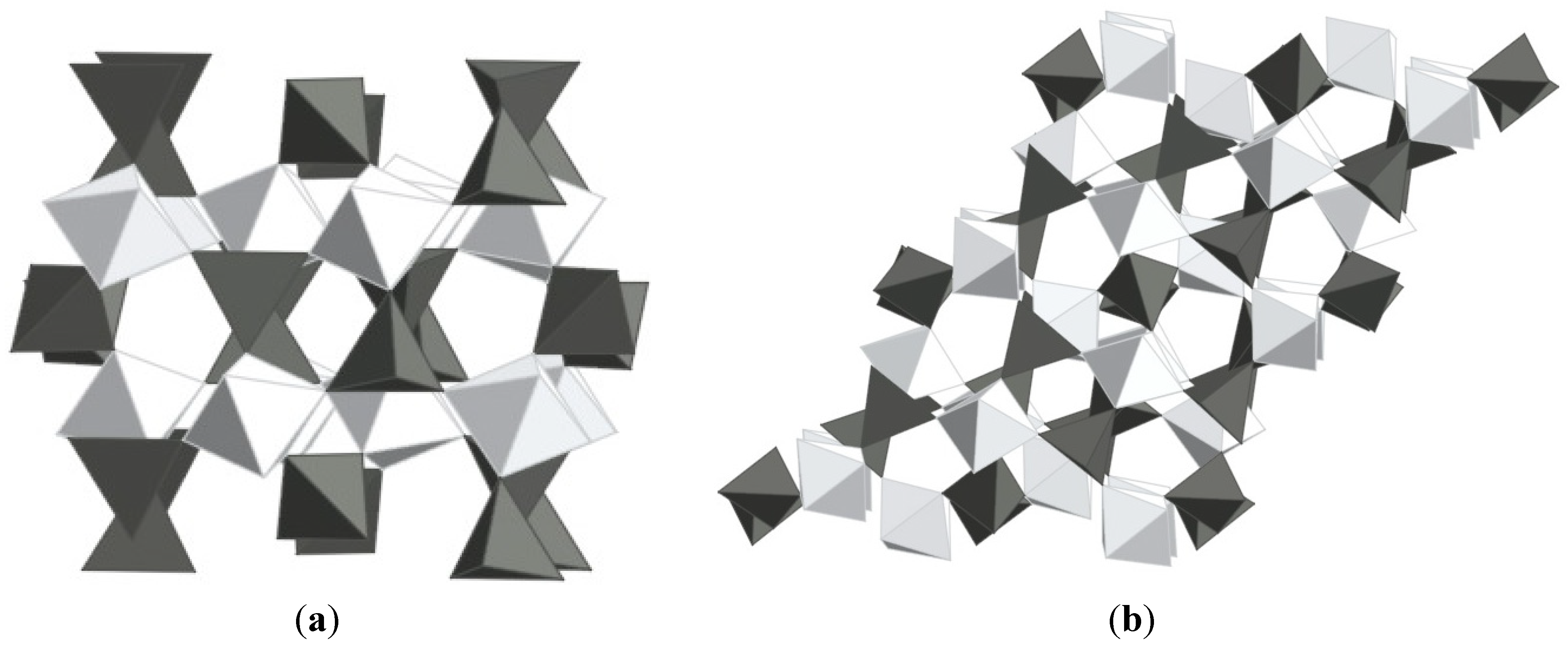
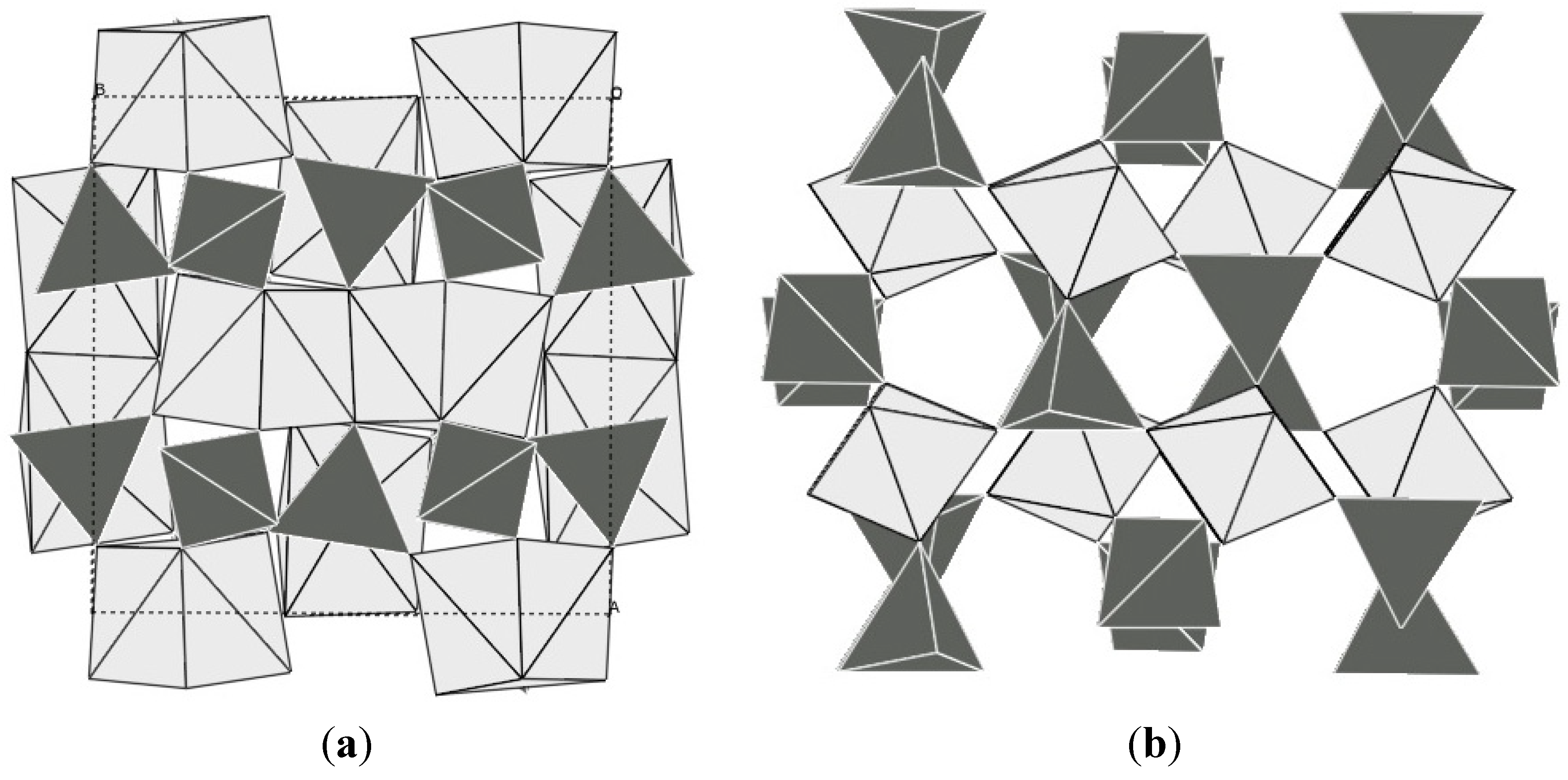
2.3.1. Substitution of Sc2W3O12
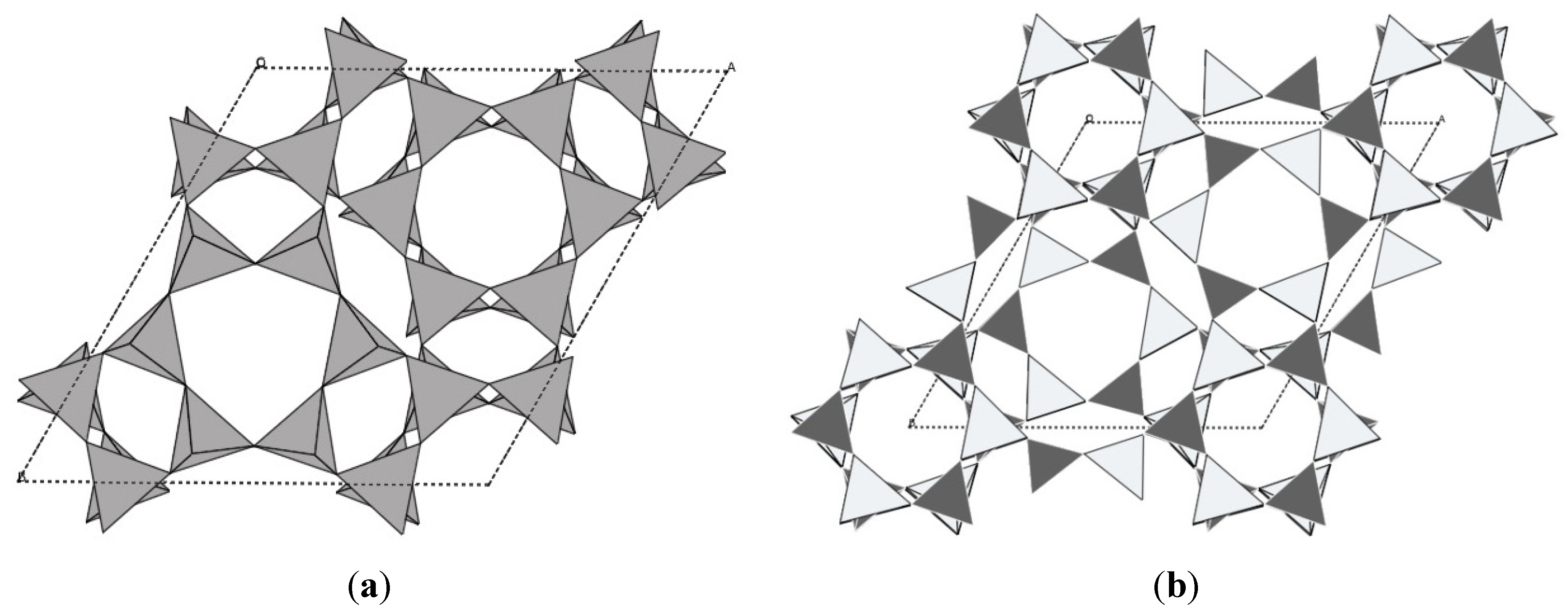
2.3.2. High Pressure Behavior
2.4. Other NTE Oxides
2.5. Metal Cyanide Networks
3. Composites
3.1. Desirable Properties of Filler Materials
3.2. Potential Problems with Known NTE Filler Materials
3.3. Literature Reports on Composites with NTE Fillers
4. Conclusions
Acknowledgments
References and Notes
- Korthuis, V.; Khosrovani, N.; Sleight, A.W.; Roberts, N.; Dupree, R.; Warren, W.W. Negative thermal expansion and phase transitions in the ZrV2−xPxO7 series. Chem. Mater. 1995, 7, 412–417. [Google Scholar] [CrossRef]
- Sleight, A.W. Thermal Contraction. Endeavour 1995, 19, 64–68. [Google Scholar] [CrossRef]
- Mary, T.A.; Evans, J.S.O.; Vogt, T.; Sleight, A.W. Negative thermal expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 1996, 272, 90–92. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Hu, Z.; Jorgensen, J.D.; Argyriou, D.N.; Short, S.; Sleight, A.W. Compressibility, phase transitions, and oxygen migration in zirconium tungstate, ZrW2O8. Science 1997, 275, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Negative thermal expansion in a large molybdate and tungstate family. J.Solid State Chem. 1997, 133, 580–583. [Google Scholar] [CrossRef]
- Ernst, G.; Broholm, C.; Kowach, G.R.; Ramirez, A.P. Phonon density of states and negative thermal expansion in ZrW2O8. Nature 1998, 396, 147–149. [Google Scholar] [CrossRef]
- Perottoni, C.A.; da Jornada, J.A.H. Pressure-induced amorphization and negative thermal expansion in ZrW2O8. Science 1998, 280, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.P.; Kowach, G.R. Large low temperature specific heat in the negative thermal expansion compound ZrW2O8. Phys. Rev. Lett. 1998, 80, 4903–4906. [Google Scholar] [CrossRef]
- Sleight, A.W. Negative thermal expansion materials. Curr. Opin. Solid State Mater. Sci. 1998, 3, 128–131. [Google Scholar] [CrossRef]
- Ravindran, T.R.; Arora, A.K.; Mary, T.A. High pressure behavior of ZrW2O8: Gruneisen parameter and thermal properties. Phys. Rev. Lett. 2000, 84, 3879–3882. [Google Scholar] [CrossRef] [PubMed]
- Reisner, B.A.; Lee, Y.; Hanson, J.C.; Jones, G.A.; Parise, J.B.; Corbin, D.R.; Toby, B.H.; Freitag, A.; Larese, J.Z.; Kahlenberg, V. Understanding negative thermal expansion and ‘trap door’ cation relocations in zeolite rho. Chem. Commun. 2000, 22, 2221–2222. [Google Scholar] [CrossRef]
- Mittal, R.; Chaplot, S.L.; Schober, H.; Mary, T.A. Origin of negative thermal expansion in cubic ZrW2O8 revealed by high pressure inelastic neutron scattering. Phys. Rev. Lett. 2001, 86, 4692–4695. [Google Scholar] [CrossRef] [PubMed]
- Beccara, S.A.; Dalba, G.; Fornasini, P.; Grisenti, R.; Sanson, A. Local thermal expansion in a cuprite structure: The case of Ag2O. Phys. Rev. Lett. 2002, 89, 025503:1–025503:4. [Google Scholar] [CrossRef]
- Birkedal, H.; Schwarzenbach, D.; Pattison, P. Observation of uniaxial negative thermal expansion in an organic crystal. Angew. Chem. 2002, 114, 780–782. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Kepert, C.J. Direct observation of a transverse vibrational mechanism for negative thermal expansion in Zn(CN)2: An atomic pair distribution function analysis. J. Am. Chem. Soc. 2005, 127, 15630–15636. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.P.; Kieffer, J. Structural origin of negative thermal expansion in high-temperature silica polymorphs. Phys. Rev. Lett. 2005, 95, 215901:1–215901:4. [Google Scholar]
- Tucker, M.G.; Goodwin, A.L.; Dove, M.T.; Keen, D.A.; Wells, S.A.; Evans, J.S.O. Negative thermal expansion in ZrW2O8: Mechanisms, rigid unit modes, and neutron total scattering. Phys. Rev. Lett. 2005, 95, 255501:1–255501:4. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Kepert, C.J. Compositional dependence of negative thermal expansion in the Prussian blue analogues (MPtIV)-PtII(CN)6 (M = Mn, Fe, Co, Ni, Cu, Zn, Cd). J. Am. Chem. Soc. 2006, 128, 7009–7014. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.L.; Calleja, M.; Conterio, M.J.; Dove, M.T.; Evans, J.S.O.; Keen, D.A.; Peters, L.; Tucker, M.G. Colossal positive and negative thermal expansion in the framework material Ag3[Co(CN)6]. Science 2008, 319, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.E.; Goodwin, A.L.; Halder, G.J.; Southon, P.D.; Kepert, C.J. Nanoporosity and exceptional negative thermal expansion in single-network cadmium cyanide. Angew. Chem. 2008, 120, 1418–1421. [Google Scholar] [CrossRef]
- Yamamura, Y.; Ikeuchi, S.; Saito, K. Characteristic Phonon spectrum of negative thermal expansion materials with framework structure through calorimetric study of Sc2M3O12 (M = W and Mo). Chem. Mater. 2009, 21, 3008–3016. [Google Scholar] [CrossRef]
- Azuma, M.; Chen, W.T.; Seki, H.; Czapski, M.; Olga, S.; Oka, K.; Mizumaki, M.; Watanuki, T.; Ishimatsu, N.; Kawamura, N.; Ishiwata, S.; Tucker, M.G.; Shimakawa, Y.; Attfield, J.P. Colossal negative thermal expansion in BiNiO3 induced by intermetallic charge transfer. Nature Commun. 2011, 2. [Google Scholar] [CrossRef]
- Mary, T.A.; Sleight, A.W. Bulk thermal expansion for tungstate and molybdates of the type A2M3O12. J. Mater. Res. 1999, 14, 912–915. [Google Scholar] [CrossRef]
- Lightfoot, P.; Woodcock, D.A.; Maple, M.J.; Villaescusa, L.A.; Wright, P.A. The widespread occurrence of negative thermal expansion in zeolites. J. Mater. Chem. 2001, 11, 212–216. [Google Scholar] [CrossRef]
- Negative or Zero Thermal Expansion Materials ; (Special Issue Name); Journal of the Chinese Ceramic Society: Beijing, China, 2009; Volume 37, pp. 651–759.
- Scheel, K. Versuche ueber die ausdehnung fester koerper, insbesondere von quarz in richtung der hauptachse, platin, palladium und quarzglas bei der temperatur der fluessigen Luft. Verh. Deutsch. Phys. Ges. 1907, 9, 3–23. [Google Scholar]
- Scheel, K. Ueber die ausdehnung des quarzglases. Verh. Deutsch. Phys. Ges. 1907, 9, 719–721. [Google Scholar]
- Hummel, F.A. Thermal expansion properties of natural Lithia minerals. Foote Prints 1948, 20, 3–11. [Google Scholar]
- Hummel, F.A. Termal expansion properties of some synthetic lithia minerals. J. Am. Ceram. Soc. 1951, 34, 235–239. [Google Scholar] [CrossRef]
- Roy, R.; Agrawal, D.K.; McKinstry, H.A. Very low thermal expansion coefficient materials. Annu. Rev. Mater. Sci. 1989, 19, 59–81. [Google Scholar] [CrossRef]
- Boilot, J.P.; Salanie, J.P. Phase transformation in Na1+xSixZr2P3−xO12 compounds. Mater. Res. Bull. 1979, 14, 1469–1477. [Google Scholar] [CrossRef]
- Limaye, S.Y.; Agrawal, D.K.; Roy, R.; Mehrotra, Y. Synthesis, sintering and thermal-expansion of Ca1−xSrxZr4P6O24—An ultra-low thermal-expansion ceramic system. J. Mater. Sci. 1991, 26, 93–98. [Google Scholar] [CrossRef]
- Lenain, G.E.; McKinstry, H.A.; Alamo, J.; Agrawal, D.K. Structural model for thermal expansion in MZr2P3O12 (M = Li, Na, K, Rb, Cs). J. Mater. Sci. 1987, 22, 17–22. [Google Scholar] [CrossRef]
- Graham, J.; Wadsley, A.D.; Weymouth, J.H.; Williams, L.S. A new ternary oxide, ZrW2O8. J. Am. Ceram. Soc. 1959, 42, 570. [Google Scholar] [CrossRef]
- Martinek, C.; Hummel, F.A. Linear thermal expansion of three tungstates. J. Am. Ceram. Soc. 1968, 51, 227–228. [Google Scholar] [CrossRef]
- Pryde, A.K.A.; Hammonds, K.D.; Dove, M.T.; Heine, V.; Gale, J.D.; Warren, M.C. Origin of the negative thermal expansion in ZrW2O8 and ZrV2O7. J. Phys. Condens. Matter 1996, 8, 10973–10982. [Google Scholar] [CrossRef]
- Pryde, A.K.A.; Hammonds, K.D.; Dove, M.T.; Heine, V.; Gale, J.D.; Warren, M.C. Rigid unit modes and the negative thermal expansion in ZrW2O8. Phase Transit. 1997, 61, 141–153. [Google Scholar] [CrossRef]
- Stillinger, F.H.; Stillinger, D.K. Negative thermal expansion in the Gaussian core model. Phys. A 1997, 244, 358–369. [Google Scholar] [CrossRef]
- David, W.I.F.; Evans, J.S.O.; Sleight, A.W. Direct evidence for a low-frequency phonon mode mechanism in the negative thermal expansion compound ZrW2O8. Europhys. Lett. 1999, 46, 661–666. [Google Scholar] [CrossRef]
- Heine, V.; Welche, P.R.L.; Dove, M.T. Geometrical origin and theory of negative thermal expansion in framework structures. J. Am. Ceram. Soc. 1999, 82, 1793–1802. [Google Scholar] [CrossRef]
- Ramirez, A.P.; Broholm, C.L.; Cava, R.J.; Kowach, G.R. Geometrical frustration, spin ice and negative thermal expansion—The physics of underconstraint. Phys. B Condens. Matter 2000, 280, 290–295. [Google Scholar] [CrossRef]
- Boerio-Goates, J.; Stevens, R.; Lang, B.; Woodfield, B.F. Heat capacity calorimetry—Detection of low frequency modes in solids and an application to negative thermal expansion materials. J. Therm. Anal. Calorim. 2002, 69, 773–783. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J. Anomalous thermal expansion of cuprites: A combined high resolution pair distribution function and geometric analysis. Chem. Mater. 2009, 21, 425–431. [Google Scholar] [CrossRef]
- Korcok, J.L.; Katz, M.J.; Leznoff, D.B. Impact of metallophilicity on “colossal” positive and negative thermal expansion in a series of isostructural dicyanometallate coordination polymers. J. Am. Chem. Soc. 2009, 131, 4866–4871. [Google Scholar] [CrossRef] [PubMed]
- Sleight, A.W.; Thundathil, M.A.; Evans, J.S.O. Negative Thermal Expansion Materials. U.S. Patent 5,514,360, 7 May 1996. [Google Scholar]
- Sleight, A.W. Compounds that contract on heating. Inorg. Chem. 1998, 37, 2854–2860. [Google Scholar] [CrossRef]
- Sleight, A.W. Isotropic negative thermal expansion. Ann. Rev. Mater. Sci. 1998, 28, 29–43. [Google Scholar] [CrossRef]
- Fleming, D.A.; Johnson, D.W.; Lemaire, P.J. Article Comprising a Temperature Compensated Optical Fiber Refractive Index Grating. U.S. Patent 5,694,503, 2 December 1997. [Google Scholar]
- Verdon, C.; Dunand, D.C. High-temperature reactivity in the ZrW2O8-Cu system. Scr. Mater. 1997, 36, 1075–1080. [Google Scholar] [CrossRef]
- Balch, D.K.; Dunand, D.C. Copper-zirconium tungstate composites exhibiting low and negative thermal expansion influenced by reinforcement phase transformations. Metall. Mater. Trans. A 2004, 35, 1159–1165. [Google Scholar] [CrossRef]
- De Buysser, K.; Lommens, P.; de Meyer, C.; Bruneel, E.; Hoste, S.; van Driessche, I. ZrO2-ZrW2O8 composites with tailor-made thermal expansion. Ceram. Silik. 2004, 48, 139–144. [Google Scholar]
- Lommens, P.; de Meyer, C.; Bruneel, E.; de Buysser, K.; van Driessche, I.; Hoste, S. Synthesis and thermal expansion of ZrO2/ZrW2O8 composites. J. Eur. Ceram. Soc. 2005, 25, 3605–3610. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Vogt, T.; Subramanian, M.A.; Sleight, A.W. Negative thermal expansion in ZrW2O8 and HfW2O8. Chem. Mater. 1996, 8, 2809–2823. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P.; Hu, Z.B.; Short, S.; Jorgensen, J.D. Synthesis and properties of the negative thermal expansion material cubic ZrMo2O8. Chem. Mater. 1998, 10, 2335–2337. [Google Scholar] [CrossRef]
- Attfield, M.P.; Sleight, A.W. Exceptional negative thermal expansion in AlPO4–17. Chem. Mater. 1998, 10, 2013–2019. [Google Scholar] [CrossRef]
- Attfield, M.P.; Sleight, A.W. Strong negative thermal expansion in siliceous faujasite. Chem. Commun. 1998, 601–602. [Google Scholar]
- Goodwin, A.L.; Chapman, K.W.; Kepert, C.J. Guest-dependent negative thermal expansion in nanoporous Prussian Blue analogues MIIPtIV(CN)6 {H2O} (0 ≤ x ≤ 2; M = Zn, Cd). J. Am. Chem. Soc. 2005, 127, 17980–17981. [Google Scholar] [CrossRef] [PubMed]
- Margadonna, S.; Prassides, K.; Fitch, A.N. Zero thermal expansion in a Prussian blue analogue. J. Am. Chem. Soc. 2004, 126, 15390–15391. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.Z.; Sleight, A.W. The role of rigid unit modes in negative thermal expansion. J. Solid State Chem. 2003, 173, 442–448. [Google Scholar] [CrossRef]
- Bieniok, A.; Hammonds, K.D. Rigid unit modes and the phase transition and structural distortions of zeolite rho. Microporous Mesoporous Mat. 1998, 25, 193–200. [Google Scholar] [CrossRef]
- Dove, M.T.; Hammonds, K.D.; Harris, M.J.; Heine, V.; Keen, D.A.; Pryde, A.K.A.; Trachenko, K.; Warren, M.C. Amorphous silica from the Rigid unit mode approach. Miner. Mag. 2000, 64, 377–388. [Google Scholar] [CrossRef]
- Dove, M.T.; Trachenko, K.O.; Tucker, M.G.; Keen, D.A. Rigid unit modes in framework structures: Theory, experiment and applications. Rev. Mineral. Geochem. 2000, 39, 1–33. [Google Scholar] [CrossRef]
- Yamamura, Y.; Nakajima, N.; Tsuji, T.; Iwasa, Y.; Saito, K.; Sorai, M. Heat capacity and Gruneisen functions of negative thermal expansion compound HfW2O8. Solid State Commun. 2002, 121, 213–217. [Google Scholar] [CrossRef]
- Mittal, R.; Chaplot, S.L. Phonon density of states and thermodynamic properties in cubic and orthorhombic phases of ZrW2O8. Solid State Commun. 2000, 115, 319–322. [Google Scholar] [CrossRef]
- Mittal, R.; Chaplot, S.L.; Schober, H.; Kolesnikov, A.I.; Loong, C.K.; Lind, C.; Wilkinson, A.P. Negative thermal expansion in cubic ZrMo2O8: Inelastic neutron scattering and lattice dynamical studies. Phys. Rev. B 2004, 70, 214303:1–214303:6. [Google Scholar] [CrossRef]
- Auray, M.; Quarton, M. Zirconium tungstate. Acta Crystallogr. C 1995, 51, 2210–2213. [Google Scholar] [CrossRef]
- Closmann, C.; Sleight, A.W.; Haygarth, J.C. Low-temperature synthesis of ZrW2O8 and Mo-substituted ZrW2O8. J. Solid State Chem. 1998, 139, 424–426. [Google Scholar] [CrossRef]
- Allen, S.; Evans, J.S.O. Negative thermal expansion and oxygen disorder in cubic ZrMo2O8. Phys. Rev. B 2003, 68, 134101:1–134101:3. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P.; Rawn, C.J.; Payzant, E.A. Preparation of the negative thermal expansion material cubic ZrMo2O8. J. Mater. Chem. 2001, 11, 3354–3359. [Google Scholar] [CrossRef]
- Lind, C.; Wilkinson, A.P.; Rawn, C.J.; Payzant, E.A. Kinetics of the cubic to trigonal transformation in ZrMo2O8 and their dependence on precursor chemistry. J. Mater. Chem. 2002, 12, 990–994. [Google Scholar] [CrossRef]
- Readman, J.E.; Lister, S.E.; Peters, L.; Wright, J.; Evans, J.S.O. Direct synthesis of cubic ZrMo2O8 followed by ultrafast in situ powder diffraction. J. Amer. Chem. Soc. 2009, 131, 17560–17562. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Hanson, P.A.; Ibberson, R.M.; Duan, N.; Kameswari, U.; Sleight, A.W. Low-temperature oxygen migration and negative thermal expansion in ZrW2−xMoxO8. J. Am. Chem. Soc. 2000, 122, 8694–8699. [Google Scholar] [CrossRef]
- Kameswari, U.; Sleight, A.W.; Evans, J.S.O. Rapid synthesis of ZrW2O8 and related phases, and structure refinement of ZrWMoO8. Int. J. Inorg. Mater. 2000, 2, 333–337. [Google Scholar] [CrossRef]
- Allen, S.; Evans, J.S.O. The kinetics of low-temperature oxygen migration in ZrWMoO8. J. Mater. Chem. 2004, 14, 151–156. [Google Scholar] [CrossRef]
- De Meyer, C.; Bouree, F.; Evans, J.S.O.; de Buysser, K.; Bruneel, E.; van Driessche, I.; Hoste, S. Structure and phase transition of Sn-substituted Zr1−xSnxW2O8. J. Mater. Chem. 2004, 14, 2988–2994. [Google Scholar]
- De Buysser, K.; van Driessche, I.; Putte, B.V.; Vanhee, P.; Schaubroeck, J.; Hoste, S. Study of negative thermal expansion and shift in phase transition temperature in Ti4+- and Sn4+-substituted ZrW2O8 materials. Inorg. Chem. 2008, 47, 736–741. [Google Scholar]
- Nakajima, N.; Yamamura, Y.; Tsuji, T. Synthesis and physical properties of negative thermal expansion materials Zr1−xMxW2O8-y (M = Sc, In, Y) substituted for Zr(IV) sites by M(III) ions. Solid State Commun. 2003, 128, 193–196. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kuwahara, J.; Yoshida, T.; Nashimoto, M.; Takahashi, Y.; Takahashi, K.; Morito, Y. Thermal conductivity of negative-thermal-expansion oxide, Zr1−xYxW2O8 (x = 0.00, 0.01): Temperature dependence and effect of structural phase transition. Solid State Commun. 2004, 131, 217–221. [Google Scholar] [CrossRef]
- Tsuji, T.; Yamamura, Y.; Nakajima, N. Thermodynamic properties of negative thermal expansion materials ZrW2O8 substituted for Zr site. Thermochim. Acta 2004, 416, 93–98. [Google Scholar] [CrossRef]
- Yamamura, Y.; Nakajima, N.; Tsuji, T.; Kojima, A.; Kuroiwa, Y.; Sawada, A.; Aoyagi, S.; Kasatani, H. Drastic lowering of the order-disorder phase transition temperatures in Zr1−xMxW2O8−y (M = Sc,Y,In) solidsolutions. Phys. Rev. B 2004, 70, 104107:1–104107:6. [Google Scholar] [CrossRef]
- Yamamura, Y.; Masago, K.; Kato, M.; Tsuji, T. Comprehensive interpretation of a substitution effect on an order-disorder phase transition in A1−xMxW2O8−y (A = Zr, Hf; M = trivalent cations) and other ZrW2O8-based solid solutions. J. Phys. Chem. B 2007, 111, 10118–10122. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tao, J.; Ma, H.; Zhao, X. Disordered structure of ZrW1.8V0.2O7.9 from a combined X-ray and neutron powder diffraction study at 530 K. Acta Crystallogr. C 2009, 65, 74–76. [Google Scholar] [CrossRef]
- Chen, X.; Deng, X.; Ma, H.; Tao, J.; Zhao, X. Hydrothermal synthesis and thermal properties of a novel cubic ZrW1.80V0.20O7.90 solid solution. J. Solid State Chem. 2011, 184, 1090–1095. [Google Scholar] [CrossRef]
- Hu, Z.; Jorgensen, J.D.; Teslic, S.; Short, S.; Argyriou, D.N.; Evans, J.S.O.; Sleight, A.W. Pressure-induced phase transformation in ZrW2O8: Compressibility and thermal expansion of the orthorhombic phase. Physica B 1997, 241, 370–372. [Google Scholar] [CrossRef]
- Jorgensen, J.D.; Hu, Z.; Teslic, S.; Argyriou, D.N.; Short, S.; Evans, J.S.O.; Sleight, A.W. Pressure-induced cubic-to-orthorhombic phase transition in ZrW2O8. Phys. Rev. B 1999, 59, 215–225. [Google Scholar] [CrossRef]
- Grzechnik, A.; Crichton, W.A.; Syassen, K.; Adler, P.; Mezouar, M. A new polymorph of ZrW2O8 synthesized at high pressures and high temperatures. Chem. Mater. 2001, 13, 4255–4259. [Google Scholar] [CrossRef]
- Keen, D.A.; Goodwin, A.L.; Tucker, M.G.; Dove, M.T.; Evans, J.S.O.; Crichton, W.A.; Brunelli, M. Structural description of pressure-induced amorphization in ZrW2O8. Phys. Rev. Lett. 2007, 98, 225501:1–225501:4. [Google Scholar] [CrossRef]
- Chen, B.; Muthu, D.V.S.; Liu, Z.X.; Sleight, A.W.; Kruger, M.B. High-pressure optical study of HfW2O8. J. Phys. Condens. Matter 2002, 14, 13911–13916. [Google Scholar] [CrossRef]
- Jorgensen, J.D.; Hu, Z.; Short, S.; Sleight, A.W.; Evans, J.S.O. Pressure-induced cubic-to-orthorhombic phase transformation in the negative thermal expansion material HfW2O8. J. Appl. Phys. 2001, 89, 3184–3188. [Google Scholar] [CrossRef]
- Lind, C.; VanDerveer, D.G.; Wilkinson, A.P.; Chen, J.H.; Vaughan, M.T.; Weidner, D.J. New high-pressure form of the negative thermal expansion materials zirconium molybdate and hafnium molybdate. Chem. Mater. 2001, 13, 487–490. [Google Scholar] [CrossRef]
- Grzechnik, A.; Crichton, W.A. Structural transformations in cubic ZrMo2O8 at high pressures and high temperatures. Solid State Sci. 2002, 4, 1137–1141. [Google Scholar] [CrossRef]
- Varga, T.; Wilkinson, A.P.; Lind, C.; Bassett, W.A.; Zha, C.S. Pressure-induced amorphization of cubic ZrMo2O8 studied in situ by X-ray absorption spectroscopy and diffraction. Solid State Commun. 2005, 135, 739–744. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Jorgensen, J.D.; Short, S.; David, W.I.F.; Ibberson, R.M.; Sleight, A.W. Thermal expansion in the orthorhombic gamma phase of ZrW2O8. Phys. Rev. B 1999, 60, 14643–14648. [Google Scholar] [CrossRef]
- Varga, T.; Wilkinson, A.P.; Jupe, A.C.; Lind, C.; Bassett, W.A.; Zha, C.S. Pressure-induced amorphization of cubic ZrW2O8 studied in situ and ex situ by synchrotron X-ray diffraction and absorption. Phys. Rev. B 2005, 72, 024117:1–024117:10. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Hanson, J.C.; Sleight, A.W. Room-temperature superstructure of ZrV2O7. Acta Crystallogr. B 1998, 54, 705–713. [Google Scholar] [CrossRef]
- Khosrovani, K.; Sleight, A.W.; Vogt, T. Structure of ZrV2O7 from −263 to 470 degrees C. J. Solid State Chem. 1997, 132, 355–360. [Google Scholar] [CrossRef]
- Withers, R.L.; Evans, J.S.O.; Hanson, J.C.; Sleight, A.W. An in-situ tempertaure dependent electron and X-ray diffraction study of the structural phase transitions in ZrV2O7. J. Solid State Chem. 1998, 137, 161–167. [Google Scholar] [CrossRef]
- Onken, H. Ueber zirkonpyroarsenat. Naturwissenschaften 1965, 52, 344. [Google Scholar] [CrossRef]
- Le Flem, G.; Lamic, J.; Hagenmuller, P. As2O5-ThO2 system. Bull. Soc. Chim. Fr. 1966, 6, 1880–1883. [Google Scholar]
- Losilla, E.R.; Cabeza, A.; Bruque, S.; Aranda, M.A.G.; Sanz, J.; Iglesias, J.E.; Alonso, J.A. Syntheses, structures, and thermal expansion of germanium pyrophosphates. J. Solid State Chem. 2001, 156, 213–219. [Google Scholar] [CrossRef]
- Fayon, F.; King, I.J.; Harris, R.K.; Gover, R.K.B.; Evans, J.S.O.; Massiot, D. Characterization of the room-temperature structure of SnP2O7 by P31 through-space and through-bond NMR correlation spectroscopy. Chem. Mater. 2003, 15, 2234–2239. [Google Scholar] [CrossRef]
- Gover, R.K.B.; Withers, N.D.; Allen, S.; Withers, R.L.; Evans, J.S.O. Structure and phase transitions of SnP2O7. J. Solid State Chem. 2002, 166, 42–48. [Google Scholar] [CrossRef]
- White, K.M.; Lee, P.L.; Chupas, P.J.; Chapman, K.W.; Payzant, E.A.; Jupe, A.C.; Bassett, W.A.; Zha, C.S.; Wilkinson, A.P. Synthesis, symmetry, and physical properties of cerium pyrophosphate. Chem. Mater. 2008, 20, 3728–3734. [Google Scholar] [CrossRef]
- Wallez, G.; Raison, P.E.; Dacheux, N.; Clavier, N.; Bykov, D.; Delevoye, L.; Popa, K.; Bregiroux, D.; Fitch, A.N.; Konings, R.J.M. Triclinic-cubic phase transition and negative expansion in the actinide IV (Th, U, Np, Pu) diphosphates. Inorg. Chem. 2012, 51, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Stinton, G.W.; Hampson, M.R.; Evans, J.S.O. The 136-atom structure of ZrP2O7 and HfP2O7 from powder diffraction data. Inorg. Chem. 2006, 45, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Birkedal, H.; Andersen, A.M.K.; Arakcheeva, A.; Chapuis, G.; Norby, P.; Pattison, P. The room-temperature superstructure of ZrP2O7 is orthorhombic: There are no unusual 180° P-O-P bond angles. Inorg. Chem. 2006, 45, 4346–4351. [Google Scholar] [CrossRef] [PubMed]
- Oyetola, S.; Verbaere, A.; Guyomard, D.; Crosnier, M.P.; Piffard, Y.; Tournoux, M. New ZrP2O7-like diphosphates of either mixed (M1/2(III)M’1/2(V)) cations (M = Sb, Bi, Nd, Eu-M’ = Sb, Nb, Ta) or M’V cations (M’ = Ta, Nb)—Synthesis and structure. Eur. J. Solid State Inorg. Chem. 1991, 28, 23–36. [Google Scholar]
- Varga, T.; Wilkinson, A.P.; Haluska, M.S.; Payzant, E.A. Preparation and thermal expansion of (MIII0.5M’V0.5)P2O7 with the cubic ZrP2O7 structure. J. Solid State Chem. 2005, 178, 3541–3546. [Google Scholar] [CrossRef]
- Yanase, I.; Kojima, T.; Kobayashi, H. Effects of Nb and Y substitution on negative thermal expansion of ZrV2−xPxO7 (0 ≤ X ≤ 0.8). Solid State Commun. 2011, 151, 595–598. [Google Scholar] [CrossRef]
- Sahoo, P.P.; Sumithra, S.; Madras, G.; Row, T.N.G. Synthesis, structure, negative thermal expansion, and photocatalytic property of Mo doped ZrV2O7. Inorg. Chem. 2011, 50, 8774–8781. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.; Andersen, A.M.K. High-pressure properties of TiP2O7, ZrP2O7 and ZrV2O7. J. Appl. Crystallogr. 2001, 34, 7–12. [Google Scholar] [CrossRef]
- Hemamala, U.L.C.; El-Ghussein, F.; Muthu, D.V.S.; Andersen, A.M.K.; Carlson, S.; Ouyang, L.; Kruger, M.B. High-pressure Raman and infrared study of ZrV2O7. Solid State Commun. 2007, 141, 680–684. [Google Scholar] [CrossRef]
- Lipinska-Kalita, K.E.; Kruger, M.B.; Carlson, S.; Andersen, A.M.K. High-pressure studies of titanium pyrophosphate by Raman scattering and infrared spectroscopy. Physica B 2003, 337, 221–229. [Google Scholar] [CrossRef]
- Petruska, E.A.; Muthu, D.V.S.; Carlson, S.; Andersen, A.M.K.; Ouyang, L.; Kruger, M.B. High-pressure Raman and infrared spectroscopic studies of ZrP2O7. Solid State Commun. 2010, 150, 235–239. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Negative thermal expansion in Sc2(WO4)3. J. Solid State Chem. 1998, 137, 148–160. [Google Scholar] [CrossRef]
- Zhou, Y.; Adams, S.; Rao, R.P.; Edwards, D.D.; Neiman, A.; Pestereva, N. Charge transport by polyatomic anion diffusion in Sc2(WO4)3. Chem. Mater. 2008, 20, 6335–6345. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A. Structural phase transitions and negative thermal expansion in Sc2(MoO4)3. Int. J. Inorg. Mater. 2000, 2, 143–151. [Google Scholar] [CrossRef]
- Wu, M.M.; Cheng, Y.Z.; Peng, J.; Xiao, X.L.; Chen, D.F.; Kiyanagi, R.; Fieramosca, J.S.; Short, S.; Jorgensen, J.; Hu, Z.B. Synthesis of solid solution Er2−xCexW3O12 and studies of their thermal expansion behavior. Mater. Res. Bull. 2007, 42, 2090–2098. [Google Scholar] [CrossRef]
- Wu, M.M.; Peng, J.; Cheng, Y.Z.; Xiao, X.L.; Hao, Y.M.; Hu, Z.B. Thermal expansion in solid solution Er2−xSmxW3O12. Mater. Sci. Eng. B 2007, 137, 144–148. [Google Scholar] [CrossRef]
- Wu, M.M.; Peng, J.; Cheng, Y.Z.; Wang, H.; Yu, Z.X.; Chen, D.F.; Hu, Z.B. Structure and thermal expansion properties of solid solution Nd2−xErxW3O12 (0.0 ≤ x ≤ 0.6 and 1.5 ≤ x ≤ 2.0). Solid State Sci. 2006, 8, 665–670. [Google Scholar] [CrossRef]
- Xiao, X.L.; Peng, J.; Wu, M.M.; Cheng, Y.Z.; Chen, D.F.; Hu, Z.B. The crystal structure and thermal expansion properties of solid solutions Ln2−xDyxW3O12 (Ln = Er and Y). J. Alloy. Compd. 2008, 465, 556–561. [Google Scholar] [CrossRef]
- Peng, J.; Wu, M.M.; Wang, H.; Hao, Y.M.; Hu, Z.; Yu, Z.X.; Chen, D.F.; Kiyanagic, R.; Fieramosca, J.S.; Short, S.; Jorgensen, J. Structures and negative thermal expansion properties of solid solutions YxNd2−xW3O12 (x = 0.0–1.0, 1.6–2.0). J. Alloy. Compd. 2008, 453, 49–54. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Structure of Zr2(WO4)(PO4)2 from powder X-ray data: Cation ordering with no superstructure. J. Solid State Chem. 1995, 120, 101–104. [Google Scholar] [CrossRef]
- Cetinkol, M.; Wilkinson, A.P.; Lee, P.L. Structural changes accompanying negative thermal expansion in Zr2(MoO4)(PO4)2. J. Solid State Chem. 2009, 182, 1304–1311. [Google Scholar] [CrossRef]
- Suzuki, T.; Omote, A. Negative thermal expansion in (HfMg)(WO4)3. J. Am. Ceram. Soc. 2004, 87, 1365–1367. [Google Scholar] [CrossRef]
- Gindhart, A.M.; Lind, C.; Green, M. Polymorphism in the negative thermal expansion material magnesium hafnium tungstate. J. Mater. Res. 2008, 23, 210–213. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Jardim, P.M.; Ari, M.; de Avillez, R.R.; Rizzo, F.; Ferreira, F.F. Low positive thermal expansion in HfMgMo3O12. Phys. Status Solidi B 2008, 245, 2514–2519. [Google Scholar] [CrossRef]
- Sleight, A.W.; Brixner, L.H. A New Ferroelastic transition in some A2(MO4)3 molybdates and tungstates. J. Solid State Chem. 1973, 7, 172–174. [Google Scholar] [CrossRef]
- Forster, P.M.; Sleight, A.W. Negative thermal expansion in Y2W3O12. Int. J. Inorg. Mater. 1999, 1, 123–127. [Google Scholar] [CrossRef]
- Suzuki, T.; Omote, A. Zero thermal expansion in (Al2x(HfMg)1−x)(WO4)3. J. Am. Ceram. Soc. 2006, 89, 691–693. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Jardim, P.M.; de Avillez, R.R.; Rizzo, F. Negative thermal expansion in Y2Mo3O12. Solid State Sci. 2005, 7, 1377–1383. [Google Scholar] [CrossRef]
- Sumithra, S.; Tyagi, A.K.; Umarji, A.M. Negative thermal expansion in Er2W3O12 and Yb2W3O12 by high temperature X-ray diffraction. Mater. Sci. Eng. B 2005, 116, 14–18. [Google Scholar] [CrossRef]
- Gates, S.D.; Lind, C. Polymorphism in yttrium molybdate Y2Mo3O12. J. Solid State Chem. 2007, 180, 3510–3514. [Google Scholar] [CrossRef]
- Varga, T.; Wilkinson, A.P.; Jorgensen, J.D.; Short, S. Neutron powder diffraction study of the orthorhombic to monoclinic transition in Sc2W3O12 on compression. Solid State Sci. 2006, 8, 289–295. [Google Scholar] [CrossRef]
- Varga, T.; Wilkinson, A.P.; Lind, C.; Bassett, W.A.; Zha, C.S. High pressure synchrotron X-ray powder diffraction study of Sc2Mo3O12 and Al2W3O12. J. Phys. Condens. Matter 2005, 17, 4271–4283. [Google Scholar] [CrossRef]
- Varga, T.; Wilkinson, A.P.; Lind, C.; Bassett, W.A.; Zha, C.S. In situ high-pressure synchrotron X-ray diffraction study of Sc2W3O12 at up to 10 GPa. Phys. Rev. B 2005, 71, 214106:1–214106:8. [Google Scholar]
- Garg, N.; Murli, C.; Tyagi, A.K.; Sharma, S.M. Phase transitions in Sc2(WO4)3 under high pressure. Phys. Rev. B 2005, 72, 064106:1–064106:7. [Google Scholar]
- Garg, N.; Panchal, V.; Tyagi, A.K.; Sharma, S.M. Pressure-induced phase transitions in Al2(WO4)3. J. Solid State Chem. 2005, 178, 998–1002. [Google Scholar] [CrossRef]
- Achary, S.N.; Mukherjee, G.D.; Tyagi, A.K.; Vaidya, S.N. Preparation, thermal expansion, high pressure and high temperature behavior of Al2(WO4)3. J. Mater. Sci. 2002, 37, 2501–2509. [Google Scholar] [CrossRef]
- Arora, A.K.; Nithya, R.; Yagi, T.; Miyajima, N.; Mary, T.A. Two-stage amorphization of scandium molybdate at high pressure. Solid State Commun. 2004, 129, 9–13. [Google Scholar] [CrossRef]
- Baiz, T.I.; Heinrich, C.P.; Banek, N.A.; Vivekens, B.L.; Lind, C. In-situ non-ambient X-ray diffraction studies of indium tungstate. J. Solid State Chem. 2012, 187, 195–199. [Google Scholar] [CrossRef]
- Liu, H.; Secco, R.A.; Imanaka, N.; Rutter, M.D.; Adachi, G.; Uchida, T. Ionic to electronic dominant conductivity in Al2(WO4)3 at high pressure and high temperature. J. Phys. Chem. Solids 2003, 64, 287–294. [Google Scholar] [CrossRef]
- Mukherjee, G.D.; Vijaykumar, V.; Achary, S.N.; Tyagi, A.K.; Godwal, B.K. Phase transitions in Al2(WO4)3: High pressure investigations of low frequency dielectric constant and crystal structure. J. Phys. Condens. Matter 2004, 16, 7321–7330. [Google Scholar] [CrossRef]
- Secco, R.A.; Liu, H.; Imanaka, N.; Adachi, G.; Rutter, M.D. Electrical conductivity and amorphization Of Sc2(WO4)3 at high pressures and temperatures. J. Phys. Chem. Solids 2002, 63, 425–431. [Google Scholar] [CrossRef]
- Cetinkol, M.; Wilkinson, A.P.; Lind, C. In situ high-pressure synchrotron X-ray diffraction study of Zr2(WO4)(PO4)2 up to 16 GPa. Phys. Rev. B 2009, 79, 224118:1–224118:10. [Google Scholar] [CrossRef]
- Gates, S.D.; Colin, J.A.; Lind, C. Non-hydrolytic sol-gel synthesis, properties, and high-pressure behavior of gallium molybdate. J. Mater. Chem. 2006, 16, 4214–4219. [Google Scholar] [CrossRef]
- Karmakar, S.; Deb, S.K.; Tyagi, A.K.; Sharma, S.M. Pressure-induced amorphization in Y2(WO4)3: In situ X-ray diffraction and Raman studies. J. Solid State Chem. 2004, 177, 4087–4092. [Google Scholar] [CrossRef]
- Miller, W.; Smith, C.W.; Mackenzie, D.S.; Evans, K.E. Negative thermal expansion: A review. J. Mater. Sci. 2009, 44, 5441–5451. [Google Scholar] [CrossRef]
- Woodcock, D.A.; Lightfoot, P.; Villaescusa, L.A.; Diaz-Cabanas, M.J.; Camblor, M.A.; Engberg, D. Negative thermal expansion in the siliceous zeolites chabazite and ITQ-4: A neutron powder diffraction study. Chem. Mater. 1999, 11, 2508–2514. [Google Scholar] [CrossRef]
- Taylor, D. Thermal expansion data V. Miscellaneous binary oxides. Trans. J. Br. Ceram. Soc. 1985, 84, 9–14. [Google Scholar]
- Sanson, A.; Rocca, F.; Dalba, G.; Fornasini, P.; Grisenti, R.; Dapiaggi, M.; Artioli, G. Negative thermal expansion and local dynamics in Cu2O and Ag2O. Phys. Rev. B 2006, 73, 214305:1–214305:13. [Google Scholar] [CrossRef]
- Li, J.; Yokochi, A.; Amos, T.G.; Sleight, A.W. Strong negative thermal expansion along the O-Cu-O linkage in CuScO2. Chem. Mater. 2002, 14, 2602–2606. [Google Scholar] [CrossRef]
- Li, J.; Sleight, A.W.; Jones, C.Y.; Toby, B.H. Trends in negative thermal expansion behavior for AMO2 (A = Cu or Ag; M = Al, Sc, In, or La) compounds with the delafossite structure. J. Solid State Chem. 2005, 178, 285–294. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Dalba, G.; Fornasini, P.; Vaccari, M.; Rocca, F.; Sanson, A.; Li, J.; Sleight, A.W. Negative thermal expansion in crystals with the delafossite structure: An extended X-ray absorption fine structure study of CuScO2 and CuLaO2. Phys. Rev. B 2009, 79, 104302:1–104302:8. [Google Scholar]
- Barreteau, C.; Bregiroux, D.; Laurent, G.; Wallez, G. Reducing ultra-low thermal expansion of beta-Zr2O(PO4)2 by substitutions? Mater. Res. Bull. 2010, 45, 1996–2000. [Google Scholar] [CrossRef]
- Clavier, N.; Wallez, G.; Dacheux, N.; Bregiroux, D.; Quarton, M.; Beaunier, P. Synthesis, Raman and Rietveld analysis of thorium diphosphate. J. Solid State Chem. 2008, 181, 3352–3356. [Google Scholar] [CrossRef]
- Wallez, G.; Bregiroux, D.; Quarton, M. Mechanism of the low thermal expansion in α-Hf2O(PO4)2 and its zirconium analog. J. Solid State Chem. 2008, 181, 1413–1418. [Google Scholar] [CrossRef]
- Wallez, G.; Clavier, N.; Dacheux, N.; Bregiroux, D. Negative thermal expansion in Th2O(PO4)2. Mater. Res. Bull. 2011, 46, 1777–1780. [Google Scholar] [CrossRef]
- Wallez, G.; Launay, S.; Quarton, M.; Dacheux, N.; Soubeyroux, J.L. Why does uranium oxide phosphate contract on heating? J. Solid State Chem. 2004, 177, 3575–3580. [Google Scholar] [CrossRef]
- Dapiaggi, M.; Fitch, A.N. Negative (and very low) thermal expansion in ReO3 from 5 to 300 K. J. Appl. Crystallogr. 2009, 42, 253–258. [Google Scholar] [CrossRef]
- Amos, T.G.; Sleight, A.W. Negative thermal expansion in orthorhombic NbOPO4. J. Solid State Chem. 2001, 160, 230–238. [Google Scholar] [CrossRef]
- Amos, T.G.; Yokochi, A.; Sleight, A.W. Phase transition and negative thermal expansion in tetragonal NbOPO4. J. Solid State Chem. 1998, 141, 303–307. [Google Scholar] [CrossRef]
- Mukherjee, G.D.; Vijaykumar, V.; Karandikar, A.S.; Godwal, B.K.; Achary, S.N.; Tyagi, A.K.; Lausi, A.; Busetto, E. Compressibility anomaly and amorphization in the anisotropic negative thermal expansion material NbOPO4 under pressure. J. Solid State Chem. 2005, 178, 8–14. [Google Scholar] [CrossRef]
- Wang, J.; Deng, J.; Yu, R.; Chen, J.; Xing, X. Coprecipitation synthesis and negative thermal expansion of NbVO5. Dalton Trans. 2011, 40, 3394–3397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Q.; Deng, J.; Yu, R.; Chen, J.; Xing, X. Phase transformation and negative thermal expansion in TaVO5. Inorg. Chem. 2011, 50, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Greve, B.K.; Martin, K.L.; Lee, P.L.; Chupas, P.J.; Chapman, K.W.; Wilkinson, A.P. Pronounced negative thermal expansion from a simple structure: Cubic ScF3. J. Am. Chem. Soc. 2010, 132, 15496–15498. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Partin, D.E.; Lincoln, F.J.; Kouvetakis, J.; O’Keeffe, M. The disordered crystal structures of Zn(CN)2 and Ga(CN)3. J. Solid State Chem. 1997, 134, 164–169. [Google Scholar] [CrossRef]
- Goodwin, A.L.; Kepert, C.J. Negative thermal expansion and low-frequency modes in cyanide-bridged framework materials. Phys. Rev. B 2005, 71, 140301:1–140301:4. [Google Scholar] [CrossRef]
- Adak, S.; Daemen, L.L.; Hartl, M.; Williams, D.; Summerhill, J.; Nakotte, H. Thermal expansion in 3d-metal Prussian blue analogs—A survey study. J. Solid State Chem. 2011, 184, 2854–2861. [Google Scholar] [CrossRef]
- Goodwin, A.L.; Kennedy, B.J.; Kepert, C.J. Thermal expansion matching via framework flexibility in Zinc dicyanometallates. J. Am. Chem. Soc. 2009, 131, 6334–6335. [Google Scholar] [CrossRef] [PubMed]
- Conterio, M.J.; Goodwin, A.L.; Tucker, M.G.; Keen, D.A.; Dove, M.T.; Peters, L.; Evans, J.S.O. Local structure in Ag3[Co(CN)6]: Colossal thermal expansion, rigid unit modes and argentophilic interactions. J. Phys. Condens. Matter 2008, 20. [Google Scholar] [CrossRef]
- Goodwin, A.L.; Keen, D.A.; Tucker, M.G.; Dove, M.T.; Peters, L.; Evans, J.S.O. Argentophilicity-dependent colossal thermal expansion in extended Prussian blue analogues. J. Am. Chem. Soc. 2008, 130, 9660–9661. [Google Scholar] [CrossRef] [PubMed]
- Kozy, L.C.; Tahir, M.N.; Lind, C.; Tremel, W. Particle size and morphology control of the negative thermal expansion material cubic zirconium tungstate. J. Mater. Chem. 2009, 19, 2760–2765. [Google Scholar] [CrossRef]
- Nikolov, V.; Koseva, I.; Stoyanova, R.; Zhecheva, E. Conditions for preparation of nanosized Al2(WO4)3. J. Alloy. Compd. 2010, 505, 443–449. [Google Scholar] [CrossRef]
- Duan, N.; Kameswari, U.; Sleight, A.W. Further contraction of ZrW2O8. J. Am. Chem. Soc. 1999, 121, 10432–10433. [Google Scholar] [CrossRef]
- Lind, C. Negative Thermal Expansion Materials Related to Cubic Zirconium Tungstate. Ph.D. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2001. [Google Scholar]
- Banek, N.A.; Baiz, H.I.; Latigo, A.; Lind, C. Autohydration of nanosized cubic zirconium tungstate. J. Amer. Chem. Soc. 2010, 132, 8278–8279. [Google Scholar] [CrossRef]
- Sullivan, L.M.; Lukehart, C.M. Zirconium tungstate (ZrW2O8)/polyimide nanocomposites exhibiting reduced coefficient of thermal expansion. Chem. Mater. 2005, 17, 2136–2141. [Google Scholar] [CrossRef]
- Sharma, G.R.; Coleman, M.R.; Lind, C. Polyimide nanocomposites for tunable coefficient of thermal expansion. In Proceedings of the 40th International SAMPE Technical Conference, Memphis, TN, USA, 8–11 September 2008.
- Lind, C.; Coleman, M.R.; Kozy, L.C.; Sharma, G.R. Zirconium tungstate/polymer nanocomposites: Challenges and opportunities. Phys. Status Solidi B 2011, 248, 123–129. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Lee, Y.M. Improvement in thermal stability of NaA zeolite composite membrane by control of intermediate layer structure. J. Membr. Sci. 2011, 366, 229–236. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Yeo, J.G.; Kim, S.K.; Lee, Y.M. Synthesis, ethanol dehydration and thermal stability of NaA zeolite/alumina composite membranes with narrow non-zeolitic pores and thin intermediate layer. J. Membr. Sci. 2010, 364, 138–148. [Google Scholar] [CrossRef]
- Akhtar, F.; Ojuva, A.; Wirawan, S.K.; Hedlund, J.; Bergstrom, L. Hierarchically porous binder-free silicalite-1 discs: A novel support for all-zeolite membranes. J. Mater. Chem. 2011, 21, 8822–8828. [Google Scholar] [CrossRef]
- Tran, K.D.; Groshens, T.J.; Nelson, J.G. Fabrication of near-zero thermal expansion (FexSc1−x)2Mo3O12-MoO3 ceramic composite using the reaction sintering process. Mater. Sci. Eng. A 2001, 303, 234–240. [Google Scholar] [CrossRef]
- Watanabe, H.; Tani, J.; Kido, H.; Mizuuchi, K. Thermal expansion and mechanical properties of pure magnesium containing zirconium tungsten phosphate particles with negative thermal expansion. Mater. Sci. Eng. A 2008, 494, 291–298. [Google Scholar] [CrossRef]
- Holzer, H.; Dunand, D.C. Phase transformation and thermal expansion of Cu/ZrW2O8 metal matrix composites. J. Mater. Res. 1999, 14, 780–789. [Google Scholar] [CrossRef]
- Yilmaz, S.; Dunand, D.C. Finite-element analysis of thermal expansion and thermal mismatch stresses in a Cu-60vol%ZrW2O8 composite. Compos. Sci. Technol. 2004, 64, 1895–1898. [Google Scholar] [CrossRef]
- Yilmaz, S. Phase transformations in thermally cycled Cu/ZrW2O8 composites investigated by synchrotron X-ray diffraction. J. Phys. Condens. Matter 2002, 14, 365–375. [Google Scholar] [CrossRef]
- Yilmaz, S. Thermal mismatch stress development in Cu-ZrW2O8 composite investigated by synchrotron X-ray diffraction. Compos. Sci. Technol. 2002, 62, 1835–1839. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, X.; Xu, G.; Wang, C.; Sun, S.; Riedel, R. Preparation and thermal properties of zirconium tungstate/copper composites. Mater. Werkst. 2008, 39, 649–653. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.F.; Zhang, Y.H.; Zhang, J.L.; Lu, F.S.; Wang, X.L. Synthesis and thermal expansion of 4J36/ZrW2O8 composites. Rare Met. 2010, 29, 371–375. [Google Scholar] [CrossRef]
- Kanamori, K.; Kineri, T.; Fukuda, R.; Kawano, T.; Nishio, K. Low-temperature sintering of ZrW2O8-SiO2 by spark plasma sintering. J. Mater. Sci. 2009, 44, 855–860. [Google Scholar] [CrossRef]
- Kofteros, M.; Rodriguez, S.; Tandon, V.; Murr, L.E. A preliminary study of thermal expansion compensation in cement by ZrW2O8 additions. Scr. Mater. 2001, 45, 369–374. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Yan, X.; Yang, J.; Fu, T.; Qiu, J. Synthesis of ZrO2/ZrW2O8 composites with low thermal expansion. Compos. Sci. Technol. 2007, 67, 1167–1171. [Google Scholar] [CrossRef]
- Yang, X.; Xu, J.; Li, H.; Cheng, X.; Yan, X. In situ synthesis of ZrO2/ZrW2O8 composites with near-zero thermal expansion. J. Am. Ceram. Soc. 2007, 90, 1953–1955. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Liu, H.F.; Cheng, X.N. Study on the technology of ZrO2-ZrW2O8 composites prepared by co-precipitation method. J. Inorg. Mater. 2008, 23, 991–995. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Li, H.; Xu, J.; Sun, X. Thermal and electric conductivity of near-zero thermal expansion ZrW2O8/ZrO2 composites. J. Ceram. Soc. Jpn. 2008, 116, 471–474. [Google Scholar] [CrossRef]
- Sun, L.; Kwon, P. ZrW2O8/ZrO2 composites by in situ synthesis of ZrO2 + WO3: Processing, coefficient of thermal expansion, and theoretical model prediction. Mater. Sci. Eng. A 2009, 527, 93–97. [Google Scholar] [CrossRef]
- Sun, L.; Kwon, P. ZrW2O8-ZrO2 continuous functionally graded materials fabricated by in situ reaction of ZrO2 and WO3. J. Am. Ceram. Soc. 2010, 93, 703–708. [Google Scholar] [CrossRef]
- Sun, L.; Sneller, A.; Kwon, P. ZrW2O8-containing composites with near-zero coefficient of thermal expansion fabricated by various methods: Comparison and optimization. Compos. Sci. Technol. 2008, 68, 3425–3430. [Google Scholar] [CrossRef]
- Tani, J.-I.; Takahashi, M.; Kido, H. Fabrication and thermal expansion properties of ZrW2O8/Zr2WP2O12 composites. J. Eur. Ceram. Soc. 2010, 30, 1483–1488. [Google Scholar] [CrossRef]
- Isobe, T.; Kato, Y.; Mizutani, M.; Ota, T.; Daimon, K. Pressureless sintering of negative thermal expansion ZrW2O8/Zr2WP2O12 composites. Mater. Lett. 2008, 62, 3913–3915. [Google Scholar] [CrossRef]
- Isobe, T.; Umezome, T.; Kameshima, Y.; Nakajima, A.; Okada, K. Preparation and properties of negative thermal expansion Zr2WP2O12 ceramics. Mater. Res. Bull. 2009, 44, 2045–2049. [Google Scholar] [CrossRef]
- Tani, J.-I.; Kimura, H.; Hirota, K.; Kido, H. Thermal expansion and mechanical properties of phenolic resin/ZrW2O8 composites. J. Appl. Polym. Sci. 2007, 106, 3343–3347. [Google Scholar] [CrossRef]
- Chu, X.; Huang, R.; Yang, H.; Wu, Z.; Lu, J.; Zhou, Y.; Li, L. The cryogenic thermal expansion and mechanical properties of plasma modified ZrW(2)O(8) reinforced epoxy. Mater. Sci. Eng. A 2011, 528, 3367–3374. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lind, C. Two Decades of Negative Thermal Expansion Research: Where Do We Stand? Materials 2012, 5, 1125-1154. https://doi.org/10.3390/ma5061125
Lind C. Two Decades of Negative Thermal Expansion Research: Where Do We Stand? Materials. 2012; 5(6):1125-1154. https://doi.org/10.3390/ma5061125
Chicago/Turabian StyleLind, Cora. 2012. "Two Decades of Negative Thermal Expansion Research: Where Do We Stand?" Materials 5, no. 6: 1125-1154. https://doi.org/10.3390/ma5061125