Optical Properties of Mg, Fe, Co-Doped Near-Stoichiometric LiTaO3 Single Crystals
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. As Grown Crystals

| Crystal no. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Growth rate (mm/h) | 0.6 | 0.4 | 0.4 | 0.2 | 0.2 |
| Rotating rate (rpm) | 6 | 12 | 9 | 9 | 9 |
| Crystal size (mm2) | Φ25 × 75 | Φ40 × 60 | Φ40 × 70 | Φ30 × 50 | Φ30 × 50 |
| Li/Ta in solution (-) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Mg/Ta in solution (mol%) | 0 | 1.2 | 1.2 | 1.2 | 1.2 |
| Fe/Ta in solution (ppm wt) | 0 | 0 | 200 | 1000 | 2000 |
| Li/Ta in feed (-) | 1.041 | 1.041 | 1.041 | 1.041 | 1.041 |
3.2. ICP-AES Measurements
| Wafer no. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Li/Ta (-) | 0.995 | 0.976 | 0.978 | 0.981 | 0.980 |
| Mg/Ta (mol%) | 0 | 1.225 | 0.926 | 0.774 | 0.704 |
| Fe/Ta (ppm wt) | 0 | 0 | 120 | 352 | 650 |
3.3. UV-Visible-NIR Spectrum


3.4. OH− Absorption Spectrum

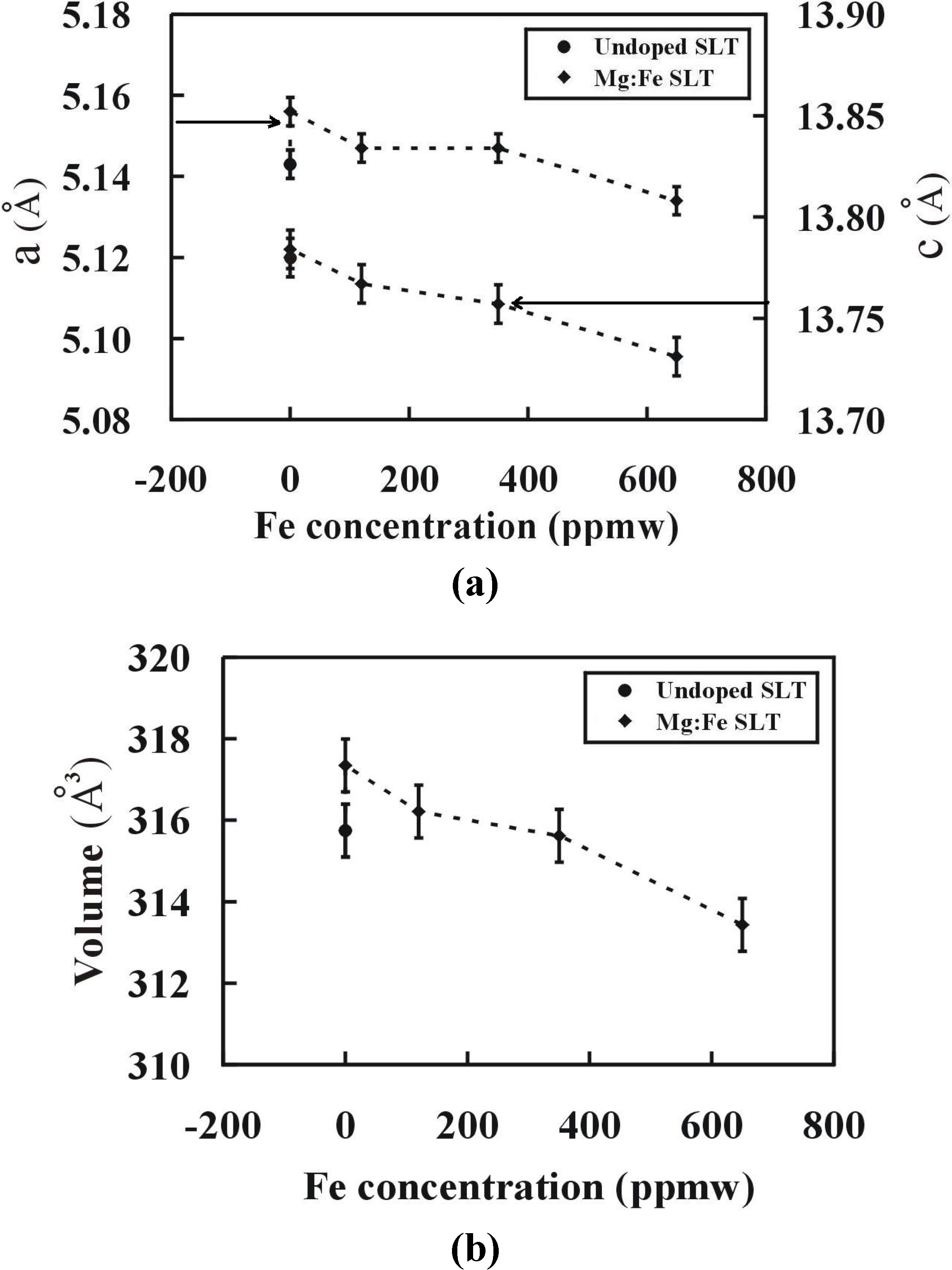
3.5. X-ray Diffraction (XRD) Measurements
3.6. Holographic Properties of Mg, Fe Co-Doped Near SLT Crystals
| At Intensity 1 W/cm2 | ||
|---|---|---|
| Holographic Parameters | Wafer-3 (200 ppm Wt) | Wafer-4 (1000 ppm Wt) |
| ηmax (%) | 14.3 | 33.4 |
| Δn (10−5) | 3.17 | 5.03 |
| τr (S) | 31.3 | 34.5 |
| τe (S) | 95.1 | 267.9 |
| M (#) | 0.9 | 3.4 |
| S (10−2 cm/J) | 2.4 | 3.2 |
4. Conclusions
References
- Holm, A.; Sturzer, Q.; Xu, Y.; Weigel, R. Investigation of surface acoustic waves on LiNbO3, quartz, and LiTaO3 by laser probing. Microelectron. Eng. 1996, 31, 123–127. [Google Scholar] [CrossRef]
- Wang, K.M.; Chen, F.; Hu, H.; Zhang, J.H.; Lu, F.; Shi, B.R.; Lu, Q.M.; Ma, C.Q. Waveguide formation in LiTaO3 and LiB3O5 by keV hydrogen ion implantation. Opt. Commun. 2001, 196, 215–219. [Google Scholar] [CrossRef]
- Kukhtarev, N.V.; Markov, V.B.; Odulov, S.G.; Soskin, M.S.; Vinetskii, V.L. Holographic storage in electrooptic crystals. i. steady state. Ferroelectrics 1978, 22, 949–960. [Google Scholar] [CrossRef]
- Fang, S.; Ma, D.; Zhang, T.; Ling, F.; Wang, B. Growth and optical properties of Mg, Fe Co-doped LiTaO3 crystal. Optik 2006, 117, 72–76. [Google Scholar] [CrossRef]
- Fang, S.; Wang, B.; Zhang, T.; Ling, F.; Wang, R. Growth and photorefractive properties of Zn, Fe double-doped LiTaO3 crystal. Opt. Mater. 2006, 28, 207–211. [Google Scholar] [CrossRef]
- Miyazawa, S.; Iwasaki, H. Congruent melting composition of lithium metatantalate. J. Cryst. Growth 1971, 10, 276–278. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kitamura, K.; Ji, Y.; Montemezzani, G.; Zgonik, M.; Medrano, C.; Gunter, P. Photorefractive properties of iron-doped stoichiometric lithium niobate. Opt. Lett. 1997, 15, 501–503. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kitamura, K.; Niwa, K.; Hatano, H.; Bernasconi, P.; Montemezzani, G.; Gunter, P. Stoichiometric LiTaO3 for dynamic holography in Near UV wavelength range. Jpn. J. Appl. Phys. 1999, 38, 1816–1819. [Google Scholar] [CrossRef]
- Hsu, W.T.; Chen, Z.B.; You, C.A.; Huang, S.W.; Liu, J.P.; Lan, C.W. Growth and photorefractive properties of Mg, Fe co-doped near-stoichiometric lithium tantalate single crystals. Opt. Mater. 2010, 32, 1071–1076. [Google Scholar] [CrossRef]
- Tsai, C.B.; Hsia, Y.T.; Shih, M.D.; Tai, C.Y.; Hsieh, C.K.; Hsu, W.C.; Lan, C.W. Zone-levelling Czochralski growth of MgO-doped near-stoichiometric lithium niobate single crystals. J. Cryst. Growth 2005, 275, 504–511. [Google Scholar] [CrossRef]
- Tsai, C.B.; Hsu, W.T.; Shih, M.D.; Lin, Y.Y.; Huang, Y.C.; Hsieh, C.K.; Hsu, W.C.; Hsu, R.T.; Lan, C.W. Growth and characterizations of ZnO-doped near-stoichiometric LiNbO3 crystals by zone-leveling Czochralski method. J. Cryst. Growth 2006, 289, 145–150. [Google Scholar] [CrossRef]
- Tsai, C.B.; Hsu, W.T.; Wang, C.C.; Shih, M.D.; Tai, C. Y.; Hsieh, C.K.; Hsu, W.C.; Hsu, R.T.; Lan, C.W. Improvements of uniformity and stoichiometry for zone-leveling Czochralski growth of MgO-doped LiNbO3 crystals. Mat. Sci. Eng. B 2006, 128, 161–167. [Google Scholar] [CrossRef]
- Hsu, W.T.; Chen, Z.B.; You, C.A.; Chou, M.M.C.; Lin, Y.Y.; Huang, Y.C.; Rai, D.K.; Lan, C.W. Zone-leveling Czochralski growth and characterization of undoped and MgO-doped near-stoichiometric lithium tantalate crystals. J. Cryst. Growth 2008, 311, 66–71. [Google Scholar] [CrossRef]
- Ashino, T.; Takada, K. Determination of Lithium and Niobium in Lithium Niobate by Inductively Coupled Plasma Atomic Emission Spectrometry after Fusion with Ammonium Hydrogensulfate. Anal. Sci. 1993, 5, 737–739. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Guo, F.; Xu, Y.; Zhao, L. Growth and spectroscopic characterization of Fe2O3 highly doped near-stoichiometric LiNbO3 single crystals. J. Cryst. Growth 2007, 303, 651–654. [Google Scholar] [CrossRef]
- Clark, M.G.; Disalvo, F.J.; Glass, A.M.; Peterson, G.E. Electronic structure and optical index damage of iron‐doped lithium niobate. J. Chem. Phys. 1973, 59. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sota, T.; Suzuki, K.; Ily, N.; Kitamura, K.; Kimura, S. Defect structures in LiNbO3. J. Phys. Condens. Matter 1995, 7, 3627–3636. [Google Scholar] [CrossRef]
- Yevdokimov, S.V.; Yatsenko, A.V. Investigation of the localization of H+ ions in stoichiometric LiNbO3. Crystallogr. Rep. 2003, 48, 542–546. [Google Scholar] [CrossRef]
- Baumer, C.; David, C.; Betzler, K.; Hesse, H.; Lengyel, K.; Kovacs, L.; Wohlecke, M. Composition dependence of the OH-stretch-mode spectrum in lithium tantalite. Phys. Stat. Sol. 2004, 201, R13–R16. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhang, G. Microscopic mechanism of suppressing photorefraction in LiNbO3:Mg, Fe crystals. Solid Stat. Commun. 1996, 98, 523–526. [Google Scholar] [CrossRef]
- Kogelnik, H. Coupled wave theory for thick hologram gratings. Bell Syst. Tech. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Zhen, X.H.; Wang, R.; Li, M.C.; Zhao, L.C.; Xu, Y.H. Optical damage resistance in Zn:Nd:LiNbO3 laser crystals. Mater. Chem. Phys. 2003, 80, 11–14. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, Y.T.; Geng, T.; Dau, Q.; Xu, Y.H. Growth and holographic data storage properties of near-stoichiometric LiTaO3 crystals doped with Mn. Mater. Chem. Phys. 2009, 114, 257–260. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsu, W.T.; Chen, Z.B.; Wu, C.C.; Choubey, R.K.; Lan, C.W. Optical Properties of Mg, Fe, Co-Doped Near-Stoichiometric LiTaO3 Single Crystals. Materials 2012, 5, 227-238. https://doi.org/10.3390/ma5020227
Hsu WT, Chen ZB, Wu CC, Choubey RK, Lan CW. Optical Properties of Mg, Fe, Co-Doped Near-Stoichiometric LiTaO3 Single Crystals. Materials. 2012; 5(2):227-238. https://doi.org/10.3390/ma5020227
Chicago/Turabian StyleHsu, Wei Tse, Zhi Bin Chen, Chien Cheng Wu, Ravi Kant Choubey, and Chung Wen Lan. 2012. "Optical Properties of Mg, Fe, Co-Doped Near-Stoichiometric LiTaO3 Single Crystals" Materials 5, no. 2: 227-238. https://doi.org/10.3390/ma5020227




