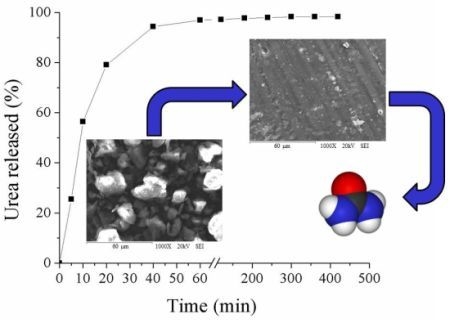
Preparation, Characterization and Release of Urea from Wheat Gluten Electrospun Membranes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Obtaining Membranes by Electrospinning
| Solution | Distance collector (cm) | Flow rate (mL h−1) | Voltage (kV) | Characteristic |
|---|---|---|---|---|
| 1 | 15 | 0.1 | 15 | Only drops |
| 1 | 15 | 0.1 | 20 | Only drops |
| 2 | 15 | 0.03 | 15 | Only drops |
| 2 | 15 | 0.03 | 20 | Only drops |
| 3 | 15 | 0.01 | 15 | Thin layer |
| 3 | 15 | 0.01 | 18 | Thin layer |
| 3 | 15 | 0.03 | 15 | Thin layer |
| 3 | 15 | 0.03 | 18 | Thin layer |
| 4 | 15 | 0.01 | 15 | Thin layer |
| 4 | 15 | 0.03 | 15 | Thin layer |
| 4 | 15 | 0.05 | 15 | Only drops |
| 5 | 15 | 0.01 | 15 | Thin layer |
| 6 | 15 | 0.01 | 15 | Thin layer with drops |
| 6 | 10 | 0.01 | 10 | Thin layer with drops |
| 7 | 10 | 0.05 | 20 | Thin layer with drops dispersed |
| 8 | 10 | 0.01 | 15 | Thin layer and homogeneous |
2.2. Microscopic Characteristics
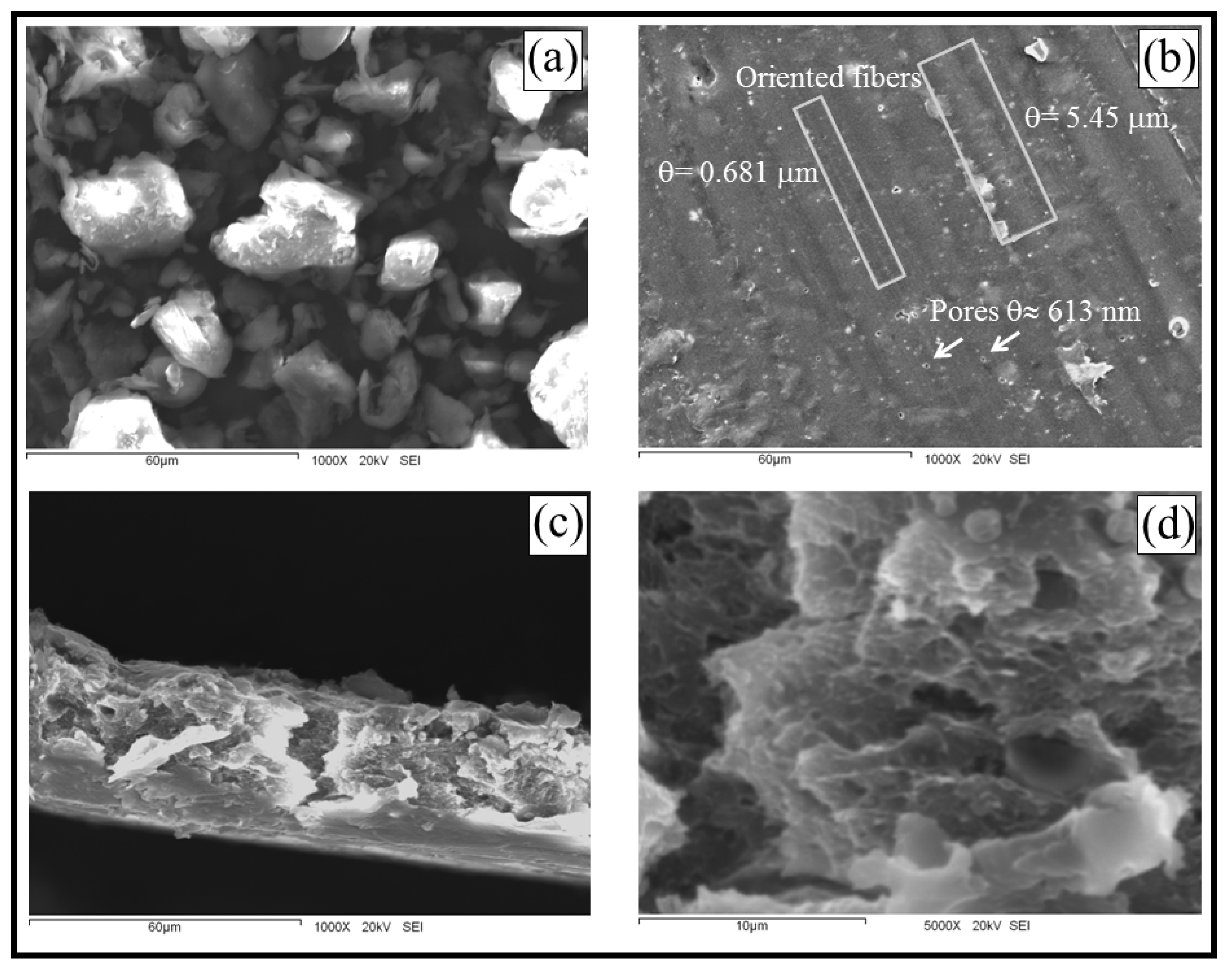
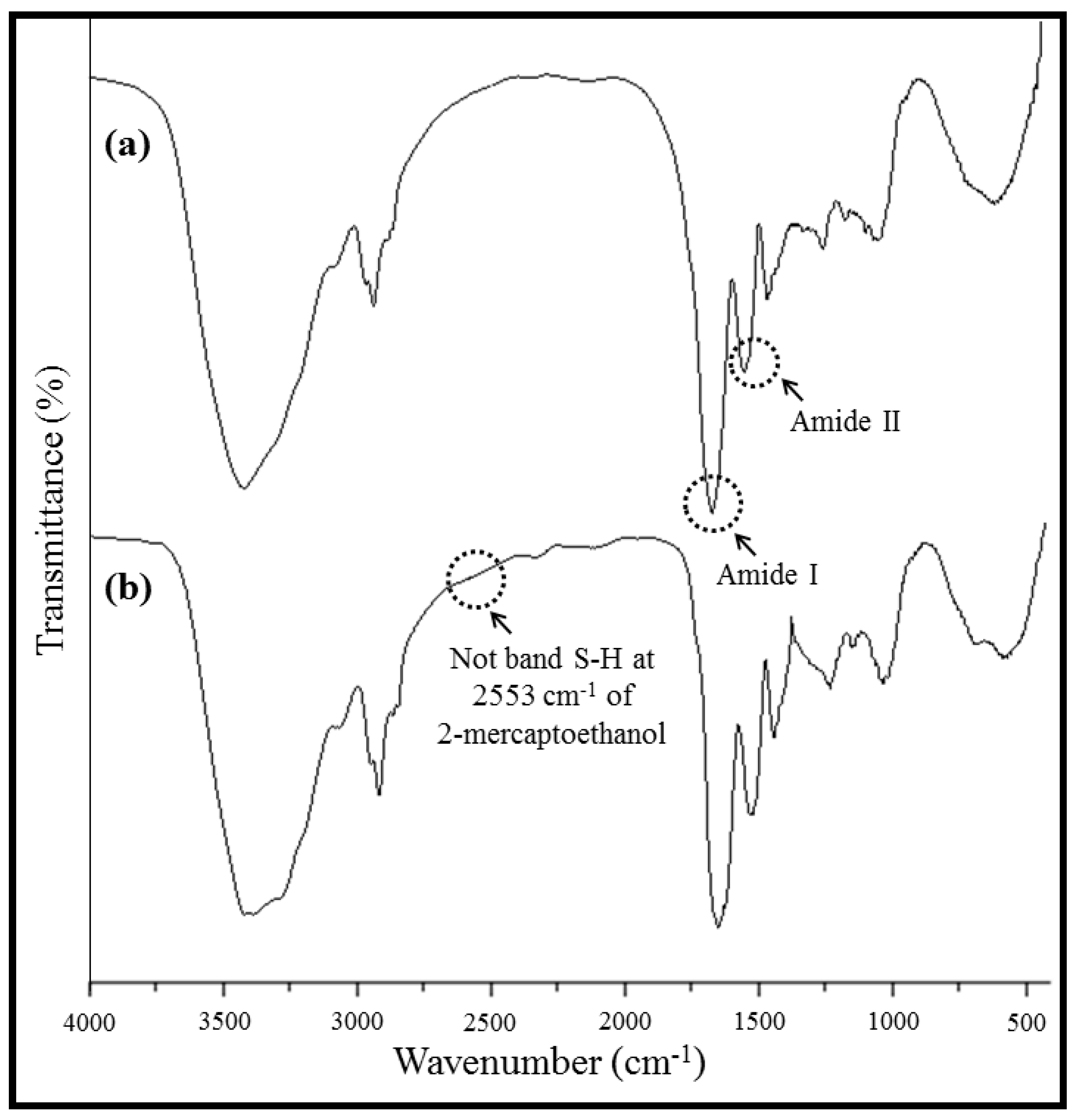
2.3. Fourier Transform-Infrared Spectroscopy, FT-IR
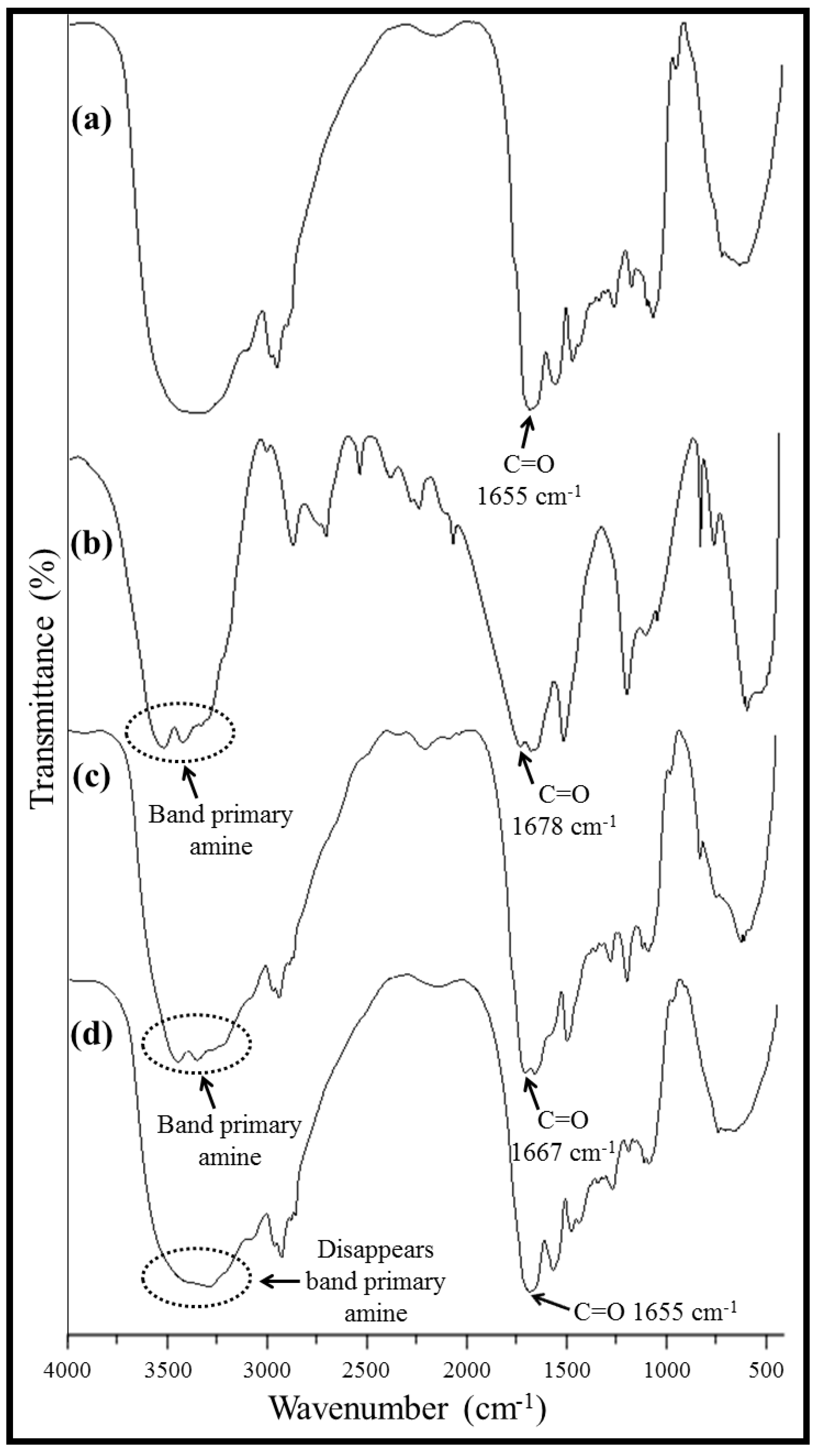
2.4. Thermogravimetric Analysis, TGA
2.5. Differential Scanning Calorimetry, DSC
| Sample | TGA | DSC | |
|---|---|---|---|
| Temperature (°C) | Loss in weight (%) | Tg (°C) | |
| WG powder | 100 | 7 | 149 |
| 160–350 | 49 | ||
| 350–600 | 37 | ||
| WG membranes | 100 | 4 | 140 |
| 100–357 | 50 | ||
| 390–568 | 40 | ||
| Pastille from WG membranes loaded with urea | 100 | 2 | 169 |
| 117–207 | 19 | ||
| 207–350 | 38 | ||
| 350–600 | 42 | ||
2.6. Urea Release Kinetic
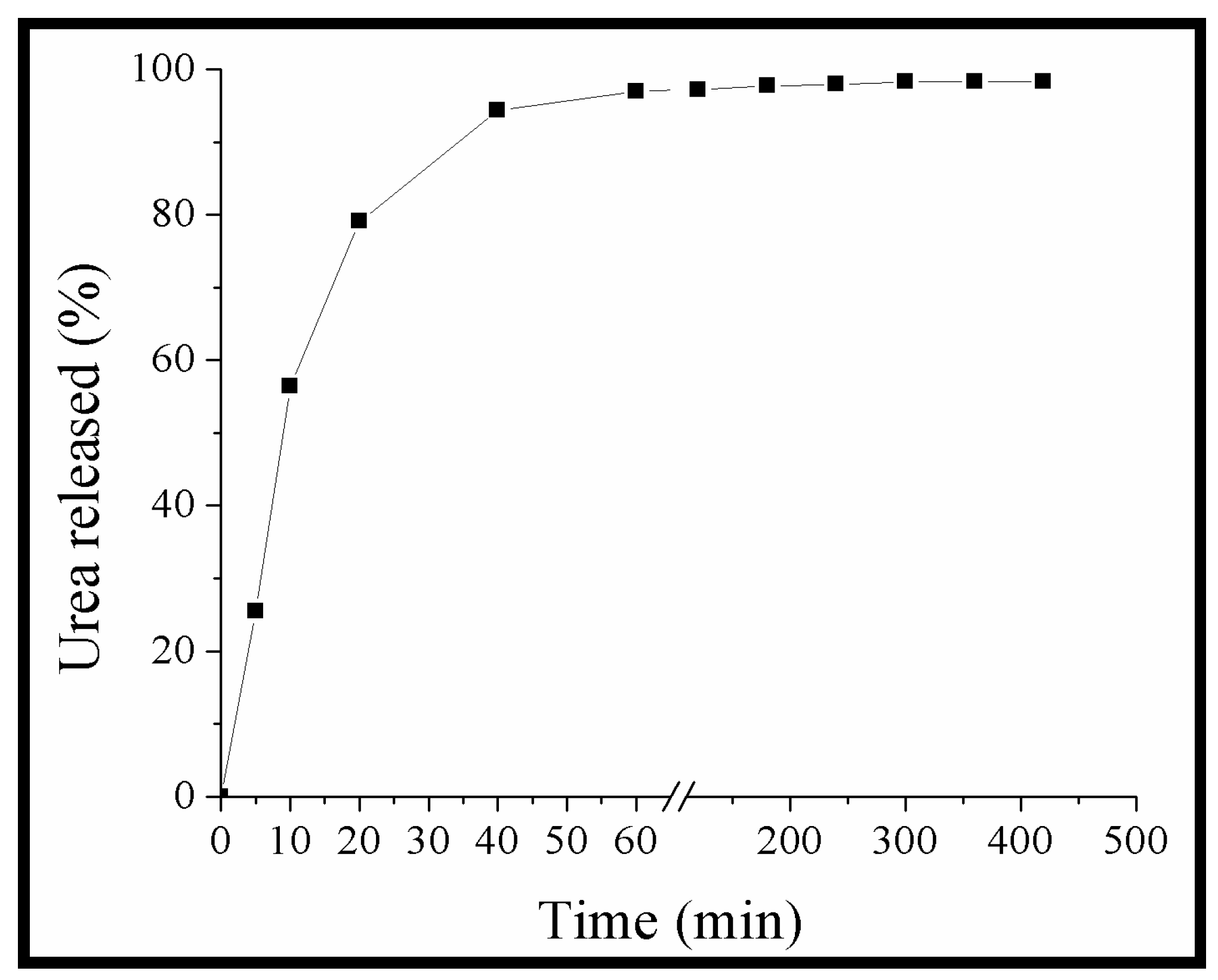
2.7. Empirical Model for the Urea Release Kinetic
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Solutions Preparation
| Solution | Solvent system | Concentration (w/v) |
|---|---|---|
| 1 | Acetic acid 70% a/Ethanol 70% a 1:1 | 8% |
| 2 | Acetic acid 70% a/Ethanol 70% a 1:1 | 12% |
| 3 | Acetic acid 70% a/2-propanol 70% a 1:3 | 12% |
| 4 | Ethanol 80% a/Acetone 80% a 1:1 | 12% |
| 5 | 1-propanol 70% a/2-mercaptoethanol 19:1 | 12% |
| 6 | Acetic acid 70% b/Ethanol 70% b/2-mercaptoethanol 95:95:10 | 12% |
| 7 | Acetic acid 80% a/Ethanol 50% a/2-mercaptoethanol 95:95:10 | 12% |
| 8 | Ethanol 50% a/2-mercaptoethanol 19:1 | 8% |
3.2.2. Fibrous Membrane Preparation
3.2.3. Characterization
3.2.4. Loading of Urea in the Wheat Gluten Films
3.2.5. Urea Release Kinetic Experiments
4. Conclusions
Acknowledgments
References
- Vashishtha, M.; Dongara, P.; Singh, D. Improvement in properties of urea by phosphogypsum coating. Int. J. ChemTech Res. 2010, 2, 36–44. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Willem, J.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.J.; Grageda, C.O.; Vera, N.J. Nitrogen fertilizer management in Mexico: Use of isotopic techniques (15N). Terra 2001, 20, 51–56. [Google Scholar]
- Liang, R.; Liu, M. Preparation and properties of a double-coated slow-release and water-retention urea fertilizer. J. Agric. Food Chem. 2006, 54, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, M.E.; Horwath, W.R. Methylene urea as a slow-release nitrogen source for processing tomatoes. Nutr. Cycl. Agroecosys. 2005, 71, 177–190. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M.; Liang, R. Preparation and properties of a double-coated slow-release NPK compound fertilizer with superabsorbent and wáter-retention. Bioresour. Technol. 2008, 99, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Morgan, K.T. Nitrogen recovery and transformation from a surface or sub-surface application of controlled-release fertilizer on a sandy soil. J. Plant Nutr. 2008, 31, 2214–2231. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Polaczek, J.; Pielichowski, K. PAA-Based hybrid organic-inorganic fertilizers with controlled release. Pol. J. Environ. Stud. 2009, 18, 475–479. [Google Scholar]
- Wang, Y.; Liu, M.; Ni, B.; Xie, L. k-Carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Eng. Chem. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Domeneck, S.; Brendel, L.; Morel, M.H.; Guilbert, S. Swelling behavior and structural characteristics of wheat gluten polypeptide films. Biomacromolecules 2004, 5, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Novel protein fibers from wheat gluten. Biomacromolecules 2007, 8, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Tatham, A.S.; Forde, J.; Kreis, H.; Miflin, B.J. The classification and nomenclature of wheat gluten proteins: A reassessment. J. Cereal Sci. 1986, 4, 97–106. [Google Scholar] [CrossRef]
- Horvat, D.; Kurtanjek, Z.; Drezner, G.; Simic, G.; Magdic, D. Effect of HMM glutenin subunits on wheat quality attributes. Food Technol. Biotechnol. 2009, 47, 353–359. [Google Scholar]
- Metakovsky, E.V.; Novoselskaya, A.Y.; Sozinov, A.A. Genetic analysis of gliadin components in winter wheat using two-dimensional polyacrylamide gel electrophoresis. Theor. Appl. Genet. 1984, 69, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Shewry, P.R. The conformation of wheat gluten proteins. The secondary structures and thermal stabilities of α-, β-, γ- and ω-gliadins. J. Cereal Sci. 1985, 3, 103–113. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S. High molecular weight subunits of wheat glutenin. J. Cereal Sci. 1992, 15, 105–120. [Google Scholar] [CrossRef]
- Siegel, R.A.; Rathbone, M.J. Overview of controlled release mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: New York, NY, USA, 2012; pp. 19–43. [Google Scholar]
- Garg, K.; Bowlin, G.L. Electrospinning jets and nanofibrous structures. Biomicrofluidics 2011, 5, 1–19. [Google Scholar] [CrossRef]
- Woerdeman, D.L.; Ye, P.; Shenoy, S.; Parnas, R.S.; Wnek, G.E.; Trofimova, O. Electrospun fibers from wheat protein: Investigation of the interplay between molecular structure and fluid dynamics of the electronspinning process. Biomacromolecules 2005, 6, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Woerdeman, D.L.; Shenoy, S.; Breger, D. Effects of hydroxyl groups versus physical entanglements on the electrospinning behavior of wheat protein. J. Biobased Mater. Bioenerg. 2007, 1, 31–36. [Google Scholar]
- Woerdeman, D.L.; Shenoy, S.; Breger, D. Role of chain entanglements in the electrospinning of wheat protein-poly(vinyl alcohol) blends. J. Adhes. 2007, 83, 785–789. [Google Scholar] [CrossRef]
- Castillo-Ortega, M.M.; Nájera-Luna, A.; Rodríguez-Félix, D.E.; Encinas, J.C.; Rodríguez-Félix, F.; Romero, J.; Herrera-Franco, P.J. Preparation, characterization and release of amoxicillin from cellulose acetate and poly(vinyl pyrrolidone) coaxial electrospun fibrous membranes. Mater. Sci. Eng. C 2011, 31, 1772–1778. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Testin, R.F. Modification of physical and barrier properties of edible wheat gluten-based films. Cereal Chem. 1993, 70, 426–429. [Google Scholar]
- Aydt, T.P.; Weller, C.L.; Testin, R.F. Mechanical and barrier properties of edible corn and wheat protein films. Trans. ASAE 1991, 34, 207–211. [Google Scholar] [CrossRef]
- Mojumdar, S.C.; Moresoli, C.; Simon, L.C.; Legge, R.L. Edible wheat gluten (WG) protein films: Preparation, thermal, mechanical and spectral properties. J. Therm. Anal. Calorim. 2011, 104, 929–936. [Google Scholar] [CrossRef]
- Irissin-Mangata, J.; Bauduin, G. New plasticizers for wheat gluten films. Eur. Polym. J. 2001, 37, 1533–1541. [Google Scholar] [CrossRef]
- Cherian, G.; Gennadios, A.; Weller, C.L.; Chinachoti, P. Thermomechanical behavior of wheat gluten films: effect of sucrose, glycerin, and sorbitol. Cereal Chem. 1995, 72, 1–6. [Google Scholar]
- Park, H.J.; Bunn, J.M.; Weller, C.L.; Vergano, P.J.; Testin, R.F. water vapor permeability and mechanical properties of grain protein-based films as affected by mixtures of polyethylene glycol and glycerin plasticizers. Trans. ASAE 1994, 37, 1281–1285. [Google Scholar] [CrossRef]
- Kuktaite, R.; Plivelic, T.S.; Cerenius, Y.; Hedenqvist, M.S.; Gällstedt, M.; Marttila, S.; Ignell, R.; Popineau, Y.; Tranquet, O.; Shewry, P.R.; Johansson, E. Structure and morphology of wheat gluten films: from polymeric protein aggregates toward superstructure arrangements. Biomacromolecules 2011, 12, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Mauricio-Iglesias, M.; Peyron, S.; Guillard, V.; Gontard, N. Wheat gluten nanocomposite films as food-contact materials: Migration tests and impact of a novel food stabilization technology (high pressure). J. Appl. Polym. Sci. 2010, 116, 2526–2535. [Google Scholar]
- Chiou, B,S.; Robertson, G.H.; Rooff, L.E.; Cao, T.; Jafri, H.; Gregorski, K.S.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Water absorbance and thermal properties of sulfated wheat gluten films. J. Appl. Polym. Sci. 2010, 116, 2638–2644. [Google Scholar]
- Li, W.; Dobraszczyk, B.J.; Dias, A.; Gil, A.M. Polymer conformation structure of wheat proteins and gluten subfractions revealed by ATR-FTIR. Cereal Chem. 2006, 83, 407–410. [Google Scholar] [CrossRef]
- Andreani, L.; Cercená, R.; Ramos, B.G.; Soldi, V. Development and characterization of wheat gluten microspheres for use in a controlled release system. Mater. Sci. Eng. C 2009, 29, 524–531. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S. Voltametric behavior of noradrenaline at 2-mercaptoethanol self-assembled monolayer modified gold electrode and its analytical application. Sensors 2003, 3, 61–68. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Zhuang, X.; Chen, X.; Jing, X. Controlled release of urea encapsulated by starch-g-poly(l-lactide). Carbohyd. Polym. 2008, 72, 342–348. [Google Scholar] [CrossRef]
- Rodríguez-Félix, D.E.; Pérez-Martínez, C.J.; Castillo-Ortega, M.M.; Pérez-Tello, M.; Romero-García, J.; Ledezma-Pérez, A.S.; Castillo-Castro, T.; Rodríguez-Félix, F. pH- and temperature-sensitive semi-interpenetrating network hydrogels composed of poly(acrylamide) and poly(γ-glutamic acid) as amoxicillin controlled-release system. Polym. Bull. 2012, 68, 197–207. [Google Scholar] [CrossRef]
- Mulder, W.J.; Gosselink, R.J.; Vingerhoeds, M.H.; Harmsen, P.F.; Eastham, D. Lignin based controlled release coatings. Ind. Crop. Prod. 2011, 34, 915–920. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II Fickian and anomalous release from swellable devices. J. Control Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, H.; Xiao, C. pH-sensitive cationic guar gum/poly (acrylic acid) polyelectrolyte hydrogels: Swelling and vitro drug release. Carbohyd. Polym. 2007, 69, 774–783. [Google Scholar] [CrossRef]
- Tapia-Albarran, M.; Villafuerte-Robles, L. Assay of amoxicillin sustained release from matrix tablets containing different proportions of Carbopol 971P NF. Int. J. Pharm. 2004, 273, 121–127. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Castro-Enríquez, D.D.; Rodríguez-Félix, F.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Castillo-Ortega, M.M.; Rodríguez-Félix, D.E.; Armenta-Villegas, L.; Ledesma-Osuna, A.I. Preparation, Characterization and Release of Urea from Wheat Gluten Electrospun Membranes. Materials 2012, 5, 2903-2916. https://doi.org/10.3390/ma5122903
Castro-Enríquez DD, Rodríguez-Félix F, Ramírez-Wong B, Torres-Chávez PI, Castillo-Ortega MM, Rodríguez-Félix DE, Armenta-Villegas L, Ledesma-Osuna AI. Preparation, Characterization and Release of Urea from Wheat Gluten Electrospun Membranes. Materials. 2012; 5(12):2903-2916. https://doi.org/10.3390/ma5122903
Chicago/Turabian StyleCastro-Enríquez, Daniela Denisse, Francisco Rodríguez-Félix, Benjamín Ramírez-Wong, Patricia Isabel Torres-Chávez, María Mónica Castillo-Ortega, Dora Evelia Rodríguez-Félix, Lorena Armenta-Villegas, and Ana Irene Ledesma-Osuna. 2012. "Preparation, Characterization and Release of Urea from Wheat Gluten Electrospun Membranes" Materials 5, no. 12: 2903-2916. https://doi.org/10.3390/ma5122903