In Vivo Degradation Behavior of the Magnesium Alloy LANd442 in Rabbit Tibiae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Clinical Investigations
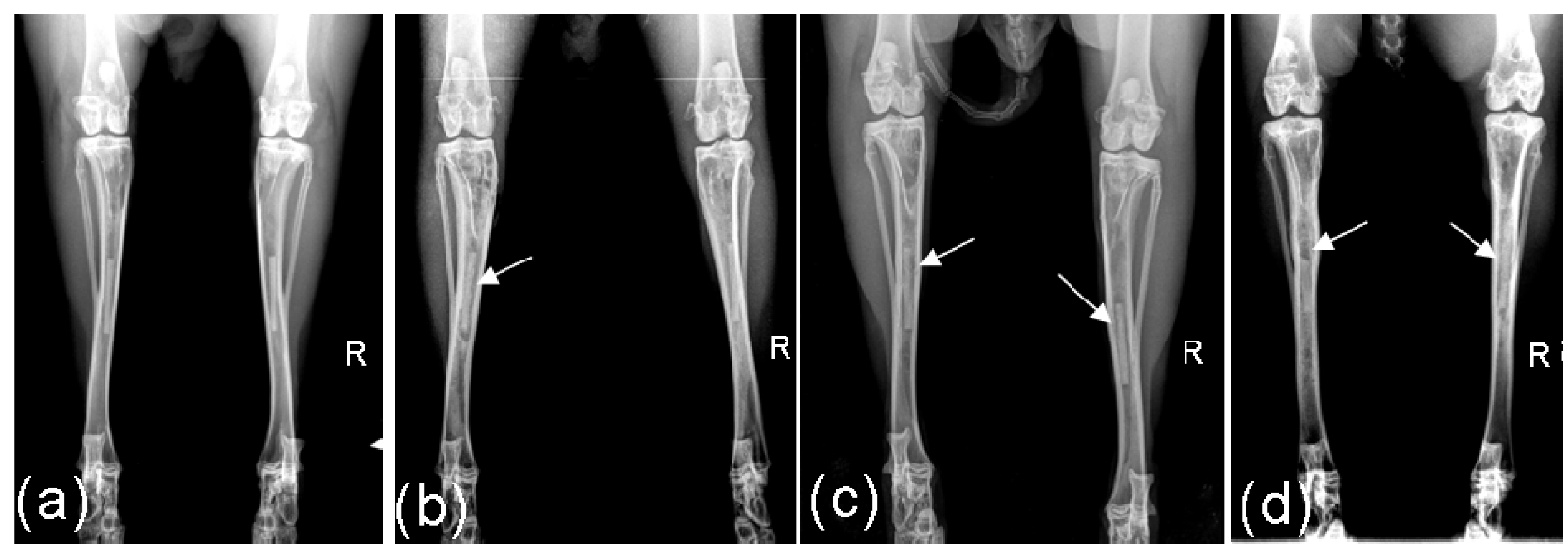
2.2. Radiological Investigations
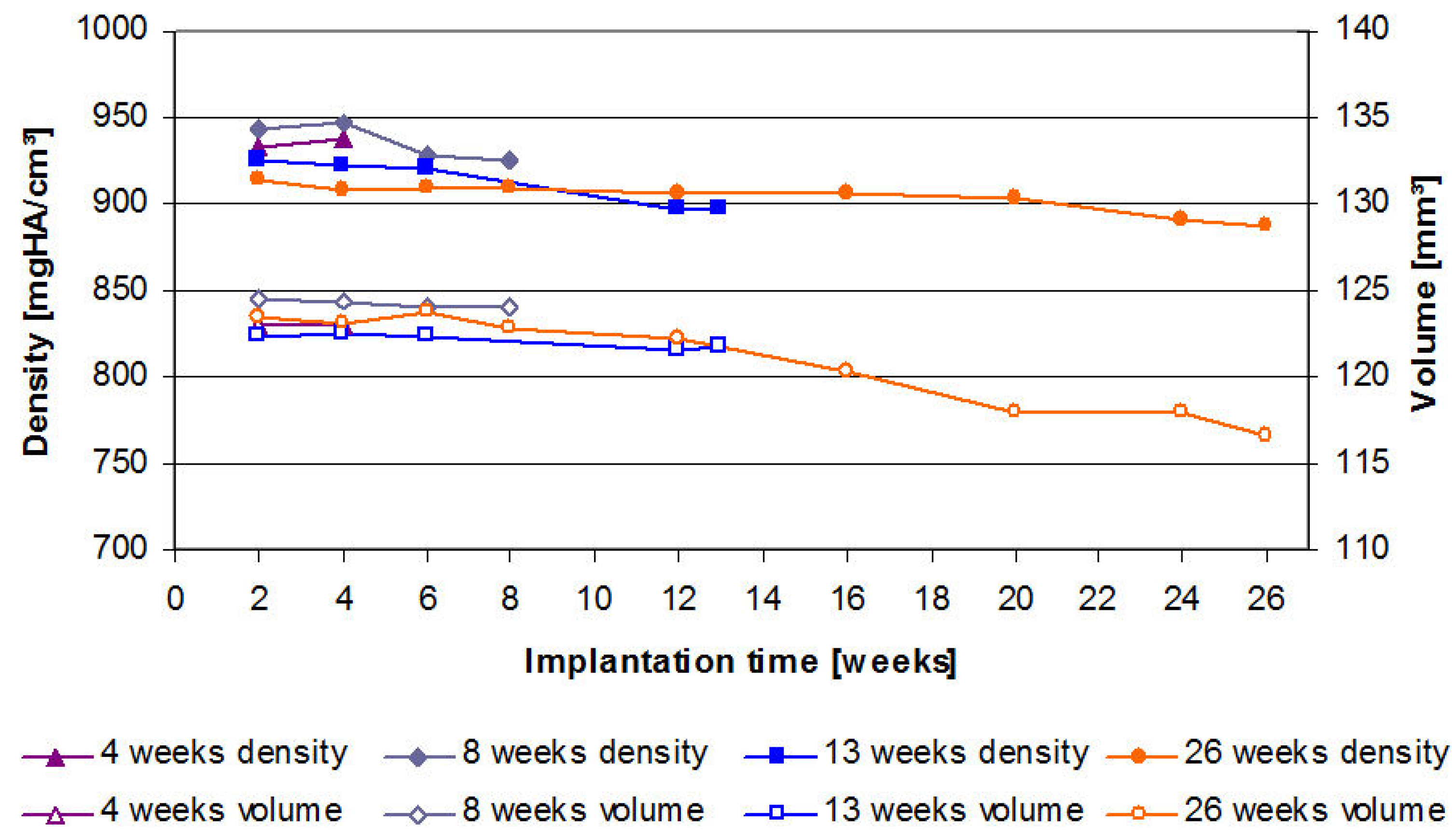
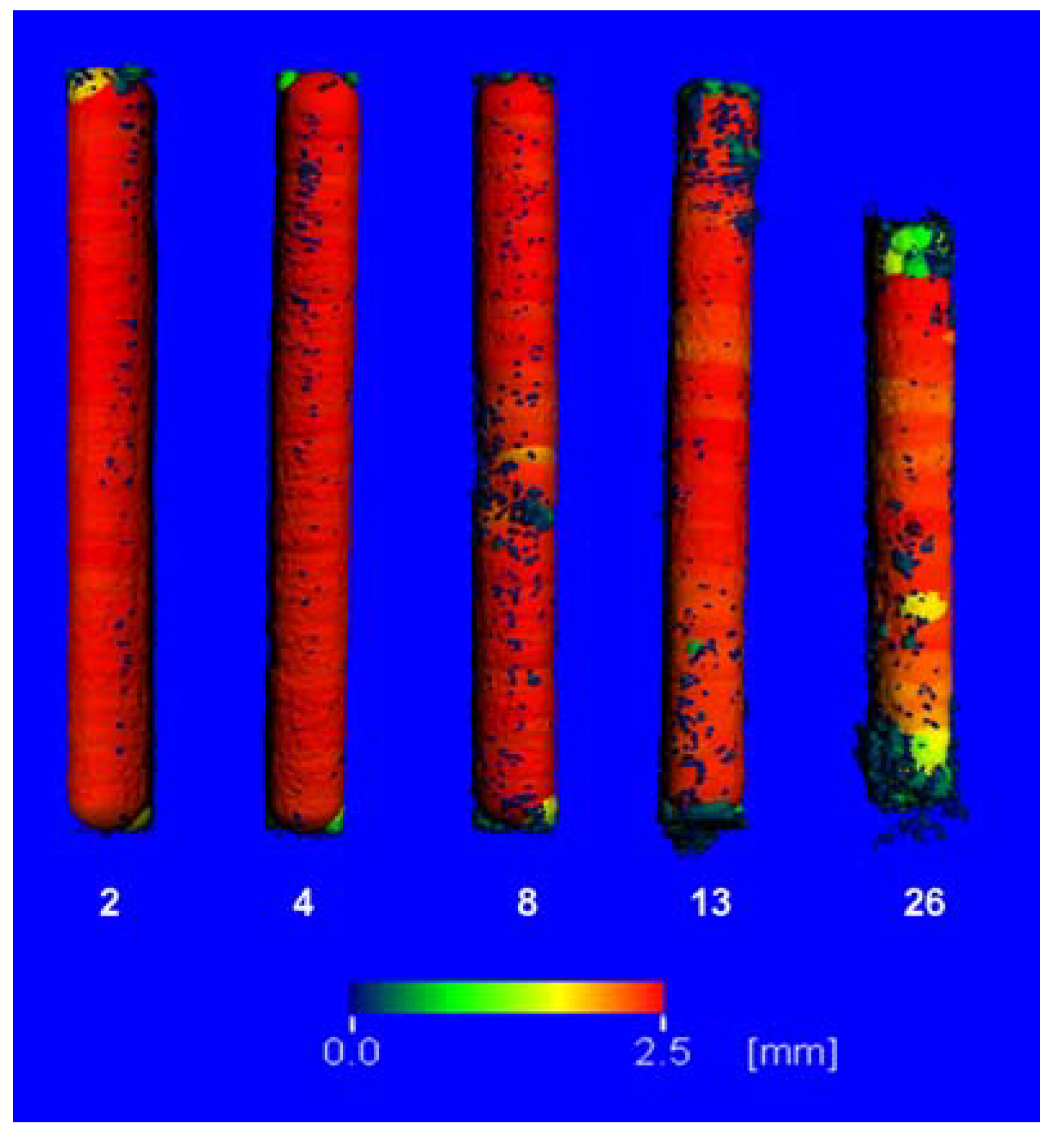
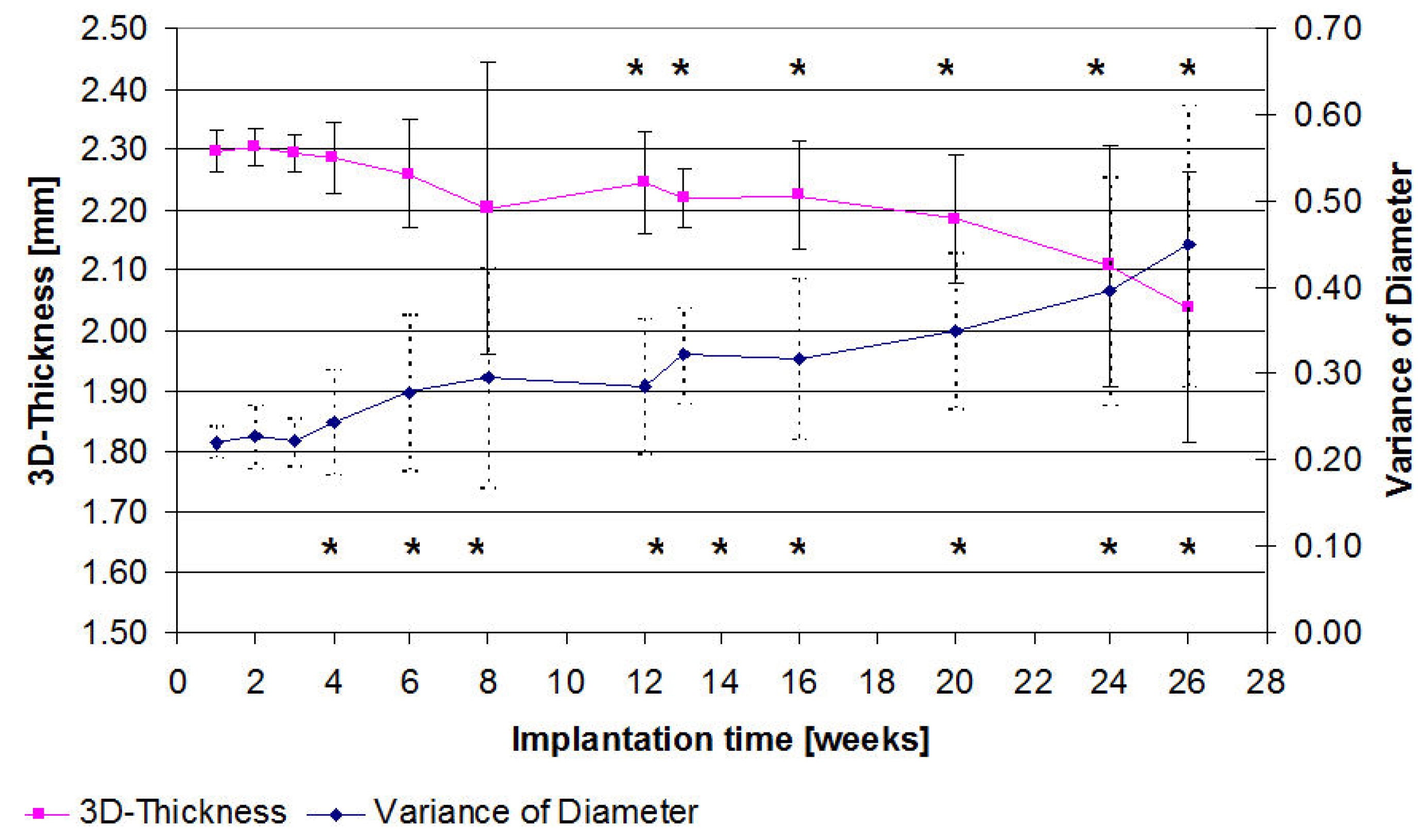
2.3. µ-Computed Tomographic Investigation of the in Vivo LANd442-Pins
2.4. X-Ray Diffractometry Following Explantation
2.5. Investigation of the Pins Using Stereomicroscopy Following Explantation

2.6. SEM Investigation and the EDX Analysis
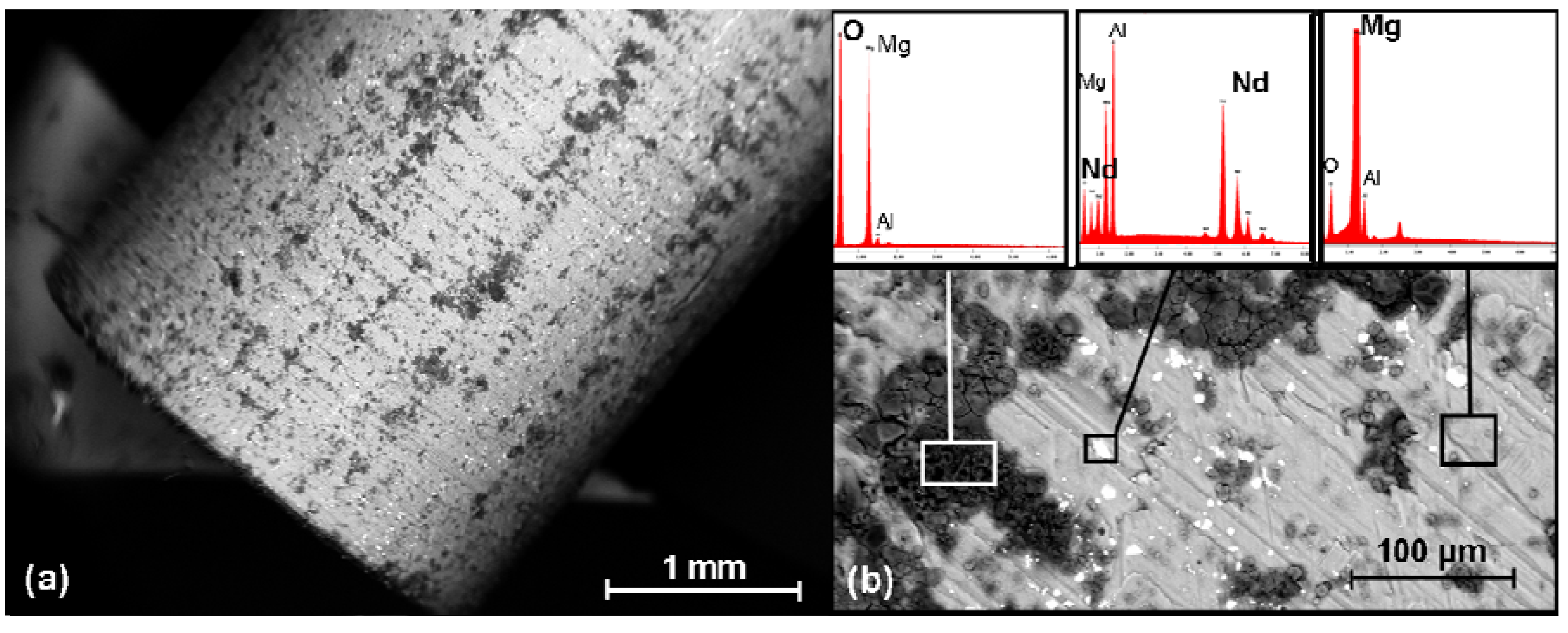
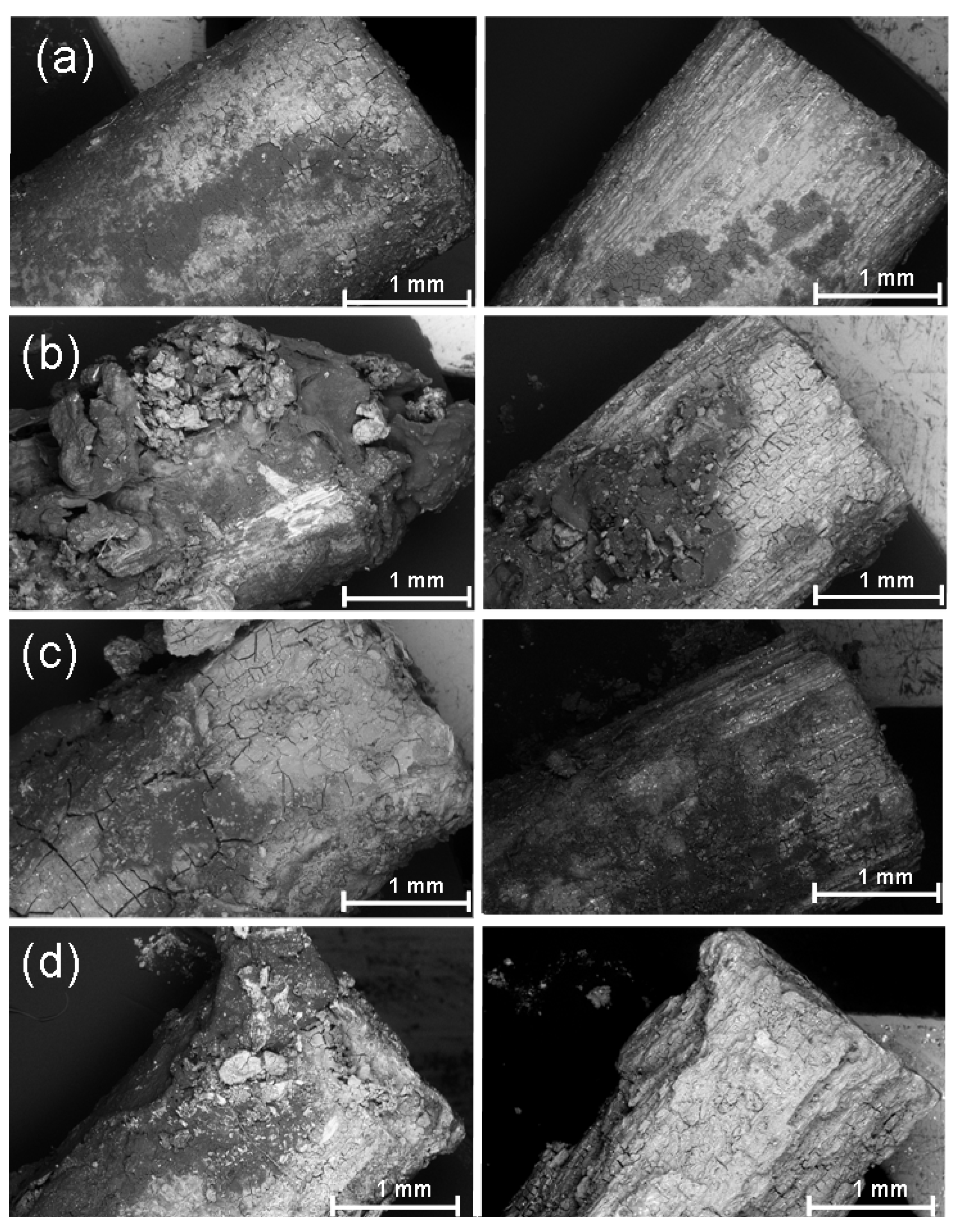
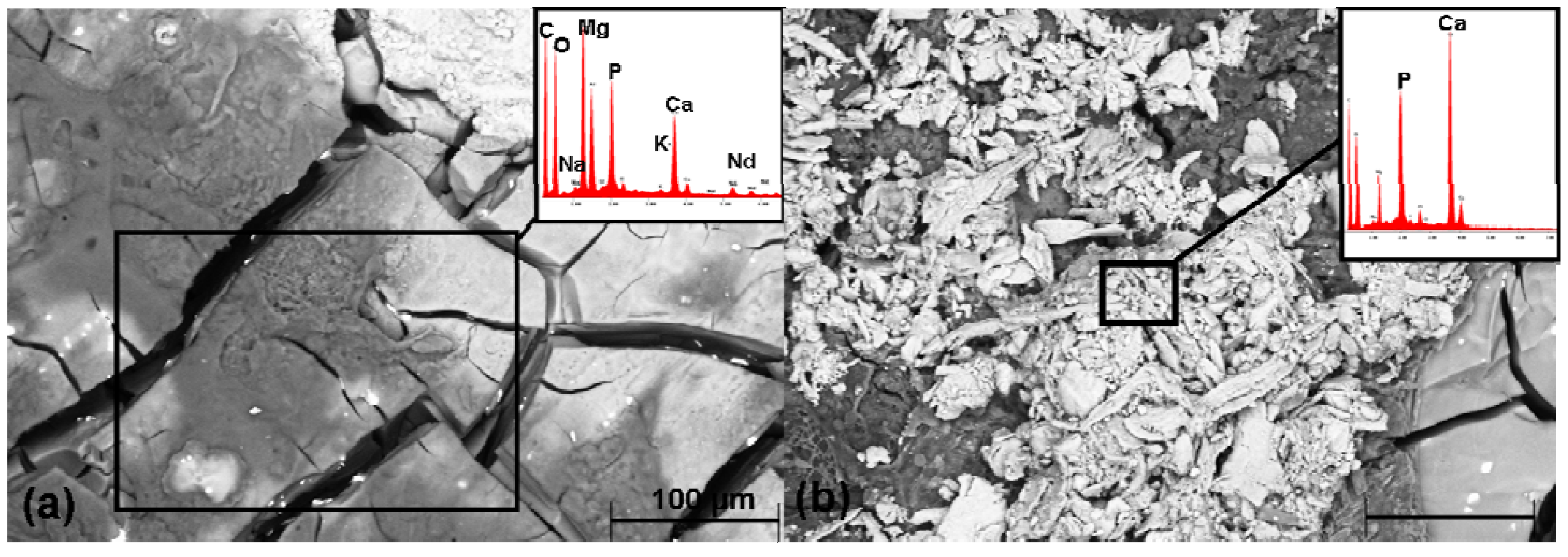
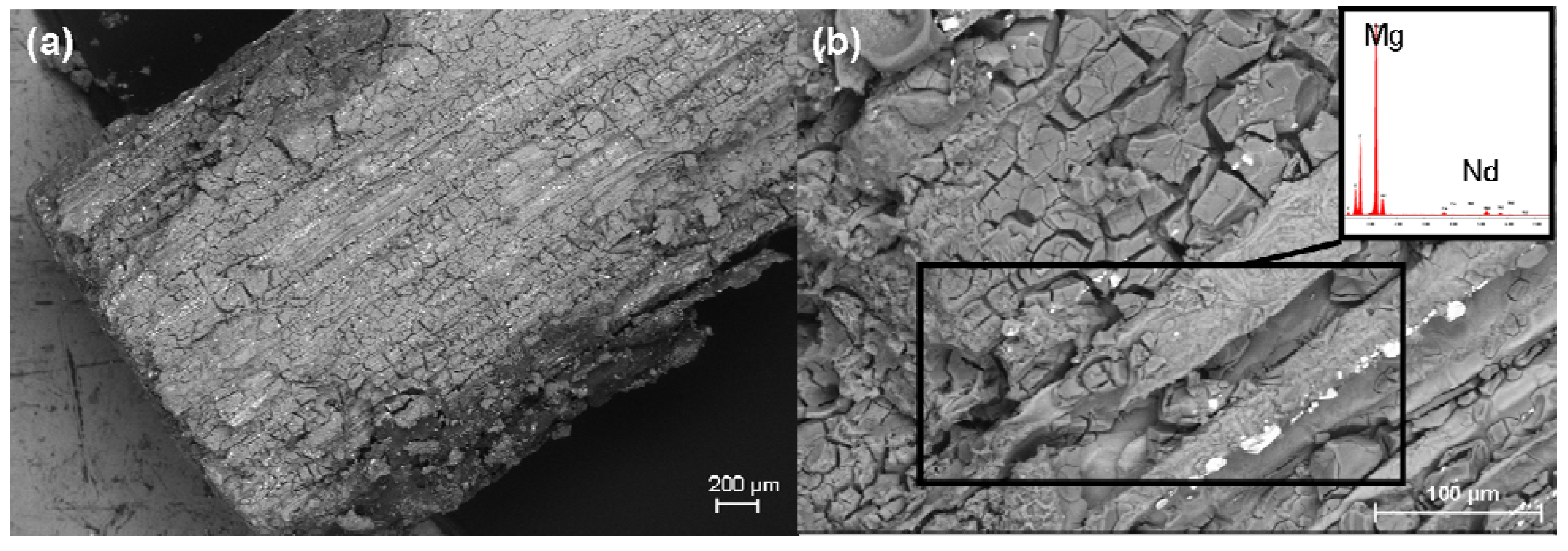
2.7. Determination of the Weight
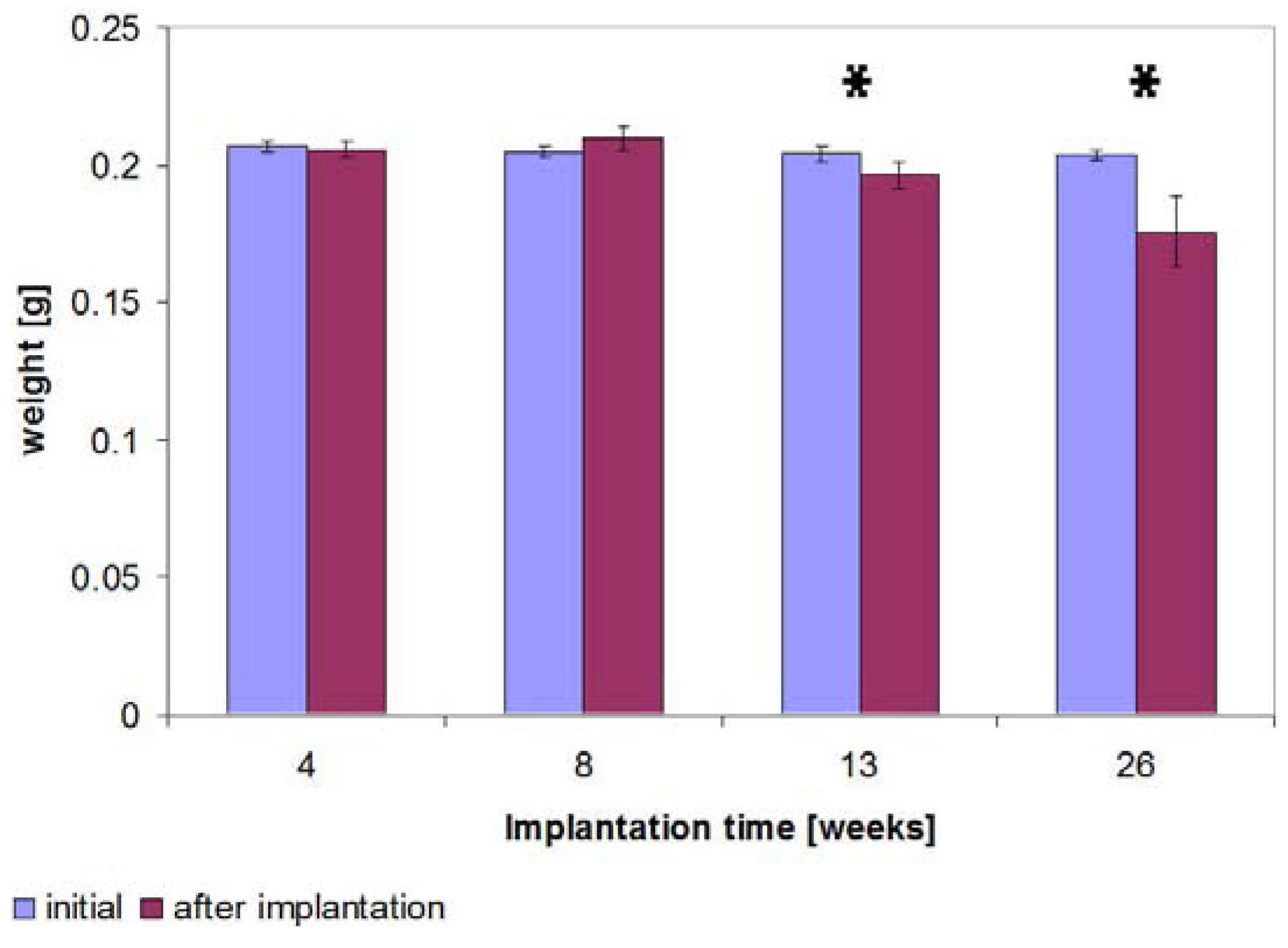
2.8. Results of the Three Point Bending
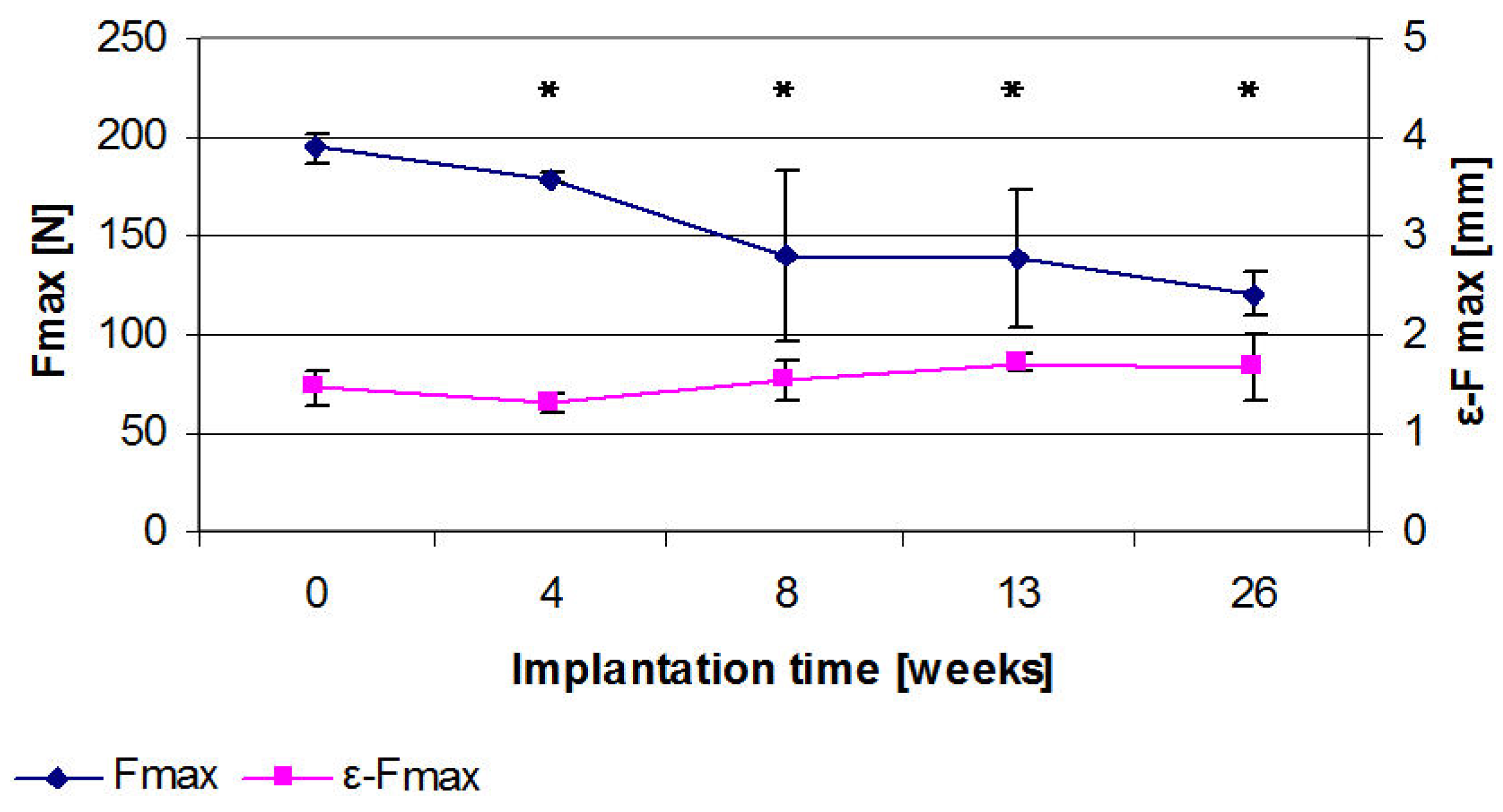
2.9. Discussion
3. Experimental Section
3.1. Manufacturing and Testing of the Implants
3.2. In Vivo Investigations
3.2.1. Test Animals
3.2.2. Surgical Procedure
3.2.3. Post-Operative Investigation Period
3.2.4. Radiological Investigation
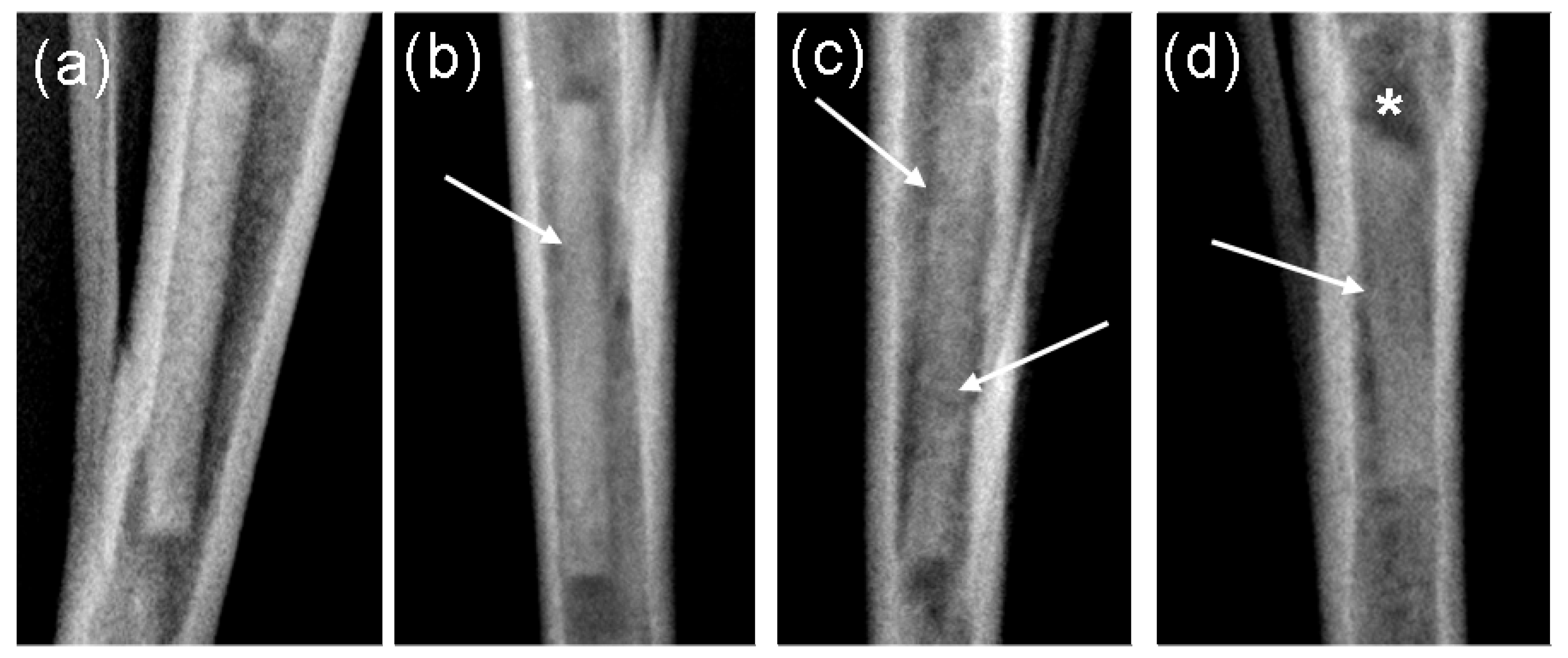
3.2.5. In Vivo µ-Computed Tomography
3.3. Investigation of the Implants Following Removal
3.3.1. X-Ray Diffractometery
3.3.2. Investigation of Implants Using Stereomicroscopy
3.3.3. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy
3.3.4. Investigation of the Implants after Treating in Hydrofluoric Acid
3.3.5. Determination of the Weight
3.4. Statistics
4. Conclusions
Acknowledgments
References
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Trans. A 2002, 396, 1399–1404. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.O. Biodegradable implants in traumatology: A review on the state-of-the-art. Arch. Orthop. Trauma Surg. 1995, 114, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Corrosion of metal orthopaedic implants. J. Bone Joint Surg. Am. 1998, 80, 268–282. [Google Scholar] [PubMed]
- Sinibaldi, K.; Rosen, H.; Liu, S.K.; DeAngelis, M. Tumors associated with metallic implants in animals. Clin. Orthop. Relat. Res. 1976, 118, 257–266. [Google Scholar] [PubMed]
- Meyer-Lindenberg, A.; Thomann, M.; Krause, A.; Bormann, D.; von Rechenberg, B.; Windhagen, H. Untersuchungen zum Einsatz einer Magnesiumbasislegierung als neues resorbierbares Implantatmaterial für die Osteosynthese. Kleintierpraxis 2010, 55, 349–363. [Google Scholar]
- Thomann, M.; Krause, C.; Bormann, D.; von der Höh, N.; Windhagen, H.; Meyer-Lindenberg, A. Comparison of the resorbable magnesium alloys LAE442 und MgCa0.8 concerning their mechanical properties, their progress of degradation and the bone-implant-contact after 12 months implantation duration in a rabbit model. Mater. Sci. Eng. Tech. 2009, 40, 82–87. [Google Scholar]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [Green Version]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Aspects Med. 2003, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lambotte, A. L’utilisation du magnesium comme materiel perdu dans l’osteosynthèse. Bull. Mém. Soc. Nat. Chir. 1932, 28, 1325–1334. [Google Scholar]
- McBride, E.D. Absorbable metal in bone surgery. J. Am. Med. Assoc. 1938, 111, 2464–2467. [Google Scholar] [CrossRef]
- Verbrugge, J. Le materiel metallique resorbable en chirurgie osseuse. La. Press Med. 1934, 23, 460–465. [Google Scholar]
- McBride, D. Magnesium screw and nail transfixion in fractures. South. Med. J. 1938, 31, 508–515. [Google Scholar] [CrossRef]
- Groves, E.W. An experimental study of the operative treatment of fractures. Br. J. Surg. 1913, 1, 438–501. [Google Scholar] [CrossRef]
- </i>Yang, Y.; Li, X. Influence of neodymium on high cycle fatigue behavior of die cast AZ91D magnesium alloy. J. Rare Earth 2010, 28, 456–460. [Google Scholar] [CrossRef]
- Drynda, A.; Deinet, N.; Braun, N.; Peuster, M. Rare earth metals used in biodegradable magnesium-based stents do not interfere with proliferation of smooth muscle cells but do induce the upregulation of inflammatory genes. J. Biomed. Mater. Res. A 2009, 91, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Reifenrath, J.; Bormann, D.; Meyer-Lindenberg, A. Magnesium alloys as promising degradable implant materials in orthopaedic research. In Magnesium Alloys—Corrosion and Surface Treatments; Czerwinski, F., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Rokhlin, L.L. Advances in metallic alloys. In Magnesium Alloys Containing Rare-Earth Metals—Structure and Properties; Fridlyander, J.N., Eskin, D.G., Eds.; Taylor & Francis Ltd.: London, UK, 2003; pp. 3–4. [Google Scholar]
- Reifenrath, J.; Krause, A.; Bormann, D.; von Rechenberg, B.; Windhagen, H.; Meyer-Lindenberg, A. Profound differences in the in-vivo-degradation and biocompatibility of two very similar rare-earth containing Mg-alloys in a rabbit model. Mater. Sci. Eng. Technol. 2010, 41, 1054–1061. [Google Scholar]
- Nakamura, Y.; Tsumura, Y.; Tonogai, Y.; Shibata, T.; Ito, Y. Differences in behavior among the chlorides of seven rare earth elements administered intravenously to rats. Fundam. Appl. Toxicol. 1997, 37, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Effect of neodymium on the as-extruded ZK20 magnesium alloy. J. Rare Earths 2010, 28, 631–635. [Google Scholar] [CrossRef]
- Gunde, P.; Hänzi, A.C.; Sologubenko, A.S.; Uggowitzer, P.J. High-strength magnesium alloys for degradable implant applications. Mater. Sci. Eng. A 2011, 528, 1047–1054. [Google Scholar] [CrossRef]
- Buchmann, F. Patient zum Röntgen—Medizinische Abbildung und Untersuchungsablauf vom Röntgenbild über MRT bis Ultraschall; LIT: Münster, Germany, 2002; p. 106. [Google Scholar]
- Huehnerschulte, T.A.; Angrisani, N.; Rittershaus, D.; Bormann, D.; Windhagen, H.; Meyer-Lindenberg, A. In vivo corrosion of two novel magnesium alloys ZEK100 and AX30 and their mechanical suitability as biodegradable implants. Materials 2011, 4, 1144–1167. [Google Scholar] [CrossRef] [Green Version]
- Seitz, J.-M.; Collier, K.; Wulf, E.; Bormann, D.; Bach, F.-W. Comparison of the corrosion behavior of coated and uncoated magnesium alloys in an in vitro corrosion environment. Adv. Biomater. 2011, 13, 313–323. [Google Scholar]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Krause, A.; Höh, N. von der; Bormann, D.; Krause, C.; Bach, F.-W.; Windhagen, H.; Meyer-Lindenberg, A. Degradation behaviour and mechanical properties of magnesium implants in rabbit tibiae. J. Mater. Sci. 2010, 45, 624–632. [Google Scholar] [CrossRef]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. A 2009, 90, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Papakyriacou, M.; Zettl, B.; Stanzl-Tschegg, S.E. Influence of porosity on the fatigue limit of die cast magnesium and aluminium alloys. Int. J. Fatigue 2003, 25, 245–256. [Google Scholar] [CrossRef]
- Kannan, M.B.; Singh Raman, R.K.; Witte, F.; Blawert, C.; Dietzel, W. Influence of circumferential notch and fatigue crack on the mechanical integrity of biodegradable magnesium-based alloy in simulated body fluid. J. Biomed. Mater. Res. B 2010, 96B, 303–309. [Google Scholar]
- Atrens, A.; Liu, M.; Zainal Abidin, N.I. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Hara, T.; Tanck, E.; Homminga, J.; Huiskes, R. The influence of microcomputed tomography threshold variations on the assessment of structural and mechanical trabecular bone properties. Bone 2002, 31, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Stahl, J.; Bäumer, W.; Seitz, J.M.; Bach, F.W.; Petersen, L.J.; Kietzmann, M. Ex vivo examination of the biocompatibility of biodegradable magnesium via microdialysis in the perfused bovine udder model. Int. J. Artif. Organs 2011, 34, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; Bian, Y. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Von Vogel, H.U. Chemiker-Kalender; Springer: Berlin, Germany, 1966; Volume III. [Google Scholar]
- Li, L.; Gao, J.; Wang, Y. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf. Coat. Technol. 2004, 185, 92–98. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.-H. Biodegradable behaviors of AZ31 magnesium alloys in simulated body fluid. Mater. Sci. Eng. C 2009, 29, 1039–1045. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, E.; Yin, D.; Zeng, S.; Yang, K. In vitro corrosion behaviour of Mg alloys in a phosphate buffered solution for bone implant application. J. Mater. Sci. Mater. Med. 2008, 19, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Thomann, M.; Krause, C.; Angrisani, N.; Bormann, D.; Hassel, T.; Windhagen, H.; Meyer-Lindenberg, A. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0.8) on degradation in a rabbit model. J. Biomed. Mater. Res. A 2010, 93, 1609–1619. [Google Scholar] [PubMed]
- García-Aznar, J.M.; Kuiper, J.H.; Gómez-Benito, M.J.; Doblaré, M.; Richardson, J.B. Computational simulation of fracture healing: Influence of interfragmentary movement on the callus growth. J. Biomech. 2007, 40, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.N.; Mishra, S. The biomechanical environment of a bone fracture and its influence upon the morphology of healing. Med. Eng. Phys. 2003, 25, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Wraighte, P.J.; Scammell, B.E. Principles of fracture healing. Surgery 2006, 24, 198–207. [Google Scholar]
- Frost, H.M. The biology of fracture healing. Clin. Orthop. Relat. Res. 1989, 248, 283–293. [Google Scholar] [PubMed]
- Yuen, C.K.; Ip, W.Y. Theoretical risk assessment of magnesium alloys as degradable biomedical implants. Acta Biomater. 2010, 6, 1808–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorczyca, J.T.; McKale, J.; Pugh, K.; Pienkowski, D. Modified tibial nails for treating distal tibia fractures. J. Orthop. Trauma 2002, 16, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Staesche, M. Über die chemische Erzeugung einer dickeren magnesiumfluorid-schutzschicht auf magnesium-legierungen. Arch. Metallkunde 1948, 3, 99–102. [Google Scholar]
- Makar, G.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Verdier, S.; van der Laak, N.; Delalande, S.; Metson, J.; Dalard, F. The surface reactivity of magnesium-aluminium alloys in acidic fluoride solutions studied by electrochemical techniques and XPS. Appl. Surf. Sci. 2004, 235, 513–524. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ullmann, B.; Reifenrath, J.; Dziuba, D.; Seitz, J.-M.; Bormann, D.; Meyer-Lindenberg, A. In Vivo Degradation Behavior of the Magnesium Alloy LANd442 in Rabbit Tibiae. Materials 2011, 4, 2197-2218. https://doi.org/10.3390/ma4122197
Ullmann B, Reifenrath J, Dziuba D, Seitz J-M, Bormann D, Meyer-Lindenberg A. In Vivo Degradation Behavior of the Magnesium Alloy LANd442 in Rabbit Tibiae. Materials. 2011; 4(12):2197-2218. https://doi.org/10.3390/ma4122197
Chicago/Turabian StyleUllmann, Berit, Janin Reifenrath, Dina Dziuba, Jan-Marten Seitz, Dirk Bormann, and Andrea Meyer-Lindenberg. 2011. "In Vivo Degradation Behavior of the Magnesium Alloy LANd442 in Rabbit Tibiae" Materials 4, no. 12: 2197-2218. https://doi.org/10.3390/ma4122197



