Laser Fabrication of 3D Gelatin Scaffolds for the Generation of Bioartificial Tissues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Methacrylamide-Modified Gelatin (GelMod) and Its in vitro Degradation Behavior
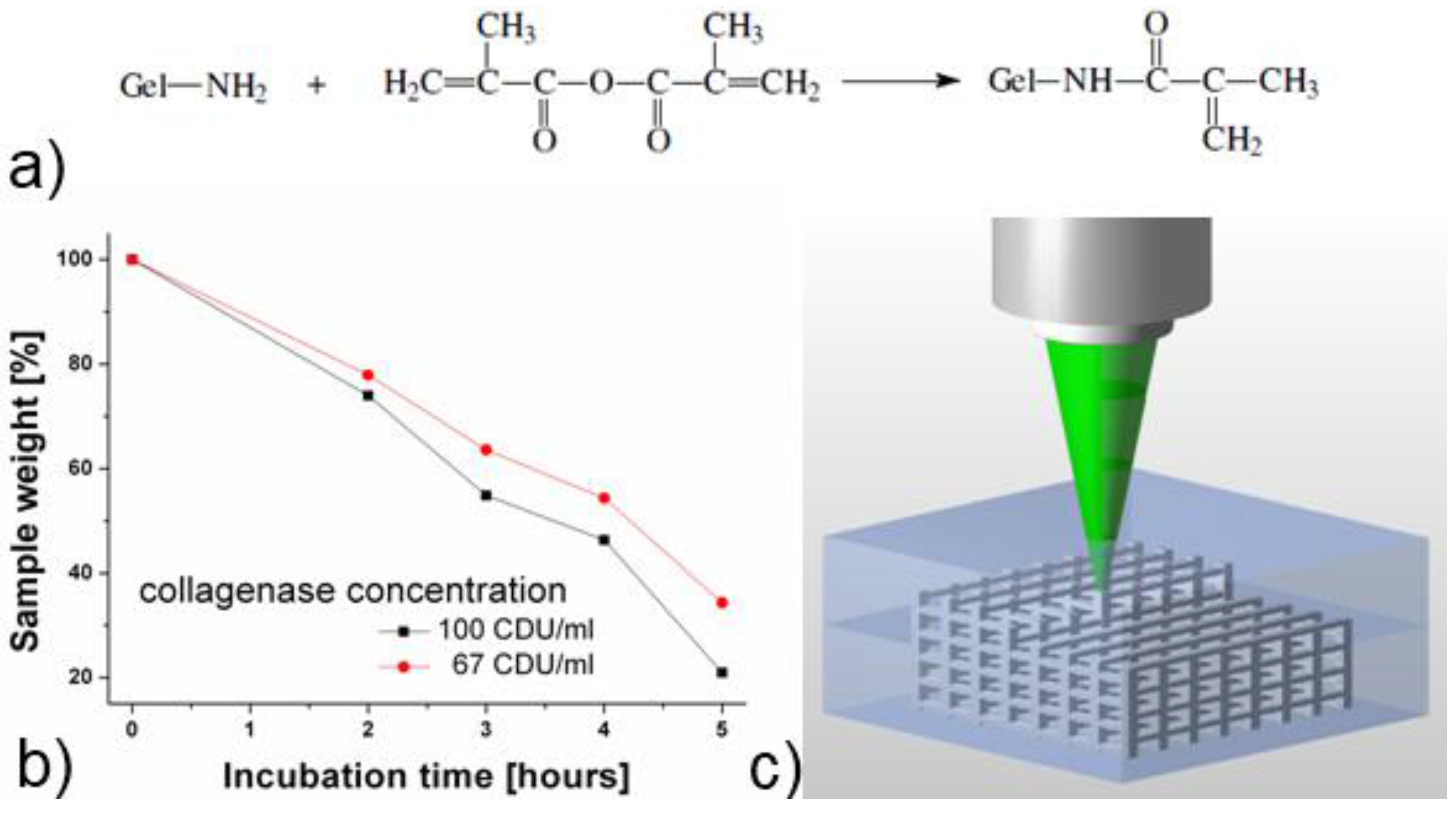
2.2. Scaffold Fabrication Using Two-Photon Polymerization (2PP)

2.3. Primary Human ASC Culture and Differentiation within the Developed Scaffolds
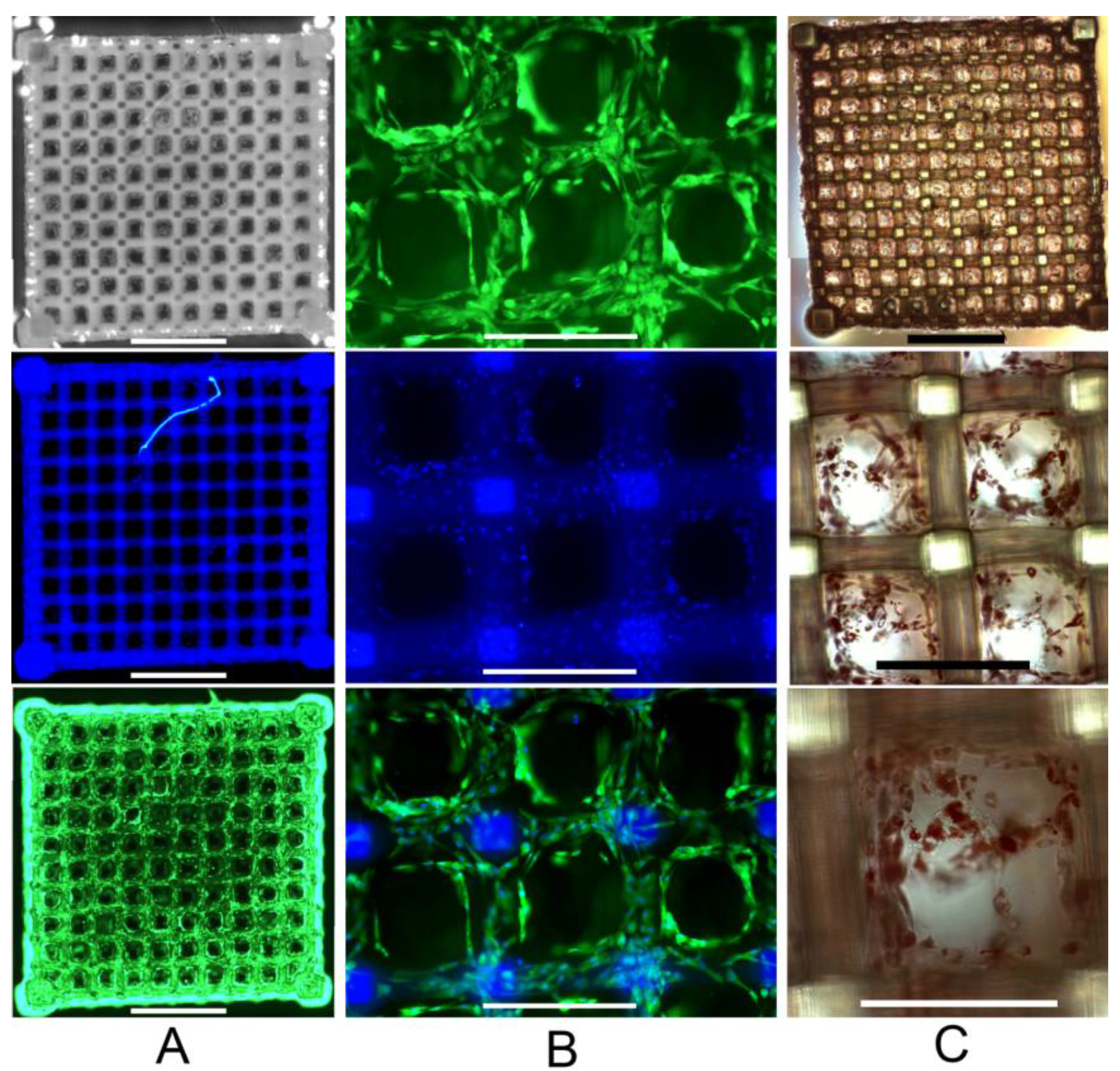
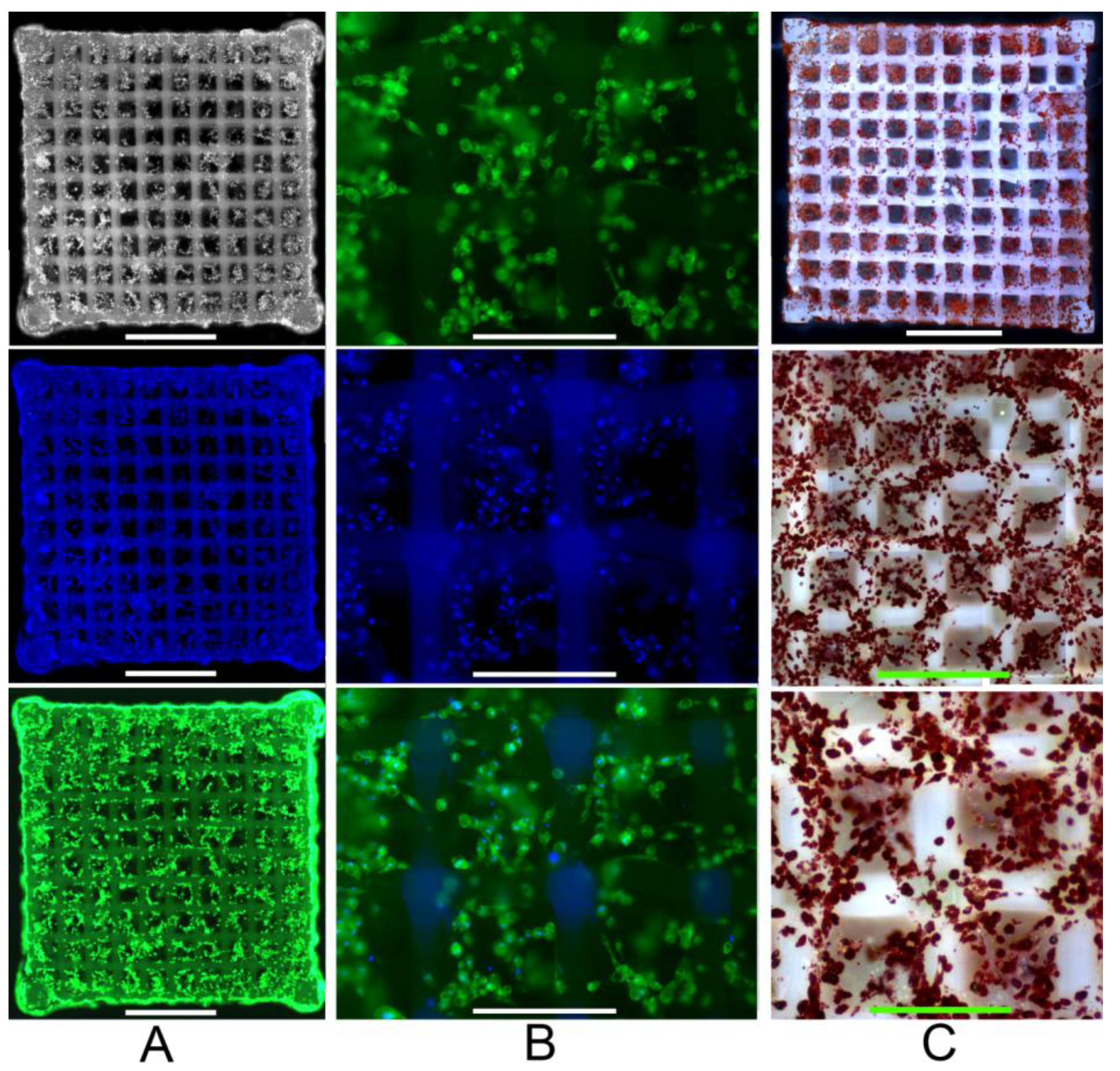
3. Experimental Section
3.1. Material Synthesis
3.2. In vitro Degradation Study
3.3. Scaffold Fabrication Using Two-Photon Polymerization (2PP)
3.4. Isolation and Culture of Human Adipose-Derived Stem Cells (ASCs)
3.5. Scaffold Seeding and ASC Differentiation
3.6. Cell Staining and Fluorescence Analysis
3.7. Oil Red O Staining for Fat Accumulation
4. Conclusions
Acknowledgements
References
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2010, 6, 41–75. [Google Scholar]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. B—Rev. 2010, 14, 61–86. [Google Scholar]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; De Wijn, J.R.; Van Blitterswijk, C.A.; De Groot, K. The effect of scaffold architecture on properties of direct 3D fiber deposition of porous Ti6Al4V for orthopedic implants. J. Biomed. Mater. Res. Part A 2010, 92A, 33–42. [Google Scholar] [CrossRef]
- Engelmayr, G.C.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Nanostructured Biomaterials for Regeneration. Adv. Funct. Mater. 2008, 18, 3568–3582. [Google Scholar] [CrossRef]
- Borenstein, J.T.; Weinberg, E.J.; Orrick, B.K.; Sundback, C.; Kaazempur-Mofrad, M.R.; Vacanti, J.P. Microfabrication of Three-Dimensional Engineered Scaffolds. Tissue Eng. 2007, 13, 1837–1844. [Google Scholar]
- Liu, T.V.; Bhatia, S.N. Three-dimensional tissue fabrication. Advan. Drug Delivery Rev. 2004, 56, 1635–1647. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.; Du, Z.; Chua, C. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2010, 8, 1–11. [Google Scholar]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends biotech. 2004, 22, 354–362. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Schlie, S.; Ngezahayo, A.; Haverich, A.; Chichkov, B.N. Two-photon polymerization technique for microfabrication of CAD-designed 3D scaffolds from commercially available photosensitive materials. J. Tissue Eng. Regen. Med. 2007, 1, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Malinauskas, M.; Schlie, S.; Chichkov, B.; Gittard, S.; Narayan, R.; Lobler, M.; Sternberg, K.; Schmitz, K.; Haverich, A. Three-dimensional laser micro- and nano-structuring of acrylated poly(ethylene glycol) materials and evaluation of their cytoxicity for tissue engineering applications. Acta Biomater. 2011, in press. [Google Scholar]
- Claeyssens, F.; Hasan, E.A.; Gaidukeviciute, A.; Achilleos, D.S.; Ranella, A.; Reinhardt, C.; Ovsianikov, A.; Shizhou, X.; Fotakis, C.; Vamvakaki, M.; Chichkov, B.N.; Farsari, M. Three-dimensional biodegradable structures fabricated by two-photon polymerization. Langmuir 2009, 25, 3219–3223. [Google Scholar] [CrossRef] [PubMed]
- Ritschdorff, E.T.; Shear, J.B. Multiphoton lithography using a high-repetition rate microchip laser. Anal. Chem. 2010, 82, 8733–8737. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.; Prestwich, G.D.; Semple, J.L.; Woodhouse, K.A. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials 2007, 28, 3834–3842. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, C.; Spruß, T.; Weiser, B.; Neubauer, M.; Becker, C.; Hacker, M.; Göpferich, A.; Blunk, T. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp. Cell Res. 2004, 300, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Patrick, C.; Chauvin, P.; Hobley, J.; Reece, G. Preadipocyte Seeded PLGA Scaffolds for Adipose Tissue Engineering. Tissue Eng. 2010, 5, 139–151. [Google Scholar] [CrossRef]
- Hong, L.; Peptan, I.; Clark, P.; Mao, J.J. Ex Vivo Adipose Tissue Engineering by Human Marrow Stromal Cell Seeded Gelatin Sponge. Ann. Biomed. Eng. 2005, 33, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, Y.; Yamashiro, H.; Yasuda, K.; Kimura, Y.; Inamoto, T.; Tabata, Y. In Situ Regeneration of Adipose Tissue in Rat Fat Pad by Combining a Collagen Scaffold with Gelatin Microspheres Containing Basic Fibroblast Growth Factor. Tissue Eng. 2010, 12, 1475–1487. [Google Scholar] [CrossRef]
- Hemmrich, K.; Von Heimburg, D.; Rendchen, R.; Di Bartolo, C.; Milella, E.; Pallua, N. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials 2005, 26, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.N.; Gobin, A.S.; West, J.L.; Patrick, C.W. Poly(ethylene glycol) Hydrogel System Supports Preadipocyte Viability, Adhesion, and Proliferation. Tissue Eng. 2010, 11, 1498–1505. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Won Rhie, J.; Mi Cho, H.; Yong Choi, C.; Kim, B. Engineering of volume-stable adipose tissues. Biomaterials 2005, 26, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Cnudde, V.; Dubruel, P.; Masschaele, B.; Cosijns, A.; De Paepe, I.; Jacobs, P.J.S.; Van Hoorebeke, L.; Remon, J.P.; Schacht, E. Porous gelatin hydrogels: 1. Cryogenic formation and structure analysis. Biomacromolecules 2007, 8, 331–337. [Google Scholar]
- Van den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.; Raeber, G.; Zisch, A.; Tirelli, N.; Hubbell, J. Cell-Responsive Synthetic Hydrogels. Adv. Mater. 2003, 15, 888–892. [Google Scholar] [CrossRef]
- Moroni, L.; Schotel, R.; Hamann, D.; De Wijn, J.; Van Blitterswijk, C. 3D fiber-deposited electrospun integrated scaffolds enhance cartilage tissue formation. Adv. Funct. Mater. 2008, 18, 53–60. [Google Scholar] [CrossRef]
- The Science and Technology of Gelatin / edited by A. G. Ward, A. Courts; Academic Press: London, UK, 1977.
- Masuda, T.; Furue, M.; Matsuda, T. Photocured, styrenated gelatin-based microspheres for de novo adipogenesis through corelease of basic fibroblast growth factor, insulin, and insulin-like growth factor I. Tissue Eng. 2010, 10, 523–535. [Google Scholar] [CrossRef]
© 2011 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Pflaum, M.; Wilhelmi, M.; Dubruel, P.; Chichkov, B. Laser Fabrication of 3D Gelatin Scaffolds for the Generation of Bioartificial Tissues. Materials 2011, 4, 288-299. https://doi.org/10.3390/ma4010288
Ovsianikov A, Deiwick A, Van Vlierberghe S, Pflaum M, Wilhelmi M, Dubruel P, Chichkov B. Laser Fabrication of 3D Gelatin Scaffolds for the Generation of Bioartificial Tissues. Materials. 2011; 4(1):288-299. https://doi.org/10.3390/ma4010288
Chicago/Turabian StyleOvsianikov, Aleksandr, Andrea Deiwick, Sandra Van Vlierberghe, Michael Pflaum, Mathias Wilhelmi, Peter Dubruel, and Boris Chichkov. 2011. "Laser Fabrication of 3D Gelatin Scaffolds for the Generation of Bioartificial Tissues" Materials 4, no. 1: 288-299. https://doi.org/10.3390/ma4010288




