Influence of the Structural Properties of Mesoporous Silica on the Adsorption of Guest Molecules
Abstract
:1. Introduction
2. Results and Discussion
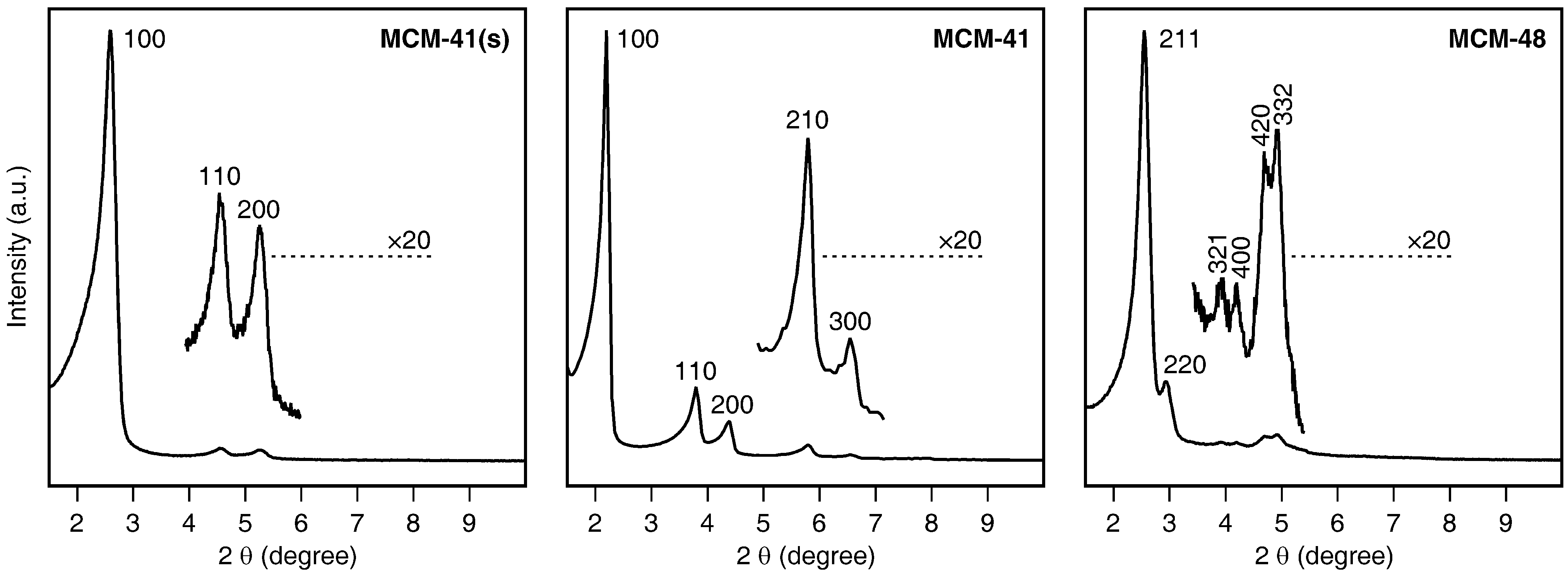
2.1. Mesoporous Silica
| dBJH [nm] | SBET [m2/g] | SExt [m2/g] | Vtot [cm3/g] | VP [cm3/g] | |
|---|---|---|---|---|---|
| MCM-41(s) | 1.96 | 776 | 35 | 0.48 | 0.44 |
| MCM-48 | 2.52 | 1320 | 173 | 0.98 | 0.86 |
| MCM-41 | 2.82 | 872 | 66 | 0.74 | 0.67 |
| SBA-15 | 6.46 | 908 | 74 | 1.21 | 1.09 |
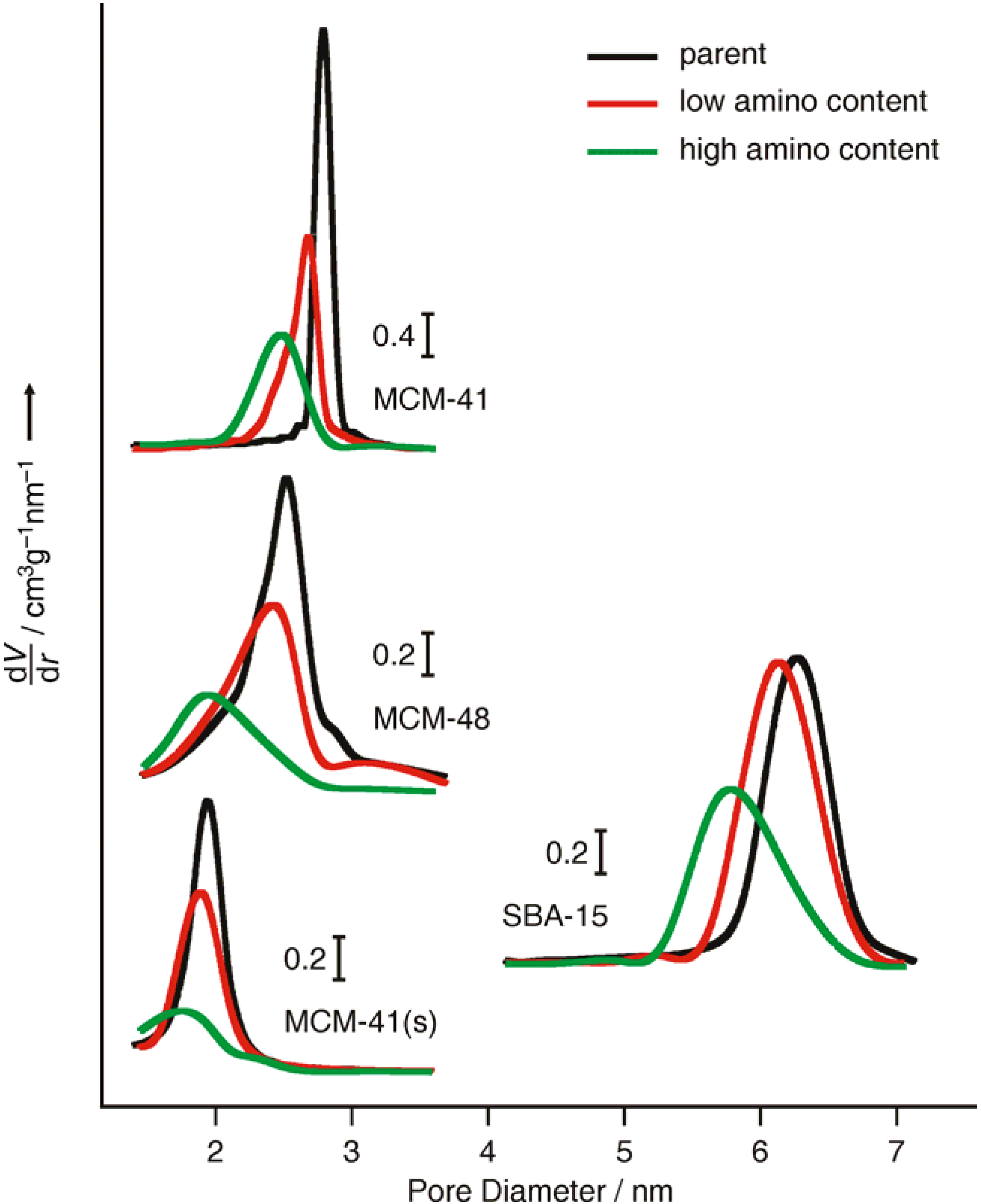
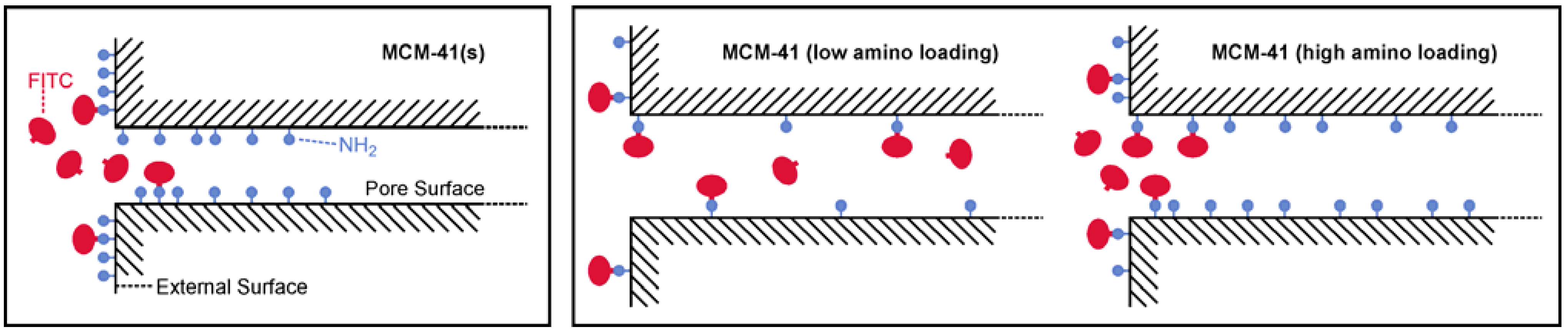
2.2. Loading with FITC and Fluorescein

3. Experimental Section
3.1. Synthesis of Mesoporous Silica
3.2. Reaction with APTMS
3.3. Loading with FITC and Fluorescein
3.4. Amino Group Analysis
3.5. Physical Measurements
4. Conclusions
Acknowledgements
References and Notes
- Moller, K.; Bein, T. Inclusion chemistry in periodic mesoporous hosts. Chem. Mater. 1998, 10, 2950–2963. [Google Scholar] [CrossRef]
- Scott, B.J.; Wirnsberger, G.; Stucky, G.D. Mesoporous and mesostructured materials for optical applications. Chem. Mater. 2001, 13, 3140–3150. [Google Scholar] [CrossRef]
- Schulz-Ekloff, G.; Wöhrle, D.; van Duffel, B.; Schoonheydt, R.A. Chromophores in porous silicas and minerals: preparation and optical properties. Microporous Mesoporous Mater. 2002, 51, 91–138. [Google Scholar] [CrossRef]
- Brühwiler, D.; Calzaferri, G.; Torres, T.; Ramm, J.H.; Gartmann, N.; Dieu, L.-Q.; López-Duarte, I.; Martínez-Díaz, M.V. Nanochannels for supramolecular organization of luminescent guests. J. Mater. Chem. 2009, 19, 8040–8067. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Delivery Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593–5604. [Google Scholar] [CrossRef]
- Hartmann, M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltrimethy lammonium-kanemite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Gao, F.; Botella, P.; Corma, A.; Blesa, J.; Dong, L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J. Phys. Chem. B 2009, 113, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Nie, S. Doping mesoporous materials with multicolor quantum dots. J. Phys. Chem. B 2003, 107, 11575–11578. [Google Scholar] [CrossRef]
- Molenkamp, W.C.; Watanabe, M.; Miyata, H.; Tolbert, S.H. Highly polarized luminescence from optical quality films of a semiconducting polymer aligned within oriented mesoporous silica. J. Am. Chem. Soc. 2004, 126, 4476–4477. [Google Scholar] [CrossRef] [PubMed]
- Brühwiler, D.; Gfeller, N.; Calzaferri, G. Resorufin in the channels of zeolite L. J. Phys. Chem. B 1998, 102, 2923–2929. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of nanoporous materials from adsorption and desorption isotherms. Colloids Surf. A: Physicochem. Eng. Aspects 2001, 187-188, 11–21. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Adsorption study of surface and structural properties of MCM-41 materials of different pore sizes. J. Phys. Chem. B 1997, 101, 583–589. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Di Renzo, F.; Fajula, F. True microporosity and surface area of mesoporous SBA-15 as a function of synthesis temperature. Langmuir 2001, 17, 8328–8335. [Google Scholar] [CrossRef]
- Gartmann, N.; Brühwiler, D. Controlling and imaging the functional-group distribution on mesoporous silica. Angew. Chem. Int. Ed. 2009, 48, 6354–6356. [Google Scholar] [CrossRef]
- Gartmann, N.; Schütze, C.; Ritter, H.; Brühwiler, D. The effect of water on the functionalization of mesoporous silica with 3-aminopropyltriethoxysilane. J. Phys. Chem. Lett. 2010, 1, 379–382. [Google Scholar] [CrossRef]
- Ramm, J.H.; Gartmann, N.; Brühwiler, D. Direct synthesis and fluorescent imaging of bifunctionalized mesoporous iodopropyl-silica. J. Colloid Interf. Sci. 2010, 345, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Wirnsberger, G.; Scott, B.J.; Stucky, G.D. pH Sensing with mesoporous thin films. Chem. Commun. 2001, 119–120. [Google Scholar]
- Lin, Y.-S.; Tsai, C.-P.; Huang, H.-Y.; Kuo, C.-T.; Hung, Y.; Huang, D.-M.; Chen, Y.-C.; Mou, C.-Y. Well-ordered mesoporous silica nanoparticles as cell markers. Chem. Mater. 2005, 17, 4570–4573. [Google Scholar] [CrossRef]
- Hsiao, J.-K.; Tsai, C.-P.; Chung, T.-H.; Hung, Y.; Yao, M.; Liu, H.-M.; Mou, C.-Y.; Yang, C.-S.; Chen, Y.-C.; Huang, D.-M. Mesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell tracking. Small 2008, 4, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-P.; Chen, C.-Y.; Hung, Y.; Chang, F.-H.; Mou, C.-Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J. Mater. Chem. 2009, 19, 5737–5743. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Haynes, C.L. Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles. Chem. Mater. 2009, 21, 3979–3986. [Google Scholar] [CrossRef]
- Ritter, H.; Brühwiler, D. Accessibility of amino groups in postsynthetically modified mesoporous silica. J. Phys. Chem. C 2009, 113, 10667–10674. [Google Scholar] [CrossRef]
- Miyajima, T.; Abry, S.; Zhou, W.; Albela, B.; Bonneviot, L.; Oumi, Y.; Sano, T.; Yoshitake, H. Estimation of spacing between 3-bromopropyl functions grafted on mesoporous silica surfaces by a substitution reaction using diamine probe molecules. J. Mater. Chem. 2007, 17, 3901–3909. [Google Scholar] [CrossRef]
- Yoshitake, H. Design of functionalization and structural analysis of organically-modified siliceous oxides with periodic structures for the development of sorbents for hazardous substances. J. Mater. Chem. 2010, 20, 4537–4550. [Google Scholar] [CrossRef]
- Brühwiler, D. Postsynthetic functionalization of mesoporous silica. Nanoscale 2010, 2, 887–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Pérez-Pariente, J.; Vallet-Regí, M. Influence of pore size of MCM-41 matrices on drug delivery rate. Microporous Mesoporous Mater. 2004, 68, 105–109. [Google Scholar] [CrossRef]
- Kanan, S.M.; Tze, W.T.Y.; Tripp, C.P. Method to double the surface concentration and control the orientation of adsorbed (3-aminopropyl)dimethylethoxysilane on silica powders and glass slides. Langmuir 2002, 18, 6623–6627. [Google Scholar] [CrossRef]
- Xu, J.; Luan, Z.; He, H.; Zhou, W.; Kevan, L. A reliable synthesis of cubic mesoporous MCM-48 molecular sieve. Chem. Mater. 1998, 10, 3690–3698. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Ritter, H.; Nieminen, M.; Karppinen, M.; Brühwiler, D. A comparative study of the functionalization of mesoporous silica MCM-41 by deposition of 3-aminopropyltrimethoxysilane from toluene and from the vapor phase. Microporous Mesoporous Mater. 2009, 121, 79–83. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ryoo, R.; Kim, J.M. Monitoring of the structure of siliceous mesoporous molecular sieves tailored using different synthesis conditions. Microporous Mater. 1997, 12, 93–106. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ritter, H.; Ramm, J.H.; Brühwiler, D. Influence of the Structural Properties of Mesoporous Silica on the Adsorption of Guest Molecules. Materials 2010, 3, 4500-4509. https://doi.org/10.3390/ma3084500
Ritter H, Ramm JH, Brühwiler D. Influence of the Structural Properties of Mesoporous Silica on the Adsorption of Guest Molecules. Materials. 2010; 3(8):4500-4509. https://doi.org/10.3390/ma3084500
Chicago/Turabian StyleRitter, Hanna, Jan Hinrich Ramm, and Dominik Brühwiler. 2010. "Influence of the Structural Properties of Mesoporous Silica on the Adsorption of Guest Molecules" Materials 3, no. 8: 4500-4509. https://doi.org/10.3390/ma3084500