A Comprehensive Review on Separation Methods and Techniques for Single-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Terminology of SWNTs
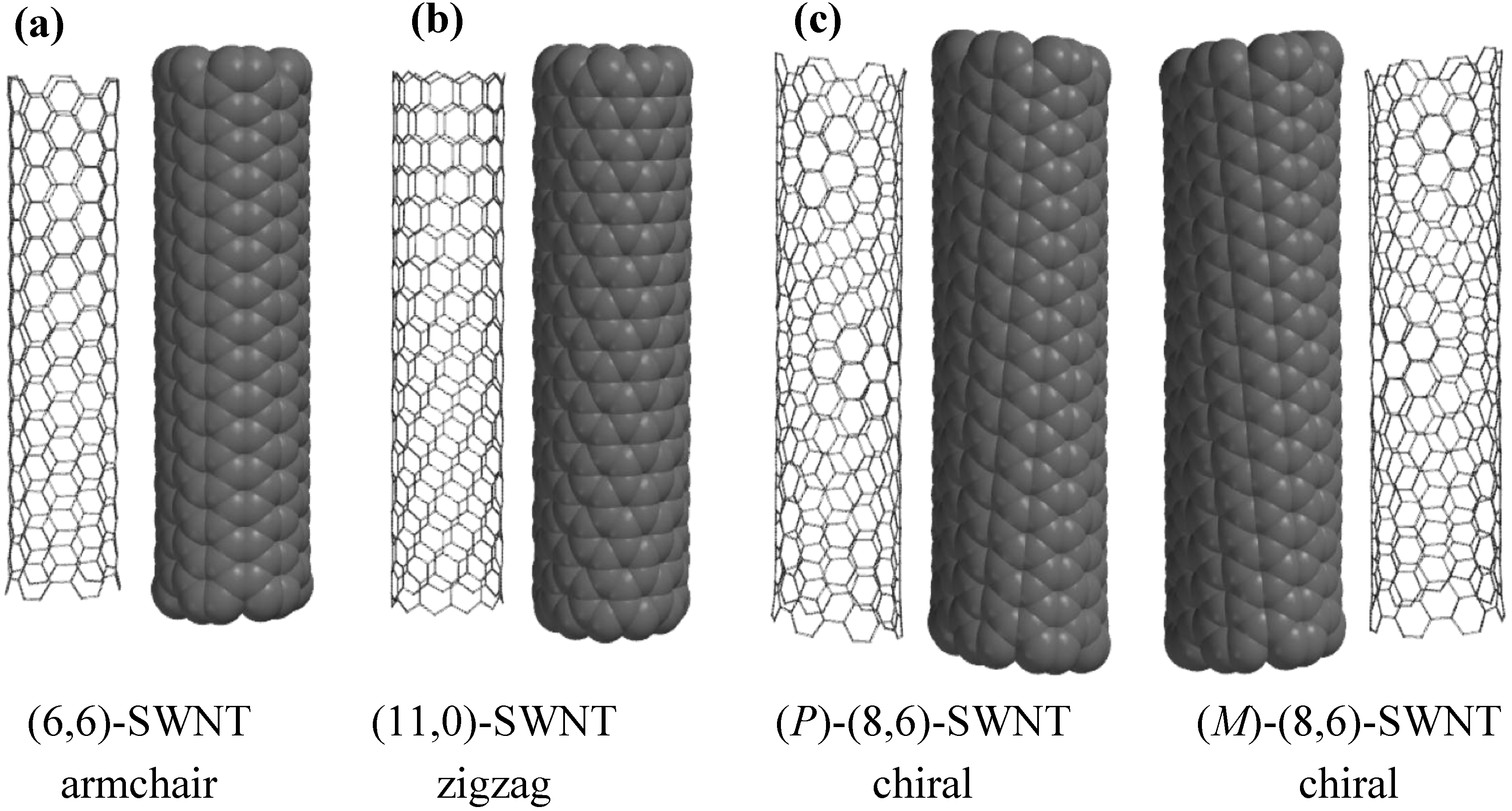
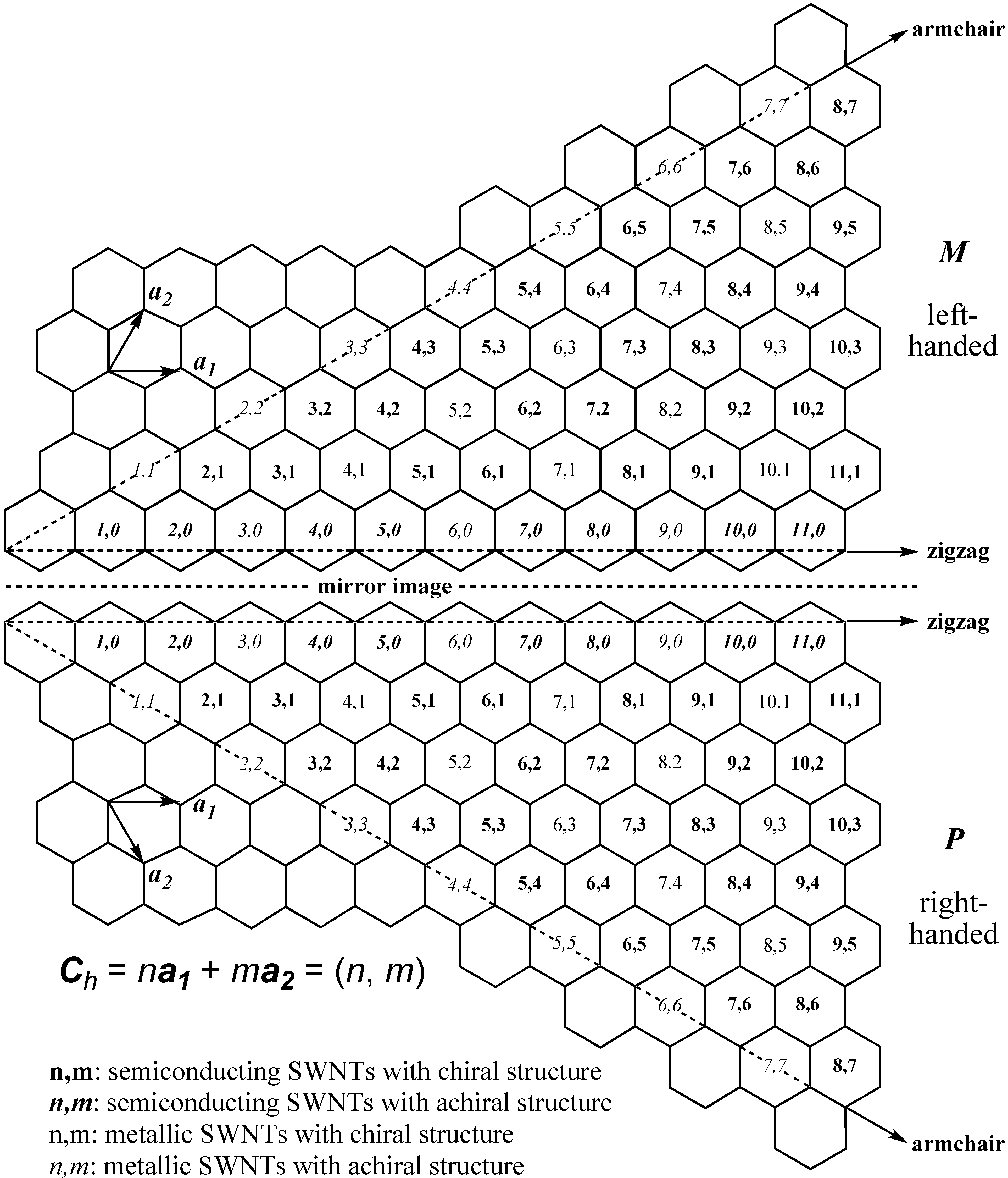

3. Physical Methods for Separation of Carbon Nanotubes
3.1. Electrophoresis
| Reference | Separated object | SWNTs | Chemicals |
| [39,40] | M/S | HiPco, LV | SDS/D2O |
| [41] | M/S | LV | DMF |
| [42] | M/S | CVD | DMF |
| [43] | M/S | LV | SC/D2O |
| [44] | length, diameter | HiPco | SC/water |
| [45] | length | LV, HiPco | polyvinyl pyrrolidone (PVP) |
| [46] | length | AD | SDS/water |
| [47] | diameter | HiPco | SDS or PVP |
| [48] | M/S | HiPco | SDS/water |
| [49] | M/S, diameter | HiPco | SDBS/water |
| [50,51,52] | M/S | HiPco | SDS/water |
| [53,54,55] | M/S | LV, AD, HiPco | SDS/water |
| [56] | M/S | AD | SDS/water |
| [57] | M/S | HiPco | SDBS/D2O |
| [58,59] | M/S | HiPco, LV | Triton X-100, water |
| [60] | M/S | HiPco | SDS, cetyltrimethylammonium bromide |
| [61] | M/S | AD | Gum Arabic or PVP |
| [62] | diameter, length | HiPco | DNA or RNA |
3.2. Centrifugation
| Reference | Separated object | SWNTs | Chemicals |
| [40,64,65,68,69,70,71,72,73,74,75,76] | M/S, diameter, (6,5), (7,5) | CoMoCAT, LV, HiPco, AD | SDS, SC/iodixanol in water, DNA/iodixanol in water |
| [77,78] | M/S | LV, HiPco, CoMoCAT | sodium deoxycholate (SDC)/ iodixanol in water |
| [25] | handedness, (6,4), (6,5) | CoMoCAT, AD | SC/iodixanol in water |
| [30] | handedness, (6,4), (6,5), (7,3), (9,1), (8,3), (9.2), (7,5), (8,4), (10,2), (7,6) | HiPco | SC, SDS/iodixanol in water |
| [66] | (7,5), (7,6), (10,5), (9,7) | LV, HiPco, CoMoCAT | fluorene-based polymer/chlorobenzene + tribromotoluene |
| [79] | M/S, diameter | HiPco | SDS, NaCl/iodixanol in water |
| [80] | M/S, diameter | AD | sodium taurodeoxycholic acid, SDS, SC/iodixanol in water |
| [81] | M/S | AD | SC, SDS/sucrose in water |
| [82] | (6,5) | Co-MCM-41 | SC, SDS/iodixanol in water |
| [83] | diameter | HiPco | SDS, PVP/water |
| [84,85] | diameter | AD | SC/iodixanol in water |
| [86] | DWNTs | Mixture of SWNTs, DWNTs and MWNTs | SC/iodixanol in water |
| [87] | short length (about 7.5 nm) | HiPco | PL-PEG/iodixanol in water |
| [88] | length | CoMoCAT, HiPco, LV | SDC/iodixanol in water |
| [53,67] | M/S | LV, AD, HiPco | SDS/agarose gel |
| [89] | (n, m) | CoMoCAT, HiPco, LV | SC/iodixanol in water |
| [90] | length | CoMoCAT, HiPco, LV | SDC/iodixanol in water |
4. Chemical Methods for Separation of Carbon Nanotubes
4.1. Chromatography
| Reference | Separated object | SWNTs | Chemicals |
| [104] | (8, 4) | FeRu-CVD | DNA, IEC |
| [105] | (6, 4), (9, 1), (6, 5) | CoMoCAT | DNA, SEC + IEC |
| [96,106] | (n, m) | HiPco | DNA, IEC |
| [94,95,107,108,109] | M/S, diameter, length | HiPco | DNA, IEC |
| [110] | (6, 5) | CoMoCAT | DNA, IEC |
| [111] | M/S, diameter, (n, m) | HiPco | DNA, SEC + IEC |
| [112,113,114] | length | DNA, SEC | |
| [115] | length | HiPco | octadecylamine/THF, GPC |
| [91,92,93,116] | length | LV, AD, MWNTs | SDS, SEC |
| [117] | length | LV | Triton X-100, SEC |
| [44,118] | length, diameter | HiPco, LV | SC, SEC |
| [119] | length | CoMoCAT, HiPco, LV, AD | DNA, SEC |
| [120] | M/S | HiPco functionalized with t-aryl groups group | SDS/o-dichlorobenzene, silica gel chromatography |
| [121] | M/S | HiPco | SDS/agarose gel beads |
| [40] | M/S | LV | SDS, SC/SEC |
| [97]a | length | LV | Triton X-100, water |
| [98]a | length | AD (SWNTs), CVD (MWNTs) | SDS, water |
| [99]a | length | AD | Triton X-100, water |
| [100]a | length | CNT (Carbolex) | Triton X-100, water |
| [101]a | length | CoMoCAT, HiPco, LV, AD | DNA, water |
| [102]a | length | functionalized MWNTs | water (pH = 10) |
| [103]b | M/S | HiPco | DNA, water |
4.2. Selective Solubilization
| Reference | Separated object | SWNTs | Chemicals |
| [53,140] | M/S | LV | SDS/water |
| [124,125,126,127] | M/S | LV, HiPco | octadecylamine, octadecylamine/THF |
| [128,129,130] | M/S (87% M) | HiPco, CoMoCAT | octylamine/THF |
| [141] | M/S | AD | amine- or phenyl-terminated SiO2 |
| [142,143] | M/S | LV, 1.1-1.6 nm | bromine, triton X-100/water |
| [130,144,145] | M/S | AD | porphyrin/CHCl3, pyrene/THF |
| [146,147] | (8, 6) 85% | HiPco | flavin mononucleotide/D2O |
| [131] | (7, 5) | Co-MCM-41 | fluorene-based polymers/toluene |
| [132,133] | (7, 5), (8, 6), (10, 5) | CoMoCAT, HiPco | fluorene-based polymers/toluene, xylene, THF, chloroform |
| [148,149] | (8, 6), (7, 6) diameter | HiPco | pentacene-quaterrylene- and naphthopentaphene-based amphiphiles, SDS/water |
| [66] | (7, 5), (7, 6), (10, 5), (9, 7) | LV, HiPco, CoMoCAT | fluorene-based polymer/chlorobenzene + tribromotoluene |
| [89] | (n, m) | LV, HiPco, CoMoCAT | fluorene-based polymer/toluene |
| [134,135,136,137] | diameter, M/S | AD, HiPco | poly(phenylenevinylene)/toluene |
| [138] | (11,6), (11,7), (12,6) | HiPco | poly(phenylenevinylene)/THF |
| [150,151] | diameter | HiPco | reversible cyclic peptide/water |
| [152] | diameter | AD | η-cyclodextrin/D2O |
| [153] | diameter | SWNTs | pentacene-based molecular tweezers/toluene |
| [154] | M/S | AD | potassium salt of coronene tetracarboxylic acid/water |
| [155] | diameter | HiPco | chitosan polymer/water |
| [156] | diameter | HiPco | porphyrinic polypeptides/DMF |
| [157] | diameter | HiPco | ruthenium metallodendrimer/DMF |
| [27] | helicity, diameter | CoMoCAT | chiral monoporphyrin/methanol |
| [26,28,29,34] | helicity, (n, m) | CoMoCAT | chiral nanotweezers/metanol |
| [158,159] | diameter | HiPco, AD | diamine-terminated oligomeric poly(ethylene glycol)/water |
| [160] | length | HiPco | tetraoctylammonium bromide/ethyl acetate or toluene |
| [161] | (8, 4), diameter | CoMoCAT, HiPco | heparin/water heparin, SDBS/water |
| [162] | M/S | CoMoCAT | DNA/water |
| [130,163] | M/S, diameter | HiPco | pyrene derivative/water |
| [164] | diameter | CoMoCAT, HiPco | pyrene derivative/water |
| [165] | diameter | HiPco | SDS, SDBS or SC in water |
| [166] | diameter | HiPco | ClSO3H/CH3SO3H |
4.3. Selective Reaction
| Reference | Separated object | SWNTs | Chemicals |
| [130,168] | M/S | HiPco | H2O2/water at 90 °C |
| [169] | diameter, (n, m) | HiPco | air at 450 °C, H2O2/water at 90 °C |
| [170] | diameter | HiPco | H2O2, light irradiation |
| [171] | (n, m) | HiPco, CoMoCAT | H2O2, SC or SC/SDS in D2O |
| [172] | diameter | HiPco | H2O2 + light |
| [173] | M/S | HiPco | OsO4, UV |
| [182] | M/S | AD | NaClOx/1-methyl-2-pyrrolidone |
| [174] | M/S, diameter | HiPco | acid mixture (H2SO4/HNO3) under microwave irradiation |
| [175] | M/S | HiPco | acid mixture (H2SO4/HNO3) |
| [176,177] | diameter | HiPco | acid mixture (H2SO4/HNO3) at 35–55 °C under sonication |
| [178] | M/S | HiPco | HNO3 at 135 °C |
| [179,180] | diameter | HiPco | ozone in methanol at −78 °C |
| [181] | (6, 5) | HiPco | AuCl4–, SC/water |
| [183] | diameter | LV | air at 350–550 °C, HNO3 at 120 °C |
| [184] | HiPco | air at 460–620 °C | |
| [185,186] | M/S | HiPco | NO2SbF6 or NO2BF4 in tetramethylene sulfone/chloroform |
| [187] | M/S, diameter | SWNTs | NO2 |
| [188,189] | M/S | HiPco | dichlorocarbene in dichlorobenzene |
| [190,194,197,198,199] | M/S | HiPco | diazonium salt/water |
| [191] | M/S | AD | diazonium salt of 4-heptadecafluorooctyl-aniline/perfluorohexane |
| [192,195] | M/S | HiPco, AD | 4-nitrobenzenediazonium salt in DMF, 4-aminobenzylamine |
| [193,200] | M/S | CVD | 4-bromobenzenediazonium tetrafluoroborate in water |
| [58,109,201] | M/S | HiPco | 4-hydroxybenzenediazonium salt |
| [196] | M/S | HiPco | SC, 4-dodecyloxybenzenediazonium tetrafluoroborate in water |
| [202] | M/S, diameter | HiPco | SDS, 4-chloro- and 4-nitrophenyldiazonium salts in water |
| [203] | M/S, diameter | HiPco | fluorine gas |
| [204] | M/S | HiPco | triethylsilane at room temperature |
| [205] | M/S | HiPco | perfluoro 2-(fluorosulfonylethoxy)propyl vinyl ether at 215 °C |
| [206] | M/S | HiPco | SO3 at 400 °C |
| [130,208] | M/S | HiPco | azomethine ylide/THF at 65 °C |
| [207] | M/S, diameter | HiPco | RLi, RMgX in cyclohexane |
| [209,210,211] | M/S | MWNTs, SWNTs | current-induced electrical breakdown |
| [212] | M/S | CVD | methane plasma at 400 °C/annealing at 600 °C |
| [213] | M/S | AD | hydrogen plasma |
| [214] | (n, m) | CVD | laser irradiation |
| [215] | M/S | CoMoCAT, HiPco, LV | laser irradiation |
| [218] | M/S | Fe-catalyzed CVD | Xe lamp |
| [216] | CoMoCAT | microwave irradiation | |
| [174,217] | M/S, diameter | HiPco | microwave irradiation |
| [219] | diameter | HiPco | Li at 473 °C |
| [220] | (n, m) | HiPco | SDS in D2O/salt (NaCl, MgSO4, ErCl3) |
| [221] | diameter | HiPco | LiClO4, (CH3)4NBF4, n-Bu4NClO4, n-Oct4NClO4, ionic liquid in CH3CN |
| [222] | M/S | AD | aromatic or aliphatic solvent with electron-withdrawing or -donating groups |
| [223] | (n, m) | HiPco | TCNQ, TFTCNQa, mordant yellow 10 and ABb |
| [224] | M/S | (10, 0), (6, 6) | naphthalene, anthracene, TCNQ and DDQ |
5. Concluding Remarks

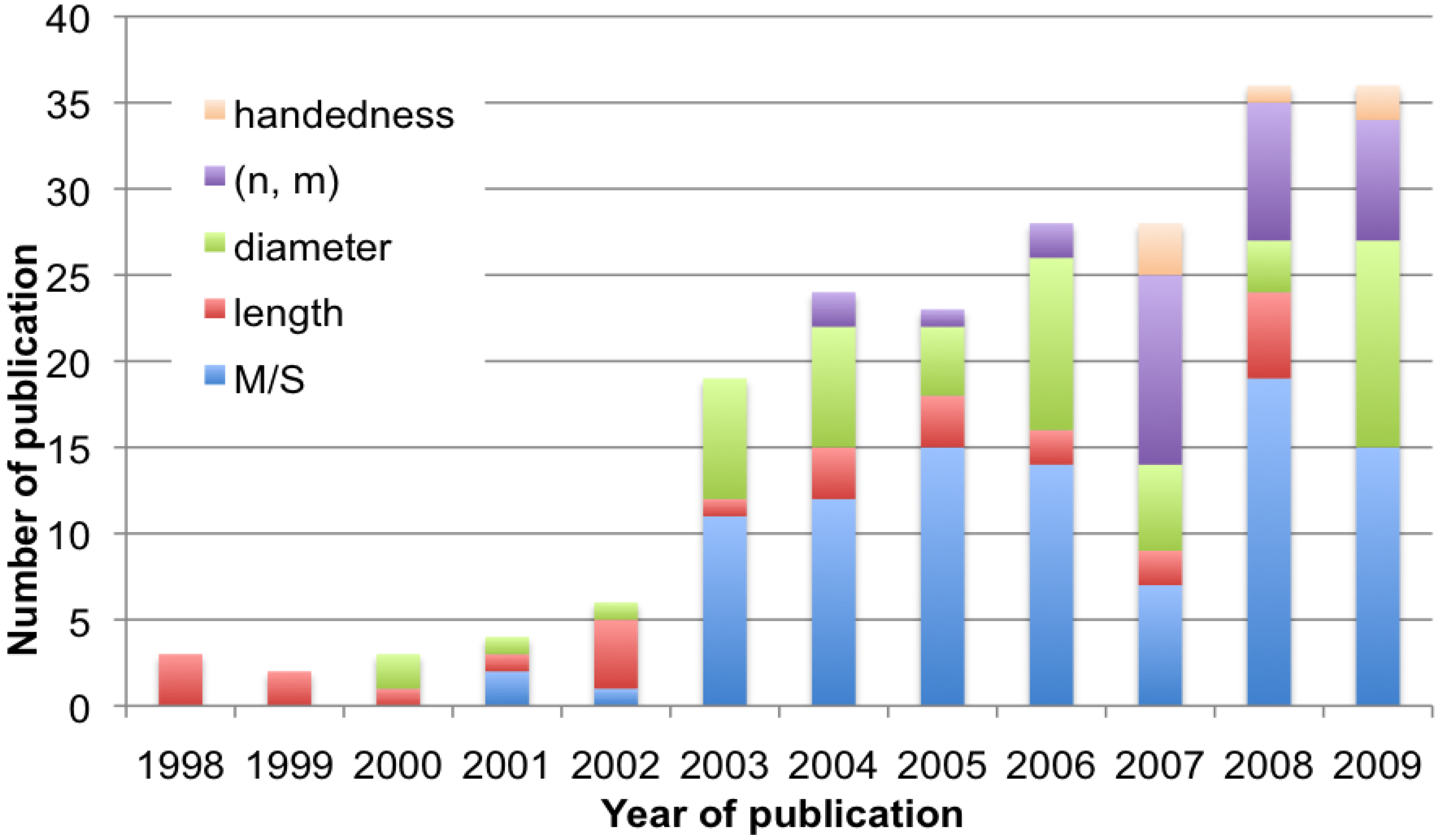
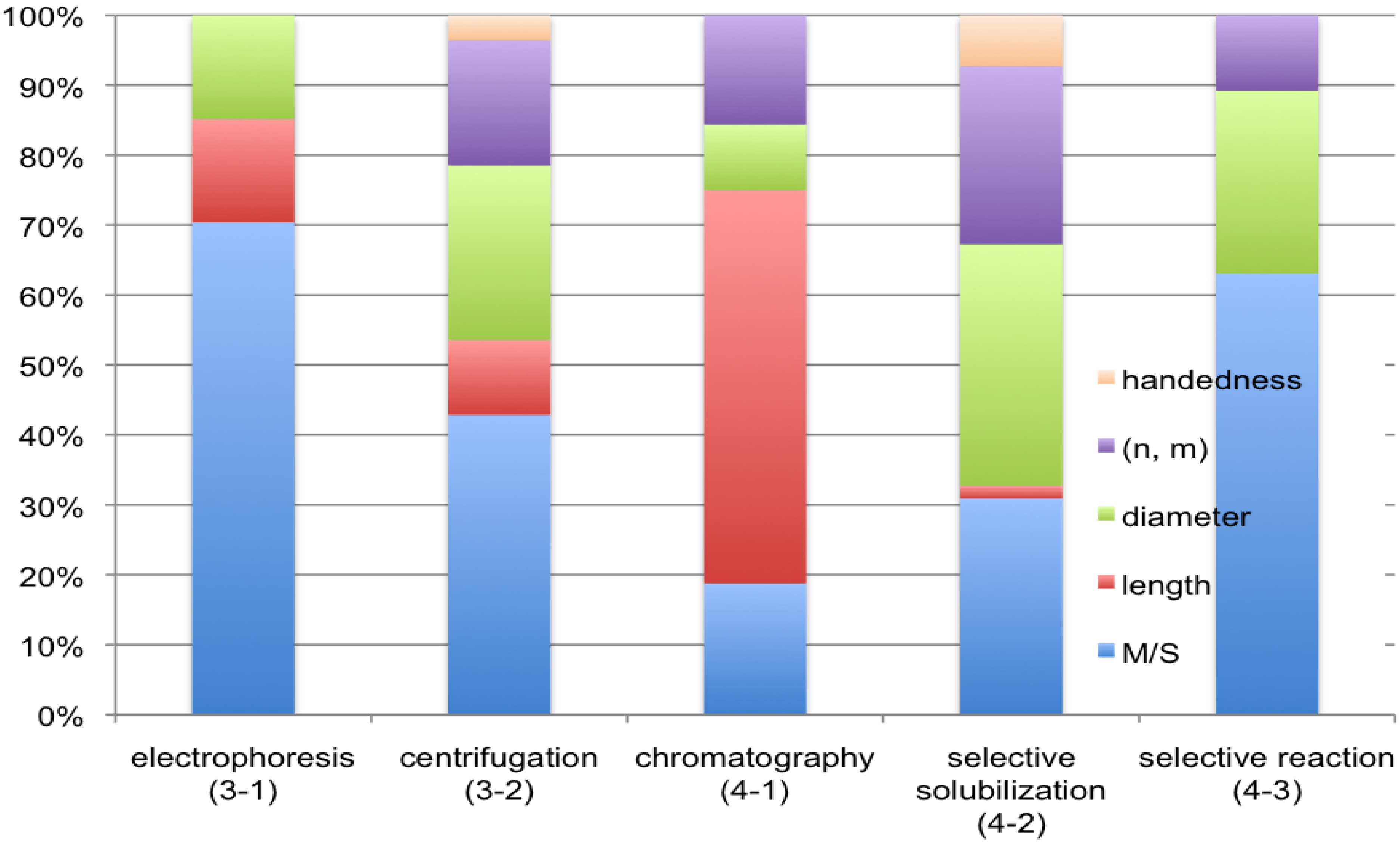
References and Notes
- Bachilo, S.M.; Balzano, L.; Herrera, J.E.; Pompeo, F.; Resasco, D.E.; Weisman, R.B. Narrow (n, m)-distribution of single-walled carbon nanotubes grown using a solid supported catalyst. J. Am. Chem. Soc. 2003, 125, 11186–11187. [Google Scholar] [CrossRef]
- Wang, B.; Poa, C.H.P.; Wei, L.; Li, L.J.; Yang, Y.; Chen, Y. (n, m) selectivity of single-walled carbon nanotubes by different carbon precursors on co-mo catalysts. J. Am. Chem. Soc. 2007, 129, 9014–9019. [Google Scholar] [CrossRef] [PubMed]
- Ciuparu, D.; Chen, Y.; Lim, S.; Haller, G.L.; Pfefferle, L. Uniform-diameter single-walled carbon nanotubes catalytically grown in cobalt-incorporated mcm-41. J. Phys. Chem. B 2004, 108, 503–507. [Google Scholar] [CrossRef]
- Luo, Z.T.; Pfefferle, L.D.; Haller, G.L.; Papadimitrakopoulos, F. (n, m) abundance evaluation of single-walled carbon nanotubes by fluorescence and absorption spectroscopy. J. Am. Chem. Soc. 2006, 128, 15511–15516. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Kobayashi, Y.; Homma, Y. Carbon nanotube growth from diamond. J. Am. Chem. Soc. 2009, 131, 6922–6923. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ren, W.; Gao, L.; Li, S.; Pei, S.; Liu, C.; Jiang, C.; Cheng, H.M. Metal-catalyst-free growth of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 2082–2083. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cai, Q.; Chen, J.; Qian, Y.; Zhang, L. Metal-catalyst-free groth of single-walled carbon nanotubes on substrates. J. Am. Chem. Soc. 2009, 131, 2094–2095. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A. Groth of single-walled carbon nanotubes without a metal catalyst—A surprising discovery. Angew. Chem. Int. Ed. 2009, 48, 5403–5404. [Google Scholar] [CrossRef]
- Liu, B.; Ren, W.; Liu, C.; Sun, C.H.; Gao, L.; Li, S.; Jiang, C.; Cheng, H.M. Growth velocity and direct length-sorted growth of short single-walled carbon nanotubes by a metal-catalyst-free chemical vapor deposition process. ACS Nano 2009, 3, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, A.R.; Chen, G.; Paronyan, T.M.; Pigos, E.M.; Kuznetsov, O.A.; Hewaparakrama, K.; Kim, S.M.; Zakharov, D.; Stach, E.A.; Sumanasekera, G.U. Preferential growth of single-walled carbon nanotubes with metallic conductivity. Science 2009, 326, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zhang, B.; Peng, B.; Zhang, J.; Choi, W.M.; Choi, J.Y.; Kim, J.M.; Liu, Z. Direct groth of semiconducting single-walled carbon nanotube array. J. Am. Chem. Soc. 2009, 131, 14642–14643. [Google Scholar] [CrossRef] [PubMed]
- Kharisov, B.I.; Kharissova, O.V.; Gutierrez, H.L.; Méndez, U.O. Recent advances on the soluble carbon noatubes. Ind. Eng. Chem. Res. 2009, 48, 572–590. [Google Scholar] [CrossRef]
- Nakashima, N.; Fujigaya, T. Fundamentals and applications of soluble carbon nanotubes. Chem. Lett. 2007, 36, 692–697. [Google Scholar] [CrossRef]
- Nakashima, N. Soluble carbon nanotubes. Int. J. Nanosci 2005, 4, 119–137. [Google Scholar] [CrossRef]
- Murakami, H.; Nakashima, N. Soluble carbon nanotubes and their applications. J. Nanosci. Nanotechnol. 2006, 6, 16–27. [Google Scholar] [PubMed]
- Liu, P. Modifications of carbon nanotubes with polymers. Eur. Polym. J. 2005, 41, 2693–2703. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Georgakilas, V.; Prato, M. Soluble carbon nanotubes. Chemistry 2003, 9, 4000–4008. [Google Scholar] [CrossRef] [PubMed]
- Hersam, M.C. Progress towards monodisperse single-walled carbon nanotubes. Nat. Nanotechnol. 2008, 3, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Campidelli, S.; Meneghetti, M.; Prato, M. Separation of metallic and semiconducting single-walled carbon nanotubes via covalent functionalization. Small 2007, 3, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Krupke, R.; Hennrich, F. Chemistry of Carbon Nanotubes; Basiuk, V.A., Basiuk, E.V., Eds.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2008; Volume 3, pp. 129–139. [Google Scholar]
- Krupke, R.; Hennrich, F. Separation techniques for carbon nanotubes. Adv. Engineering Mater. 2005, 7, 111–116. [Google Scholar] [CrossRef]
- Papadimitrakopoulos, F.; Ju, S.Y. Purity rolled up in a tube. Nature 2007, 450, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Haddon, R.C.; Sippel, J.; Rinzler, A.G.; Papadimitrakopoulos, F. Purification and separation of carbon nanotubes. MRS Bull. 2004, 29, 252–259. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Voggu, R.; Govindaraj, A. Selective generation of single-walled carbon nanotubes with metallic, semiconducting and other unique electronic properties. Nanoscale 2009, 1, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Duch, M.C.; Hersam, M.C. Isolation of single-walled carbon nanotube enantiomers by density differentiation. Nano Res. 2009, 2, 69–77. [Google Scholar] [CrossRef]
- Peng, X.; Komatsu, N.; Kimura, T.; Osuka, A. Simultaneous enrichments of optical purity and (n, m) abundance of swnts through extraction with 3,6-carbazolylene-bridged chiral diporphyrin nanotweezers. ACS Nano 2008, 2, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Komatsu, N.; Kimura, T.; Osuka, A. Optical resolution and diameter-based enrichment of single-walled carbon nanotubes through simultaneous recognition of their helicity and diameter with chiral monoporphyrin. J. Phys. Chem. C 2009, 113, 9108–9113. [Google Scholar] [CrossRef]
- Peng, X.; Komatsu, N.; Kimura, T.; Osuka, A. Improved optical enrichment of swnts through extraction with chiral nano-tweezers of 2,6-pyridylene-bridged diporphyrins. J. Am. Chem. Soc. 2007, 129, 15947–15953. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Komatsu, N.; Bhattacharya, S.; Shimawaki, T.; Aonuma, S.; Kimura, T.; Osuka, A. Optically active single-walled carbon nanotubes. Nat. Nanotechnol. 2007, 2, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bachilo, S.M.; Weisman, R.B. Advanced sorting of single-walled carbon nanotubes by nonlinear density-gradient ultracentrifugation. Nat. Nanotechnol. 2010, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Samsonidze, G.G.; Grüneis, A.; Saito, R.; Jorio, A.; Souza Filho, A.G.; Dresselhause, G.; Dresselhause, M.S. Interband optical transitions in left- and right-handed single-walled carbon nanotubes. Phys. Rev. B 2004, 69. [Google Scholar] [CrossRef]
- Sánchez-Castillo, A.; Román-Velázquez, C.E.; Noguez, C. Optical circular dichroism of single-wall carbon nanotubes. Phys. Rev. B 2006, 73, 1–7. [Google Scholar] [CrossRef]
- Moss, G.P. Basic terminology of stereochemistry. Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]
- Strano, M.S. Sorting out left from right. Nat. Nanotechnol. 2007, 2, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Surugau, N.; Urban, P.L. Electrophoretic methods for separation of nanoparticles. J. Sep. Sci. 2009, 32, 1889–1906. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.J.; Schmidt, H.K.; Pasquali, M. Brownian dynamics simulations of single-walled carbon nanotube separation by type using dielectrophoresis. J. Phys. Chem. B 2008, 112, 7467–7477. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Akita, S.; Nakayama, Y. Orientation of carbon nanotubes using electrophoresis. Jpn. J. Appl. Phys. 1996, 35, L917–L918. [Google Scholar] [CrossRef]
- Yamamoto, K.; Akita, S.; Nakayama, Y. Orientation and purification of carbon nanotubes using ac electrophoresis. J. Phys. D: Appl. Phys. 1998, 31, L34–L36. [Google Scholar] [CrossRef]
- Krupke, R.; Hennrich, F.; Löhneysen, H.V.; Kappes, M.M. Separation of metallic from semiconducting single-walled carbon nanotubes. Science 2003, 301, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Moshammer, K.; Hennrich, F.; Kappes, M.M. Selective suspension in aqueous sodium dodecyl sulfate according to electronic structure type allows simple separation of metallic from semiconducting single-walled carbon nanotubes. Nano Res. 2009, 2, 599–606. [Google Scholar] [CrossRef]
- Krupke, R.; Hennrich, F.; Weber, H.B.; Kappes, M.M.; Löhneysen, H.V. Simultaneous deposition of metallic bundles of single-walled carbon nanotubes using ac-dielectrophoresis. Nano Lett. 2003, 3, 1019–1023. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Tong, L.; Pan, H.; Liu, Z. Simultaneous dielectrophoretic separation and assembly of single-walled carbon nanotubes on multigap nanoelectrodes and their thermal sensing properties. Anal. Chem. 2006, 78, 8069–8075. [Google Scholar] [CrossRef] [PubMed]
- Krupke, R.; Linden, S.; Rapp, M.; Hennrich, F. Thin film of metallic carbon nanotubes prepared by dielectrophoresis. Adv. Mater. 2006, 18, 1468–1470. [Google Scholar] [CrossRef]
- Heller, D.A.; Mayrhofer, R.M.; Baik, S.; Grinkova, Y.V.; Usrey, M.L.; Strano, M.S. Concomitant length and diameter separation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 14567–14573. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; Boul, P.; Ericson, L.M.; Huffman, C.; Wang, Y.; Haroz, E.; Kuper, C.; Tour, J.; Ausman, K.D.; Smalley, R.E. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 2001, 342, 265–271. [Google Scholar] [CrossRef]
- Doorn, S.K.; Fields, R.E.; Hu, H.; Hamon, M.A.; Haddon, R.C.; Selegue, J.P.; Majidi, V. High resolution capillary electrophoresis of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Doorn, S.K.; Strano, M.S.; O’Connell, M.; Haroz, E.H.; Railon, K.L.; Hauge, R.H.; Smalley, R.E. Capillary electrophoresis separation of bundled and individual carbon nanotubes. J. Phys. Chem. B 2003, 107, 6063–6069. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, D.W.; Kim, H.S.; Lee, S.W.; Jhang, S.H.; Park, Y.W.; Campbell, E.E.B. Extraction of semiconducting cnts by repeated dielectrophoretic filtering. Appl. Phys. A 2005, 80, 5–8. [Google Scholar] [CrossRef]
- Peng, H.; Alvarez, N.T.; Kittrell, C.; Hauge, R.H.; Schmidt, H.K. Dielectrophoresis field flow fractionation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2006, 128, 8396–8397. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.; Usrey, M.; Rotkina, L.; Strano, M.S. Using the selective functionalization of metallic single-walled carbon nanotubes to control dielectrophoretic mobility. J. Phys. Chem. B 2004, 108, 15560–15564. [Google Scholar] [CrossRef]
- Krupke, R.; Hennrich, F. Comment on “Using the selective functionalization of metallic single-walled carbon nanotubes to control dielectrophoretic mobility”. J. Phys. Chem. B 2005, 109, 17014–17015. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Strano, M.S. Reply to “Comment on ‘Using the selective functionalization of metallic single-walled carbon nanotubes to control dielectrophoretic mobility’”. J. Phys. Chem. B 2005, 109, 17016–17018. [Google Scholar] [CrossRef]
- Tanaka, T.; Jin, H.; Miyata, Y.; Fujii, S.; Nishide, D.; Kataura, H. Mass separation of metallic and semiconducting single-wall carbon nanotubes using agarose gel. Phys. Stat. Sol. (b) 2009, 246, 2490–2493. [Google Scholar] [CrossRef]
- Tanaka, T.; Jin, H.; Miyata, Y.; Kataura, H. High-yield separation of metallic and semiconducting single-walled carbon nanotubes by agarose gel electrophoresis. Appl. Phys. Express 2008, 1. [Google Scholar] [CrossRef]
- Dimaki, M.; Bøggild, P. Dielectrophoresis of carbon nanotubes using microelectrodes: A numerical study. Nanotechnology 2004, 15, 1095–1102. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, D.S.; Yu, H.Y.; Campbell, E.E.B.; Park, Y.W. Production of individual suspended single-walled carbon nanotubes using the ac electrophoresis technique. Appl. Phys. A 2004, 78, 283–286. [Google Scholar] [CrossRef]
- Krupke, R.; Hennrich, F.; Kappes, M.M.; Lóhneysen, H.V. Surface conductance induced dielectrophoresis of semiconducting single-walled carbon nanotubes. Nano Lett. 2004, 4, 1395–1399. [Google Scholar] [CrossRef]
- Kim, W.J.; Usrey, M.L.; Strano, M.S. Selective functionalization and free solution electrophoresis of single-walled carbon nanotubes: Separate enrichment of metallic and semiconducting swnt. Chem. Mater. 2007, 19, 1571–1576. [Google Scholar] [CrossRef]
- Mureau, N.; Watts, P.C.P.; Tison, Y.; Silva, S.R.P. Bulk electrical properties of single-walled carbon nanotubes immobilized by dielectrophoresis: Evidence of metallic or semiconductor behavior. Electrophoresis 2008, 29, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hong, S.; Jung, S.; Strano, M.S.; Choi, J.; Baik, S. Dielectrophoresis of surface conductance modulated single-walled carbon nanotubes using cationic surfactants. J. Phys. Chem. B 2006, 110, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.; Donovan, K.J. Macroscopic scale separation of metallic and semiconducting nanotubes by dielectrophoresis. Carbon 2005, 43, 2508–2513. [Google Scholar] [CrossRef]
- Vetcher, A.A.; Srinivasan, S.; Vetcher, I.A.; Abramov, S.M.; Kozlov, M.; Baughman, R.H.; Levene, S.D. Fractionation of swnt/nucleic acid complexes by agarose gel electrophoresis. Nanotechnology 2006, 17, 4263–4269. [Google Scholar] [CrossRef] [PubMed]
- Martel, R. Sorting carbon nanotubes for electronics. ACS Nano 2008, 2, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.S.; Stupp, S.I.; Hersam, M.C. Enrichment of single-walled carbon nanotubes by diameter in density gradients. Nano Lett. 2005, 5, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.S.; Green, A.A.; Hulvat, J.F.; Stupp, S.I.; Hersam, M.C. Sorting carbon nanotubes by electronic structure using density differentiation. Nat. Nanotechnol. 2006, 1, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Stürtl, N.; Hennrich, F.; Lebedkin, S.; Kappes, M.M. Near monochiral single-walled carbon nanotube dispersions in organic solvents. J. Phys. Chem. C 2009, 113, 14628–14632. [Google Scholar] [CrossRef]
- Fujii, S.; Tanaka, T.; Miyata, Y.; Suga, H.; Naitoh, Y.; Minari, T.; Miyadera, T.; Tsukagoshi, K.; Kataura, H. Performance enhancement of thin-film transisters by using high-purity semiconducting single-wall carbon nanotubes. Appl. Phys. Express 2009, 2. [Google Scholar] [CrossRef]
- Green, A.A.; Hersam, M.C. Colored semitransparent conductive coatings consisting of monodisperse metallic single-walled carbon nanotubes. Nano Lett. 2008, 8, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Yanagi, K.; Maniwa, Y.; Kataura, H. Highly stabilized conductivity of metallic single wall carbon nanotube thin films. J. Phys. Chem. C 2008, 112, 3591–3596. [Google Scholar] [CrossRef]
- Miyata, Y.; Yanagi, K.; Maniwa, Y.; Kataura, H. Optical evaluation of the metal-to-semiconductor ratio of single-wall carbon nanotubes. J. Phys. Chem. C 2008, 112, 13187–13191. [Google Scholar] [CrossRef]
- Hennrich, F.; Arnold, K.; Lebedkin, S.; Quintilla, A.; Wenzel, W.; Kappes, M.M. Diameter sorting of carbon nanotubes by gradient centrifugation: Role of endohedral water. Phys. Stat. Sol. (b) 2007, 244, 3896–3900. [Google Scholar] [CrossRef]
- Nair, N.; Kim, W.J.; Braatz, R.D.; Strano, M.S. Dynamics of surfactant-suspended single-walled carbon nanotubes in a centrifugal field. Langmuir 2008, 24, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Crochet, J.; Arnold, M.S.; Hersam, M.C.; Ulbricht, H.; Resasco, D.; Hertel, T. Pump-probe spectroscopy of exciton dynamics in (6, 5) carbon nanotubes. J. Phys. Chem. C 2007, 111, 3831–3835. [Google Scholar] [CrossRef]
- Qian, H.; Georgi, C.; Anderson, N.; Green, A.A.; Hersam, M.C.; Novotny, L.; Hartschuh, A. Exiton energy transfer in pairs of single-walled carbon nanotubes. Nano Lett. 2008, 8, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Crochet, J.; Clemens, M.; Hertel, T. Quantum yield heterogeneities of aqueous single-walled carbon nanotube suspensins. J. Am. Chem. Soc. 2007, 129, 8058–8059. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Hersam, M.C. Ultracentrifugation of single-walled nanotubes. Mater. Today 2007, 10, 59–60. [Google Scholar] [CrossRef]
- Yanagi, K.; Miyata, Y.; Kataura, H. Optical and conductive characteristics of metallic single-wall carbon nanotubes with three basic colors; cyan, magenta, and yellow. Appl. Phys. Express 2008, 1. [Google Scholar] [CrossRef]
- Sato, Y.; Yanagi, K.; Miyata, Y.; Suenaga, K.; Kataura, H.; Iijima, S. Chiral-angle distribution for separated single-walled carbon nanotubes. Nano Lett. 2008, 8, 3151–3154. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Densmore, C.G.; Doorn, S.K. Electrolyte tuning of surfactant interfacial behavior for enhanced density-based separations of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.I.; Obraztsova, E.D. Metallic single-wall carbon nanotubes separated by density gradient ultracentrifugation. Phys. Stat. Sol. (b) 2009, 246, 2477–2481. [Google Scholar] [CrossRef]
- Yanagi, K.; Iitsuka, T.; Fujii, S.; Kataura, H. Separation of metallic and semiconducting carbon nanotubes by using sucrose as a gradient medium. J. Phys. Chem. C 2008, 112, 18889–18894. [Google Scholar] [CrossRef]
- Wei, L.; Wang, B.; Goh, T.H.; Li, L.J.; Yang, Y.; Chan-Park, M.B.; Chen, Y. Selective enrichment of (6, 5) and (8, 3) single-walled carbon nanotubes via cosurfactant extraction from narrow (n, m) distribution samples. J. Phys. Chem. B 2008, 112, 2771–2774. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Kim, K.K.; Geng, H.Z.; Park, H.K.; Lim, S.C.; Chae, S.J.; Kim, S.M.; Lee, Y.H.; Nayhouse, M.; Yum, M. Strategy for high concentration nanodispersion of single-walled carbon nanotubes with diameter selectivity. J. Phys. Chem. C 2009, 113, 10044–10051. [Google Scholar] [CrossRef]
- Fleurier, R.; Lauret, J.S.; Lopez, U.; Loiseau, A. Transmission electron microscopy and uv-vis-ir spectroscopy analysis of the diameter sorting of carbon nanotubes by gradient density ultracentrifugation. Adv. Funct. Mater. 2009, 19, 2219–2223. [Google Scholar] [CrossRef]
- Fleurier, R.; Lauret, J.S.; Flahaut, E.; Loiseau, A. Sorting and transmission electron microscopy analysis of single or double wall carbon nanotubes. Phys. Stat. Sol. (b) 2009, 246, 2675–2678. [Google Scholar] [CrossRef] [Green Version]
- Green, A.A.; Hersam, M.C. Processing and properties of highly enriched double-wall carbon nanotubes. Nat. Nanotechnol. 2009, 4, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zaric, S.; Daranciang, D.; Welsher, K.; Lu, Y.; Li, X.; Dai, H. Optical properties of ultrashort semiconducting single-walled carbon nanotube capsules down to sub-10 nm. J. Am. Chem. Soc. 2008, 130, 6551–6555. [Google Scholar] [CrossRef] [PubMed]
- Fagan, J.A.; Becker, M.L.; Chun, J.; Hobbie, E.K. Length fractionation of carbon nanotubes using centrifugation. Adv. Mater. 2008, 20, 1609–1613. [Google Scholar] [CrossRef]
- Hennrich, F.; Lebedkin, S.; Kappes, M.M. Improving separation techniques for single-walled carbon nanotubes: Towards monodisperse samples. Phys. Stat. Sol. (b) 2008, 245, 1951–1953. [Google Scholar] [CrossRef]
- Fagan, J.A.; Becker, M.L.; Chun, J.; Nie, P.; Bauer, B.J.; Simpson, J.R.; Hight-Waker, A.; Hobbie, E.K. Centrifugal length separation of carbon nanotubes. Langmuir 2008, 24, 13880–13889. [Google Scholar] [CrossRef] [PubMed]
- Duesberg, G.S.; Muster, J.; Krstic, V.; Burghard, M.; Roth, S. Chromatographic size separation of single-walled carbon nanotubes. Appl. Phys. A 1998, 67, 117–119. [Google Scholar] [CrossRef]
- Duesberg, G.S.; Blau, W.; Byrne, H.J.; Muster, J.; Burghard, M.; Roth, S. Chromatography of carbon nanotubes. Synth. Metals 1999, 103, 2484–2485. [Google Scholar] [CrossRef]
- Duesberg, G.S.; Burghard, M.; Muster, J.; Philipp, G.; Roth, S. Separation of carbon nanotubes by size extrusion chromatography. Chem. Commun. 1998, 435–436. [Google Scholar] [CrossRef]
- Zheng, M.; Jogota, A.; Semke, E.D.; Diner, B.A.; Mclean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nature Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Zheng, M.; Jogota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhause, M.S.; Mclean, R.S.; Onoa, G.B.; Samsonidze, G.G.; Semke, E.D.; Usrey, M.; Walls, D.J. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 2003, 302, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Manohar, S.; Jagota, A.; Zheng, M. DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature 2009, 460, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rinzler, A.G.; Dai, H.; Hafner, J.H.; Bradley, R.K.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; Rodriguez-Macias, F.; Shon, Y.S.; Lee, T.R.; Colbert, D.T.; Smalley, R.E. Fullerene pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Selegue, J.P. Separation and characterization of singler-walled and multiwalled carbon nnoatubes by using flow field-flow fraction. Anal. Chem. 2002, 74, 4774–4780. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Choi, W.B.; Lee, N.S.; Chung, D.S.; Kang, J.H.; Han, I.T.; Kim, J.M.; Moon, M.H.; Kim, J.S. Purification and characterization of single-walled carbon nanotubes. Mater. Res. Soc. Symp. Proc. 2000, 593, 123–127. [Google Scholar] [CrossRef]
- Moon, M.H.; Kang, D.; Jung, J.; Kim, J. Separation of carbon nanotubes by frit inlet asymmeterical flow field-flow fractionation. J. Sep. Sci. 2004, 27, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Fagan, J.A.; Hobbie, E.K.; Bauer, B.J. Size separation of single-wall carbon nanotubes by flow-field flow fractionation. Anal. Chem. 2008, 80, 2514–2523. [Google Scholar] [CrossRef] [PubMed]
- Tagmatarchis, N.; Zattoni, A.; Reschiglian, P.; Prato, M. Separation and purification of functionalised water-soluble multi-walled carbon nanotubes by flow field-flow fractionation. Carbon 2005, 43, 1984–1989. [Google Scholar] [CrossRef]
- Tan, S.; Lopez, H.A.; Cai, C.W.; Zhang, Y. Optical trapping of single-walled carbon nanotubes. Nano Lett. 2004, 4, 1415–1419. [Google Scholar] [CrossRef]
- Li, X.; Tu, X.; Zaric, S.; Welsher, K.; Seo, W.S.; Zhao, W.; Dai, H. Selective synthesis combined with chemical separation of single-walled carbon nanotubes for chirality selection. J. Am. Chem. Soc. 2007, 129, 15770–15771. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Semke, E.D. Enrichment of single chirality carbon nanotubes. J. Am. Chem. Soc. 2007, 129, 6084–6085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, X.; Welsher, K.; Wang, X.; Zheng, M.; Dai, H. Optical characterization and electoric devices of nearly pure (10,5) single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 2454–2455. [Google Scholar] [CrossRef] [PubMed]
- Strano, M.S.; Zheng, M.; Jagota, A.; Onoa, G.B.; Heller, D.A.; Barone, P.W.; Usrey, M.L. Understanding the nature of the DNA-assisted separation of single-walled carbon nanotubes using fluorescence and raman spectroscopy. Nano Lett. 2004, 4, 543–550. [Google Scholar] [CrossRef]
- Lustig, S.R.; Jagota, A.; Khripin, C.; Zheng, M. Theory of structure-based carbon nanotube separations by ion-exchange chromatography of DNA/cnt hybrids. J. Phys. Chem. B 2005, 109, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Usrey, M.; Kim, W.J.; Braatz, R.D.; Strano, M.S. Estimation of the (n, m) concentration distribution of single-walled carbon nanotubes from photoabsorption spectra. Anal. Chem. 2006, 78, 7689–7696. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Diner, B.A. Solution redox chemistry of carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 15490–15494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zaric, S.; Tu, X.; Wang, X.; Zhao, W.; Dai, H. Assessment of chemically separated carbon nanotubes for nanoelectronics. J. Am. Chem. Soc. 2008, 130, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mclean, R.S.; Zheng, M. High-resolution length sorting and purification of DNA-wrapped carbon nanotubes by size-exclusion chromatography. Anal. Chem. 2005, 77, 6225–6228. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.J.; Fagan, J.A.; Hobbie, E.K.; Chun, J.; Bajpai, V. Chromatographuc fractionation of swnt/DNA dispersions with on-line multi-angle light scattering. J. Phys. Chem. C 2008, 112, 1842–1850. [Google Scholar] [CrossRef]
- Becker, M.L.; Fagan, J.A.; Gallant, N.D.; Bauer, B.J.; Bajpai, V.; Hobbie, E.K.; Lacerda, S.H.; Migler, K.B.; Jakupciak, J.P. Length-dependent uptake of DNA-wrapped single-walled carbon nanotubes. Adv. Mater. 2007, 19, 939–945. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Lastella, S.; Kim, S.; Papadimitrakopoulos, F. Length separation of zwitterion-functionalized single wall carbon nanotubes by gpc. J. Am. Chem. Soc. 2002, 124, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Duesberg, G.S.; Blau, W.J.; Byrne, H.J.; Muster, J.; Burghard, M.; Roth, S. Experimental observation of individual single-wall nanotube speicies by raman microscopy. Chem. Phys. Lett. 1999, 310, 8–14. [Google Scholar] [CrossRef]
- Farkas, E.; Anderson, M.E.; Chen, Z.; Rinzler, A.G. Length sorting cut single wall carbon nanotubes by high performance liquid chromatography. Chem. Phys. Lett. 2002, 363, 111–116. [Google Scholar] [CrossRef]
- Arnold, K.; Hennrich, F.; Krupke, R.; Lebedkin, S.; Kappes, M.M. Length separation studies of single-walled carbon nanotubes dispersions. Phys. Stat. Sol. (b) 2006, 243, 3073–3076. [Google Scholar] [CrossRef]
- Fagan, J.A.; Simpson, J.R.; Bauer, B.J.; Lacerda, S.H.D.P.; Becker, M.L.; Chun, J.; Migler, K.B.; Walker, A.R.H.; Hobbie, E.K. Length-dependent optical effects in single-wall carbon nanotubes. J. Am. Chem. Soc. 2007, 129, 10607–10612. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.A.; Stewart, M.P.; Tour, J.M. Separation of single-walled carbon nanotubes on silica gel. Materials morphology and raman excitation wavelength affect data interpretation. J. Am. Chem. Soc. 2005, 127, 4497–4509. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Urabe, Y.; Nishide, D.; Kataura, H. Continuous separation of metallic and semiconducting carbon nanotubes using agarose gel. Appl. Phys. Express 2009, 2. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; Ma, J.; Hauge, R.H.; Weisman, R.B.; Smalley, R.E. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.A.; Tour, J.M. Covalent functionalization of single-walled carbon nanotubes for materials applications. J. Phys. Chem. A 2004, 108, 11151–11159. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Galeska, I.; Papadimitrakopoulos, F. A route for bulk separation of semiconducting from metallic single-wall carbon nanotubes. J. Am. Chem. Soc. 2003, 125, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Ulz, M.; Papadimitrakopoulos, F. Enrichment mechanism of semiconducting single-walled carbon nanotubes by surfactant amines. J. Am. Chem. Soc. 2009, 131, 6775–6784. [Google Scholar] [CrossRef] [PubMed]
- Samsonidze, G.G.; Chou, S.G.; Santos, A.P.; Brar, V.W.; Dresselhaus, G.; Dresselhaus, M.S.; Selbst, A.; Swan, A.K.; Ünuü, M.S.; Goldberg, B.B.; Chattopadhyay, D.; Kim, S.N.; Papadimitrakopoulos, F. Quatitative evaluation of the octadecylamine-assisted bulk separation of semiconducting and metallic single-wall carbon nanotubes by resonance raman spectroscopy. Appl. Phys. Lett. 2004, 85, 1006–1008. [Google Scholar] [CrossRef]
- Kim, S.N.; Luo, Z.; Papadimitrakopoulos, F. Diameter and metallicity dependent redox influences on the separation of single-wall carbon nanotubes. Nano Lett. 2005, 5, 2500–2504. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kimura, S.; Kanda, M.; Hirashima, Y.; Hasegawa, T.; Wakahara, T.; Lian, Y.; Nakahodo, T.; Tsuchiya, T.; Akasaka, T.; Lu, J.; Zhang, X.; Gao, Z.; Yu, Y.; Nagase, S.; Kazaoui, S.; Minami, N.; Shimizu, T.; Tokumoto, H.; Saito, R. Large-scale separation of metallic and semiconducting single-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 10287–10290. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kanda, M.; Hirashima, Y.; Hasegawa, T.; Kimura, S.; Lian, Y.; Wakahara, T.; Akasaka, T.; Kazaoui, S.; Minami, N.; Okazaki, T.; Hayamizu, Y.; Hata, K.; Lu, J.; Nagase, S. Dispersion and separation of small-diameter single-walled carbon nanotubes. J. Am. Chem. Soc. 2006, 128, 12239–12242. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lai, L.; Luo, G.F.; Zhou, J.; Qin, R.; Wang, D.; Wang, L.; Mei, W. N.; Li, G.; Gao, Z.; Nagase, S.; Maeda, Y.; Akasaka, T.; Yu, D. Why semiconducting single-walled carbon nanotubes are separated from their metallic counterparts. Small 2007, 3, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, B.; Chen, Y.; Li, L.J. Toward the extraction of single species of single-walled carbon nanotubes using fluorene-based polymers. Nano Lett. 2007, 7, 3013–3017. [Google Scholar] [CrossRef] [PubMed]
- Nish, A.; Hwang, J.Y.; Doig, J.; Nicholas, R.J. Highly selective dispersion of single-walled carbon nanotubes using aromatic polymers. Nat. Nanotechnol. 2007, 2, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Nish, A.; Doig, J.; Douven, S.; Chen, C.W.; Chen, L.C.; Nicholas, R.J. Polymer structure and solvent effects on the selective dispersion of single-walled carbon nanotubes. J. Am. Chem. Soc. 2008, 130, 3543–3553. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.B.; Stephan, C.; Coleman, J.N.; McCarthy, B.; Ajayan, P.M.; Lefrant, S.; Bernier, P.; Blau, W.J.; Byrne, H.J. Selective interaction of a semiconjugated organic polymer with single-wall nanotubes. J. Phys. Chem. B 2000, 104, 10012–10016. [Google Scholar] [CrossRef]
- Gregan, E.; Keogh, S.M.; Maguire, A.; Hedderman, T.G.; Neill, L.O.; Chambers, G.; Byrne, H.J. Purification and isolation of swnts. Carbon 2004, 42, 1031–1035. [Google Scholar] [CrossRef]
- Keogh, S.M.; Hedderman, T.G.; Lynch, P.; Farrell, G.F.; Byrne, H.J. Bundling and diameter selectivity in hipco swnts poly(p-phenylenevinylene-co-2,5-dioctyloxy-m-phenylene) composites. J. Phys. Chem. B 2006, 110, 19369–19374. [Google Scholar] [CrossRef] [PubMed]
- Keogh, S.M.; Hedderman, T.G.; Gregan, E.; Farrell, G.; Chambers, G.; Byrne, H.J. Spectroscopic analysis of single-walled carbon nanotubes and semiconjugated polymer composites. J. Phys. Chem. B 2004, 108, 6233–6241. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Malkovskiy, A.; Chu, Q.; Sokolov, A.P.; Colon, M.L.; Meador, M.; Pang, Y. Wrapping of single-walled carbon nanotubes by a π-conjugated polymer: The role of polymer conformation-controlled size selectivity. J. Phys. Chem. B 2008, 112, 12263–12269. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Castillo, A.; Noguez, C. Understanding optical activity in single-walled carbon nanotubes from first-principles studies. J. Phys. Chem. C 2010, 114, 9640–9644. [Google Scholar] [CrossRef]
- Tanaka, T.; Jin, H.; Miyata, Y.; Fujii, S.; Suga, H.; Naitoh, Y.; Minari, T.; Miyadera, T.; Tsukagoshi, K.; Kataura, H. Simple and scalable gel-based separation of metallic and semiconducting acrbon nanotubes. Nano Lett. 2009, 9, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, M.C.; Roberts, M.; Barman, S.; Jin, Y.W.; Kim, J.M.; Bao, Z. Self-sorted, aligned nanotube networks for thin-film transistors. Science 2008, 321, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Du, X.; Du, M.H.; Rancken, C.D.; Cheng, H.P.; Rinzler, A.G. Bulk separation enrichment in metallic or semiconducting single-walled carbon nanotubes. Nano Lett. 2003, 3, 1245–1249. [Google Scholar] [CrossRef]
- Park, N.; Miyamoto, Y.; Lee, K.; Choi, W.I.; Ihm, J.; Yu, J.; Han, S. Band gap sensitivity of bromine adsorption at carbon nanotubes. Chem. Phys. Lett. 2005, 403, 135–139. [Google Scholar] [CrossRef]
- Li, H.; Zhou, B.; Lin, Y.; Gu, L.; Wang, W.; Fernando, K.A.S.; Kumar, S.; Allard, L.F.; Sun, Y.P. Selective interactions of porphyrins with semiconducting single-walled carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fernando, K.A.S.; Lin, Y.; Meziani, M.J.; Veca, L.M.; Cao, L.; Zhang, P.; Kimani, M.M.; Sun, Y.P. Metallic single-walled carbon nanotubes for conductive nanocomposites. J. Am. Chem. Soc. 2008, 130, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Doll, J.; Sharma, I.; Papadimitrakopoulos, F. Seletion of carbon nanotubes with specific chiralities using helical assemblies of flavin mononucleotide. Nat. Nanotechnol. 2008, 3, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ogunro, O.O.; Wang, X.Q. Quantum electronic stability in selective enrichment of carbon nanotubes. Nano Lett. 2009, 9, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.; Greco, C.; Sadokierska, I.; Lebedkin, S.; Kappes, M.M.; Michel, T.; Alvarez, L.; Sauvajol, J.L.; Meunier, S.; Mioskowski, C. Supramolecular discrimination of carbon nanotubes according to their helicity. Nano Lett. 2008, 8, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.; Kulikiewicz, K.; Lebedkin, S.; Kappes, M.M.; Mioskowski, C.; Meunier, S.; Wagner, A. Axially chiral facial amphiphiles with a dihydronaphthopentaphene structure as molecular tweezers for swnts. Chem. Eur. J 2009, 15, 11187–11196. [Google Scholar] [CrossRef] [PubMed]
- Ortis-Acevedo, A.; Xie, H.; Zorbas, V.; Sampson, W.M.; Dalton, A.B.; Baughman, R.H.; Draper, R.K.; Musselman, I.H.; Dieckmann, G.R. Diameter-selective solubilization of single-walled carbon nanotubes by reversible cyclic peptides. J. Am. Chem. Soc. 2005, 127, 9512–9517. [Google Scholar] [CrossRef] [PubMed]
- Becraft, E.J.; Klimenko, A.S.; Dieckmann, G.R. Influence of alternating l-/d-amino acid chiralities and disulfide bond geometry on the capacity of cysteine-containing reversible cyclic peptides to disperse carbon nanotubes. Pept. Science 2009, 92, 212–221. [Google Scholar] [CrossRef]
- Dodziuk, H.; Ejchart, A.; Anczewski, W.; Ueda, H.; Krinichnaya, E.; Dolgonos, G.; Kutner, W. Water solubilization, determination of the number of different types of single-walled carbon nanotubes and their partial separation with respect to diameters by complexation with eta-cyclodextrin. Chem. Commun. 2003, 986–987. [Google Scholar] [CrossRef]
- Tromp, R.M.; Afzali, A.; Freitag, M.; Mitzi, D.B.; Chen, Z. Novel strategy for diameter-selective separation and functionalization of single-walled carbon nanotubes. Nano Lett. 2008, 8, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Voggu, R.; Rao, K.V.; George, S.J.; Rao, C.N.R. A simple method of separating metallic and semiconducting single-walled carbon nanotubes based on molecular charge transfer. J. Am. Chem. Soc. 2010. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.C.; Mercier, P.; Akins, D.L. Diameter-selective dispersion of single-walled carbon nanotubes using a water-soluble, biocompatible polymer. Chem. Commun. 2006, 1425–1427. [Google Scholar] [CrossRef]
- Saito, K.; Troiani, V.; Qiu, H.; Solladie, N.; Sakata, T.; Mori, H.; Ohama, M.; Fukuzumi, S. Nondestructive formation of supramolecular nanohybrids of single-walled carbon nanotubes with flexible porphyrinic polypeptides. J. Phys. Chem. C 2007, 111, 1194–1199. [Google Scholar] [CrossRef]
- Chaturvedi, H.; Giordano, A.N.; Kim, M.J.; MacDonnell, F.M.; Subaran, S.S.; Poler, J.C. “Mechanically docked” Metallodendrimers about single-walled carbon nanotubes. J. Phys. Chem. C 2009, 113, 11254–11261. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Lin, Y.; Wang, W.; Cao, L.; Meziani, M. J.; Wang, X.; Veca, L.M.; Zhang, P.; Quinn, R.A.; Allard, L.F.; Sun, Y.P. Diameter-selective fractionation of hipco single-walled carbon nanotubes in repeated functionalization reactions. J. Phys. Chem. C 2007, 111, 10254–10259. [Google Scholar] [CrossRef]
- Huang, W.; Fernando, K.A.S.; Lin, Y.; Zhou, B.; Allard, L.F.; Sun, Y.P. Preferential solubilization of smaller single-walled carbon nanotubes in sequential functionalization reactions. Langmuir 2003, 19, 7084–7088. [Google Scholar] [CrossRef]
- Ziegler, K.J.; Schmidt, D.J.; Rauwald, U.; Shah, K.N.; Flor, E.L.; Hauge, R.H.; Smalley, R.E. Length-dependent extraction of single-walled carbon nanotubes. Nano Lett. 2005, 5, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.Y.; Li, W.; Fan, X.F.; Wei, L.; Chen, Y.; Kuo, J.L.; Li, L.J.; Kwak, S.K.; Mu, Y.; Chan-Park, M.B. Enrichment of (8,4) single-walled carbon nanotubes through coextraction with heparin. Small 2010, 6, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Fantini, C.; Jorio, A.; Santos, A.P.; Peressinotto, V.S.T.; Pimenta, M.A. Characterization of DNA-wrapped carbon nanotubes by resonance raman and optical absorption spectroscopies. Chem. Phys. Lett. 2007, 439, 138–142. [Google Scholar] [CrossRef]
- Tomonari, Y.; Murakami, H.; Nakashima, N. Solubilization of single-waleed carbon nanotubes by using polycyclic aromatic ammonium amphiphiles in water-strategy for the design of high-performance solubilizers. Chem. Eur. J. 2006, 12, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Mundloch, U.; Ebel, A.; Hauke, F.; Hirsch, A. Dispersion of hipco and comocat single-walled nanotubes (swnts) by water soluble pyrene derivatives-depletion of small diameter swnts. Chem. Eur. J 2010, 16, 3314–3317. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.J.; Engtrakul, C.; Jones, M.; Rumbles, G.; Heben, M.J. Kinetics of pl quenching during single-waleed carbon nanotubes rebundling and diameter-dependent surfactant interactions. J. Phys. Chem. B 2006, 110, 25339–25346. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Shan, H.; Haroz, E.; Billups, W.E.; Hauge, R.; Adams, W.W.; Smalley, R.E. Diameter selection of single-walled carbon nanotubes through programmable solvation in binary sulfonic acid mixtures. J. Phys. Chem. C 2007, 111, 17827–17834. [Google Scholar] [CrossRef]
- Joselevich, E. Electronic structure and chemical reactivity of carbon nanotubes: A chemist’s view. Chem. Phys. Chem. 2004, 5, 619–624. [Google Scholar] [PubMed]
- Miyata, Y.; Maniwa, Y.; Kataura, H. Selective oxidation of semiconducting single-wall carbon nanotubes by hydrogen peroxide. J. Phys. Chem. B 2006, 110, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Kawai, T.; Miyamoto, Y.; Yanagi, K.; Maniwa, Y.; Kataura, H. Chirality-dependent combustion of single-walled carbon nanotubes. J. Phys. Chem. C 2007, 111, 9671–9677. [Google Scholar] [CrossRef]
- Yudasaka, M.; Zhang, M.; Iijima, S. Diameter-selective removal of single-wall carbon nanotubes through light-assisted oxidation. Chem. Phys. Lett. 2003, 374, 132–136. [Google Scholar] [CrossRef]
- McDonald, T.J.; Blackburn, J.L.; Metzger, W.K.; Rumbles, G.; Heben, M.J. Chiral-selective protection of single-walled carbon nanotube photoluminescence by surfactant selection. J. Phys. Chem. C 2007, 111, 17894–17900. [Google Scholar] [CrossRef]
- Zhang, M.; Yudasaka, M.; Miyauchi, Y.; Maruyama, S.; Iijima, S. Changes in the fluorescence spectrum of indivisual single-wall carbon nanotubes induced by light-assisted oxidation with hydroperoxide. J. Phys. Chem. B 2006, 110, 8935–8940. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Wong, S.S. Selective metallic tube reactivity in the solution-phase osmylation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Qui, H.; Maeda, Y.; Akasaka, T. Facile and scalable route for highly efficient enrichment of semiconducting single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 16529–16533. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Park, J.S.; An, K.H.; Lim, S.C.; Seo, K.; Kim, B.; Park, K.A.; Han, S.; Park, C.Y.; Lee, Y.H. Selective removal of metallic single-walled carbon nanotubes with small diameters by using nitric and sulfuric acids. J. Phys. Chem. B 2005, 109, 19242–19248. [Google Scholar] [CrossRef] [PubMed]
- Menna, E.; Negra, F.D.; Fontana, M.D. Selectivity of chemical oxidation attack of single-wall carbon nanotubes in solution. Phys. Rev. B 2003, 68. [Google Scholar] [CrossRef]
- Wiltshire, J.G.; Khlobystov, A.N.; Li, L.J.; Lyapin, S.G.; Briggs, G.A.D.; Nicholas, R.J. Comparative studies on acid and thermal based selective purification of hipco produced single-walled carbon nanotubes. Chem. Phys. Lett. 2004, 386, 239–243. [Google Scholar] [CrossRef]
- Bergeret, C.; Cousseau, J.; Fernandez, V.; Mevellec, J.Y.; Lefrant, S. Spectroacopic evidence of carbon nanotubes’ metallic character loss induced by covalent functionalization via nitric acid purification. J. Phys. Chem. C 2008, 112, 16411–16416. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Wong, S.S. Demonstration of diameter-selective reactivity in the sidewall ozonation of swnts by resonance raman spectroscopy. Nano Lett. 2004, 4, 1445–1450. [Google Scholar] [CrossRef]
- Banerjee, S.; Wong, S.S. Rational sidewall functionalization and purification of single-walled carbon nanotubes by solution-phase ozonolysis. J. Phys. Chem. B 2002, 106, 12144–12151. [Google Scholar] [CrossRef]
- Kato, Y.; Niidome, Y.; Nakashima, N. Efficient separation of (6,5) single-walled carbon nanotubes using a “Nanometal sinker”. Angew. Chem. Int. Ed. 2009, 48, 5435–5438. [Google Scholar] [CrossRef]
- Yoon, S.M.; Kim, S.J.; Shin, H.J.; Benayad, A.; Choi, S.J.; Kim, K.K.; Kim, S.M.; Park, Y.J.; Kim, G.; Choi, J.Y.; Lee, Y.H. Selective oxidation on metallic carbon nanotubes by halogen oxoanions. J. Am. Chem. Soc. 2008, 130, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, S.; Yudasaka, M.; Hirahara, K.; Ichihashi, T.; Iijima, S. Effect of oxidation on single-wall carbon nanotubes. Chem. Phys. Lett. 2000, 328, 374–380. [Google Scholar] [CrossRef]
- Zhou, W.; Ooi, Y.H.; Russo, R.; Papanek, P.; Luzzi, D.E.; Fischer, J.E.; Bronikowski, M.J.; Willis, P.A.; Smalley, R.E. Structural characterization and diameter-dependent oxidative stability of single wall carbon nanotubes synthesized by the catalytic decomposition of co. Chem. Phys. Lett. 2001, 350, 6–14. [Google Scholar] [CrossRef]
- An, K.H.; Park, J.S.; Yang, C.M.; Jeong, S.Y.; Lim, S.C.; Kang, C.; Son, J.H.; Jeong, M.S.; Lee, Y.H. A diameter-selective attack of metallic carbon nanotubes by nitronium ions. J. Am. Chem. Soc. 2005, 127, 5196–5203. [Google Scholar] [CrossRef] [PubMed]
- An, K.H.; Yang, C.M.; Lee, J.Y.; Lim, S.C.; Kang, C.; Son, J.H.; Jeong, M.S.; Lee, Y.H. A diameter-selective chiral separation of single-wall carbon nanotubes using nitronium ions. J. Electronic Mater. 2006, 35, 235–242. [Google Scholar] [CrossRef]
- Seo, K.; Park, K.A.; Kim, C.; Han, S.; Kim, B.; Lee, Y.H. Chirality- and diameter-dependent reactivity of NO2 on carbon nanotube walls. J. Am. Chem. Soc. 2005, 127, 15724–15729. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, B.; Hamon, M.A.; Kamaras, K.; Itkis, M.E.; Haddon, R.C. Sidewall functionalization of single-walled carbon nanotubes by addition of dichlorocarbene. J. Am. Chem. Soc. 2003, 125, 14893–14900. [Google Scholar] [CrossRef] [PubMed]
- Kamaras, K.; Itkis, M.E.; Hu, H.; Zhao, B.; Haddon, R.C. Covalent bond formation to a carbon nanotube metal. Science 2003, 301, 1501. [Google Scholar] [CrossRef] [PubMed]
- Strano, M.S.; Dyke, C.A.; Usrey, M.L.; Barone, P.W.; Allen, M.J.; Shan, H.; Kittrell, C.; Hauge, R.H.; Tour, J.M.; Smalley, R.E. Electronic structure control of single-walled carbon nanotube functionalization. Science 2003, 301, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rao, C.N.R. Separation of metallic and semiconducting single-walled carbon nanotubes through fluorous chemistry. Nano Res. 2009, 2, 183–191. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Sordan, R.; Burghard, M.; Kern, K. A selective electrochemical approach to carbon nanotube field-effect transistes. Nano Lett. 2004, 4, 827–830. [Google Scholar] [CrossRef]
- An, L.; Fu, Q.; Lu, C.; Liu, J. A simple chemical route to selectively eliminate metallic carbon nanotubes in nanotube network devices. J. Am. Chem. Soc. 2004, 126, 10520–10521. [Google Scholar] [CrossRef] [PubMed]
- Strano, M.S. Probing chiral selective reactions using a revised kataura plot for the interpretation of single-walled carbon nanotube spectroscopy. J. Am. Chem. Soc. 2003, 125, 16148–16153. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K.; Friedrich, M.; Jiang, C.; Fan, Y.; Mews, A.; Burghard, M.; Kern, K. Electrical transport and confocal raman studies of electrochemically modified individual carbon nanotubes. Adv. Mater. 2003, 15, 1515–1518. [Google Scholar] [CrossRef]
- Toyoda, S.; Yamaguchi, Y.; Hiwatashi, M.; Tomonari, Y.; Murakami, H.; Nakashima, N. Separation of semiconducting single-walled carbon nanotubes by using a long-alkyl-chain benzenediazonium compound. Chem. Asian J. 2007, 2, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Usrey, M.L.; Lippmann, E.S.; Strano, M.S. Evidence for a two-step mechanism in electronically selective single-walled carbon nanotube reactions. J. Am. Chem. Soc. 2005, 127, 16129–16135. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.D.; Rocha, J.D.R.; Weisman, R.B.; Tour, J.M. Structure-dependent reactivity of semiconducting single-walled carbon nanotubes with benzenediazonium salts. J. Am. Chem. Soc. 2008, 130, 6795–6800. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, B.G.; Jiang, D.E.; Meunier, V. New insight into cqrbon nanotube electronic-structure selectivity. Small 2008, 4, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, Q.; Ozel, T.; Gaur, A.; Rogers, J.A.; Shim, M. Electronically selective chemical functionalization of carbon nanotubes: Correlation between raman spectral and electrical responses. J. Am. Chem. Soc. 2005, 127, 11460–11468. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Kim, W.J.; Usrey, M.L.; Strano, M.S. A structure-reactivity relationship for single walled carbon nanotubes reacting with 4-hydroxybenzene diazonium salt. J. Am. Chem. Soc. 2007, 129, 3946–3954. [Google Scholar] [CrossRef] [PubMed]
- Fantini, C.; Usrey, M.; Strano, M.S. Investigation of electronic and vibrational properties of single-walled carbon nanotubes functionalized with diazonium salts. J. Phys. Chem. C 2007, 111, 17941–17946. [Google Scholar] [CrossRef]
- Yang, C.M.; An, K.H.; Park, J.S.; Park, K.A.; Lim, S.C.; Cho, S.H.; Lee, Y.S.; Park, W.; Park, C.Y.; Lee, Y.H. Preferential etching of metallic single-walled carbon nanotubes with small diameter by fluorine gas. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef]
- Lee, Y.; Jeon, K.S.; Lim, H.; Shin, H.S.; Jin, S.M.; Byon, H.R.; Suh, Y.D.; Choi, H.C. Silencing of metallic single-walled carbon nanotubes via spontaneous hydrosilylation. Small 2009, 5, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, M.; Lu, H.; Malliaras, G.G.; Blanchet, G.B. Suppression of metallic conductivity of single-walled carbon nanotubes by cycloaddition reactions. Science 2009, 323, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Cao, L.; Wei, D.; Wang, Y.; Kajiura, H.; Li, Y.; Noda, K.; Luo, G.; Wang, L.; Zhou, J.; Lu, J.; Gao, Z. A facile low-cost, and scalable method of selective ething of semiconducting single-walled carbon nanotubes by a gas reaction. Adv. Mater. 2009, 21, 813–816. [Google Scholar] [CrossRef]
- Wunderlich, D.; Hauke, F.; Hirsch, A. Preferred functionalization of metallic and small-diameter single-walled carbon nanotubes by nucleophilic addition of organolithium and magnesium compounds followed by reoxidation. Chem. Eur. J. 2008, 14, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- M.-Moyon, C.; Izard, N.; Doris, E.; Mioskowski, C. Separation of semiconducting from metallic carbon nanotubes by selective functionalization with azomethine ylide. J. Am. Chem. Soc. 2006, 128, 6552–6553. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering carbon nanotubes and nanotube circuits using electrical breakdown. Science 2001, 292, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Hersam, M.C.; Arnold, M.S.; Martel, R.; Avouris, P. Current saturation and electrical breakdown in multiwalled carbon nanotubes. Phys. Rev. Lett. 2001, 86, 3128–3131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gaur, A.; Hur, S.H.; Kocabas, C.; Meitl, M.A.; Shim, M.; Rogers, J.A. P-channel, n-channel thin film transisters and p-n diodes based on single wall carbon nanotube networks. Nano Lett. 2004, 4, 2031–2035. [Google Scholar] [CrossRef]
- Zhang, G.; Qi, P.; Wang, X.; Lu, Y.; Li, X.; Tu, R.; Bangsaruntip, S.; Mann, D.; Zhang, L.; Dai, H. Selective ething of metallic carbon nanotubes by gas-phase reaction. Science 2006, 314, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, A.; Tokumoto, M.; Umek, P.; Vrbanic, D.; Mozetic, M.; Mihailovic, D.; Venturini, P.; Pejovnik, S. Selective etching of metallic single-wall carbon nanotubes with hydrogen plasma. Nanotechnology 2005, 16, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Maehashi, K.; Ohno, Y.; Inoue, K.; Matsumoto, K. Chirality selection of single-walled carbon nanotubes by laser resonance chirality selection method. Appl. Phys. Lett. 2004, 85, 858–860. [Google Scholar] [CrossRef]
- Huang, H.; Maruyama, R.; Noda, K.; Kajiura, H.; Kadono, K. Preferential destruction of metallic single-walled carbon nanotubes by laser irradiation. J. Phys. Chem. B 2006, 110, 7316–7320. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Seo, H.W.; Park, J.K.; Kim, J.E.; Choi, D.G.; Han, C.S. Selective removal of metallic swnts using microwave radiation. Curr. Appl. Phys. 2008, 8, 725–728. [Google Scholar] [CrossRef]
- Priya, B.R.; Byrne, H.J. Quantitative analyses of microwave-treated hipco carbon nanotubes using absorption and raman spectroscopy. J. Phys. Chem. C 2009, 113, 7134–7138. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xian, X.; Zhang, J.; Liu, Z. Sorting out semiconducting single-walled carbon nanotube arrays by preferential destruction of metallic tubes using xenon-lamp irradiation J. Phys. Chem. C 2008, 112, 3849–3856. [Google Scholar] [CrossRef]
- Kalbác, M.; Kavan, L.; Dunsch, L. Selective ething of thin single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Boukhalfa, S.; Chikkannanavar, S.B.; McDonald, T.J.; Heben, M.J.; Doorn, S.K. Selective aggregation of single-walled carbon nanotubes via salt addition. J. Am. Chem. Soc. 2007, 129, 1898–1899. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Dunsch, L. Diameter-selective electrochemical doping of hipco single-walled carbon nanotubes. Nano Lett. 2003, 3, 969–972. [Google Scholar] [CrossRef]
- Shim, H.J.; Kim, S.M.; Yoon, S.M.; Benayad, A.; Kim, K.K.; Kim, S.J.; Park, H.K.; Choi, J.Y.; Lee, Y.H. Tailoring electronic structures of carbon nanotubes by solvent with electron-donating and -withdrawing groups. J. Am. Chem. Soc. 2008, 130, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; Eibergen, E.E.; Doorn, S.K. Chiral selectivity in the charge-transfer bleaching of single-walled carbon naotube spectra. Nature Mater. 2005, 4, 412–418. [Google Scholar] [CrossRef]
- Lu, J.; Nagase, S.; Zhang, X.; Wang, D.; Ni, M.; Maeda, Y.; Wakahara, T.; Nakahodo, T.; Tsuchiya, T.; Akasaka, T.; Gao, Z.; Yu, D.; Ye, H.; Mei, W.N.; Zhou, Y. Selective interaction of large or charge-transfer aromatic molecules with metallic single-walled carbon nanotubes: Critical role of the molecular size and orientation. J. Am. Chem. Soc. 2006, 128, 5114–5118. [Google Scholar] [CrossRef] [PubMed]
- Bonard, J.M.; Stora, T.; Salvetat, J.P.; Maier, F.; Stöckli, T.; Duschl, C.; Forró, L.; Heer, W.A.D.; Chatelain, A. Purification and size-selection of carbon nanotubes. Adv. Mater. 1997, 9, 827–831. [Google Scholar] [CrossRef]
- Bandow, S.; Rao, A.M.; Williams, K.A.; Thess, A.; Smalley, R.E.; Eklund, P.C. Purification of single-wall carbon nanotubes by microfilteration. J. Phys. Chem. B 1997, 101, 8839–8842. [Google Scholar] [CrossRef]
- Bachilo, S.M.; Strano, M.S.; Kittrell, C.; Hauge, R.H.; Smalley, R.E.; Weisman, R.B. Structure-assigned optical spectra of single-walled carbon nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Komatsu, N.; Wang, F. A Comprehensive Review on Separation Methods and Techniques for Single-Walled Carbon Nanotubes. Materials 2010, 3, 3818-3844. https://doi.org/10.3390/ma3073818
Komatsu N, Wang F. A Comprehensive Review on Separation Methods and Techniques for Single-Walled Carbon Nanotubes. Materials. 2010; 3(7):3818-3844. https://doi.org/10.3390/ma3073818
Chicago/Turabian StyleKomatsu, Naoki, and Feng Wang. 2010. "A Comprehensive Review on Separation Methods and Techniques for Single-Walled Carbon Nanotubes" Materials 3, no. 7: 3818-3844. https://doi.org/10.3390/ma3073818




