Thermotropic and Thermochromic Polymer Based Materials for Adaptive Solar Control
Abstract
:1. Introduction
- -
- No additional energy consumption, no electrical power supply
- -
- High switching range between off- and on-state
- -
- Variety in shape and size—three-dimensional convex glasses
- -
- Low cost product and low effort for installation as well
- -
- Self-regulating, i.e., no active control is required for operation
- -
- High long-term stability, maintenance-free
- -
- High potential to replace conventional sun protection systems like sun-blinds, roller blinds, etc.
2. Thermotropic Systems
2.1. Theoretical Considerations
2.2. Switching Mechanisms
- -
- Phase separation (e.g., polymer blends and polymer gels with “Lower Critical Solution Temperature”. In the context of gel-based smart windows, the term Lower Critical Solution Temperature is often used. However, chemically crosslinked gels are not dissolved at any time, and thus, some authors in the more scientifically affected literature call this temperature for gels the “Volume Transition Temperature”);
- -
- Change of the particle size (e.g., thermotropic nanoparticles);
- -
- Aggregation (e.g., block copolymers);
- -
- Phase transition (e.g., casting resins with fixed domains).
2.3. Phase-Separating Polymer Blends
2.4. Phase-Separating Thermotropic Gels
2.5. Thermotropic Nanoparticles and Aggregates

2.6. Polymer Blends with Phase Transition
| Sample | Composition [%] | Normal-normal transmittance at 560 nm [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| PA | P(E-co-GMA) | 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | |
| 1 | 100 | 0 | 85 | 85 | 85 | 85 | 85 | 85 |
| 2A | 98 | 2 | 77 | 75 | 70 | 60 | 50 | 45 |
| 2B | 95 | 5 | 65 | 60 | 55 | 40 | 30 | 25 |
| 2C | 91 | 9 | 45 | 35 | 20 | 8 | 2 | 0 |
2.7. Thermotropic Casting Resins

2.8. Thermotropic Polyolefine Films
| Thermotropic Film | Additive [%] | Thickness [µm] | Tvis nn [%] | ΔTvis nn [%] | |
|---|---|---|---|---|---|
| off | on | ||||
| TF 13 | 10 | 120 | 58 | 22 | 36 |
| TF 11 | 5 | 120 | 64 | 37 | 27 |
| TF 39 | 5 | 300 | 18 | 9.9 | 8.1 |
| TF 34 | 4 | 500 | 16 | 7.2 | 8.8 |
| TF 35 | 3 | 300 | 26 | 13 | 13 |
| PE | 0 | 600 | 43 | 42 | 1.0 |
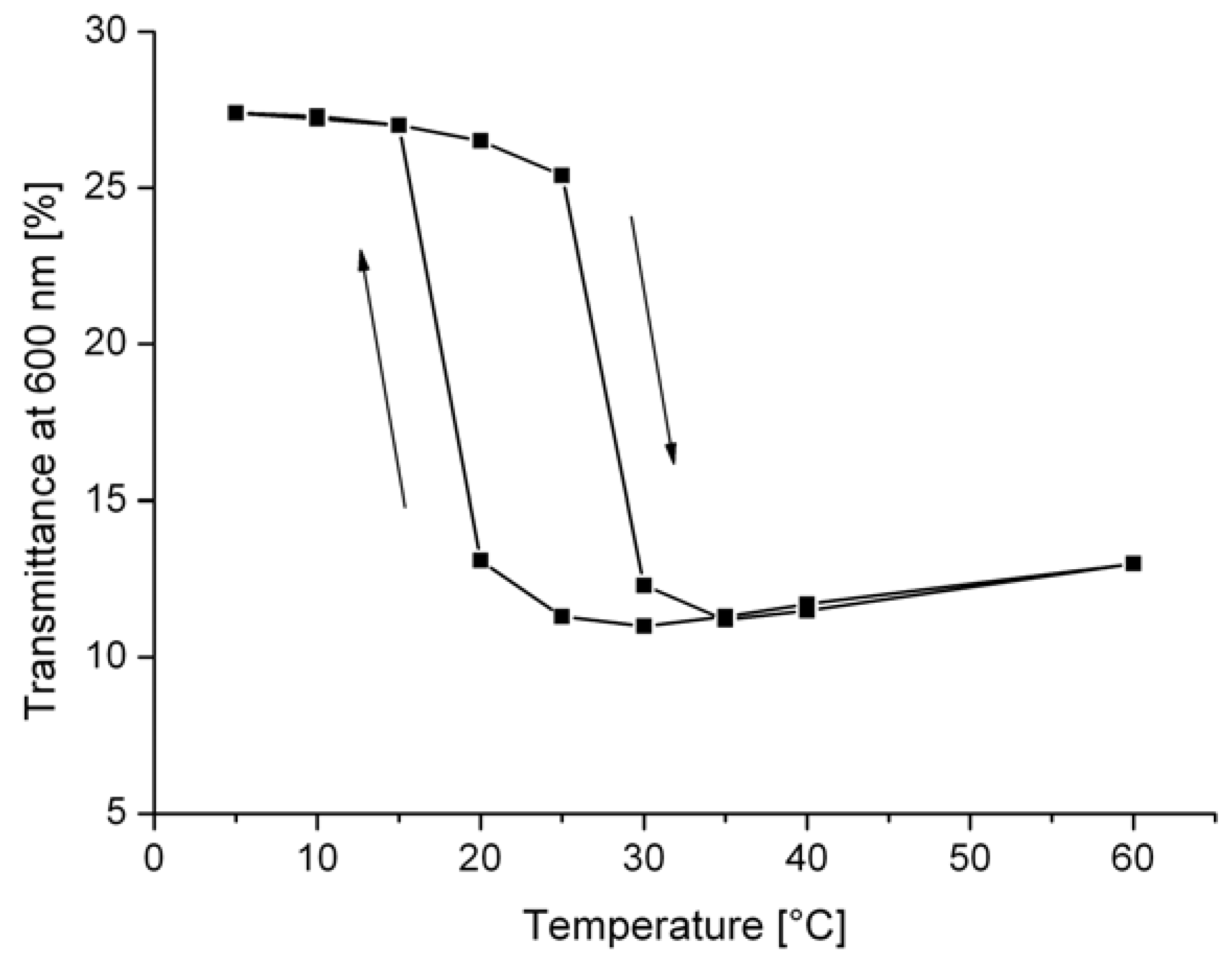
3. Thermochromic Polymer Systems
3.1. Ligand-Exchange Thermochromic Systems (LETC)
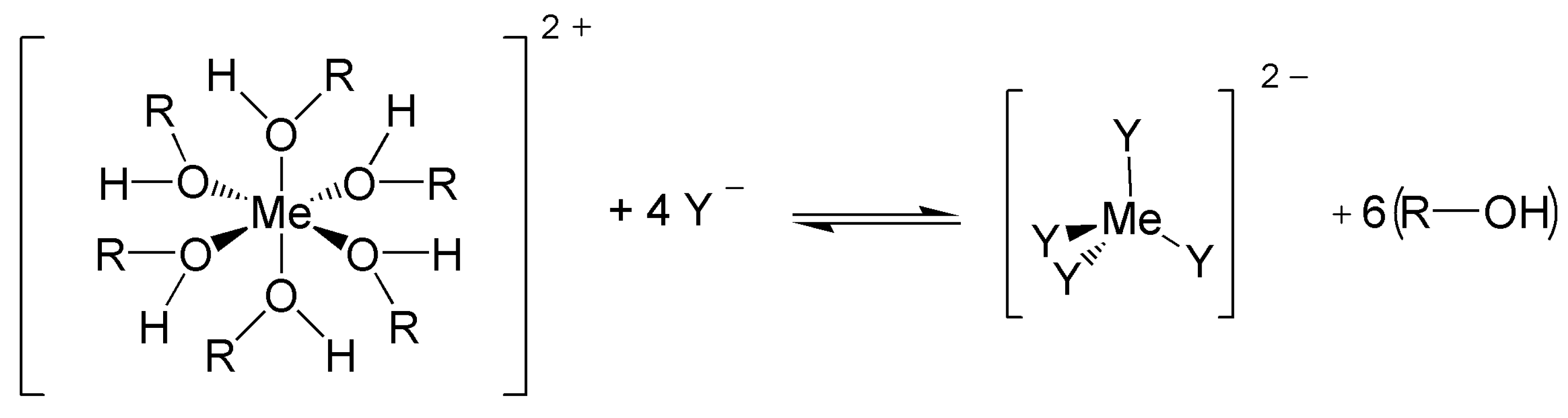
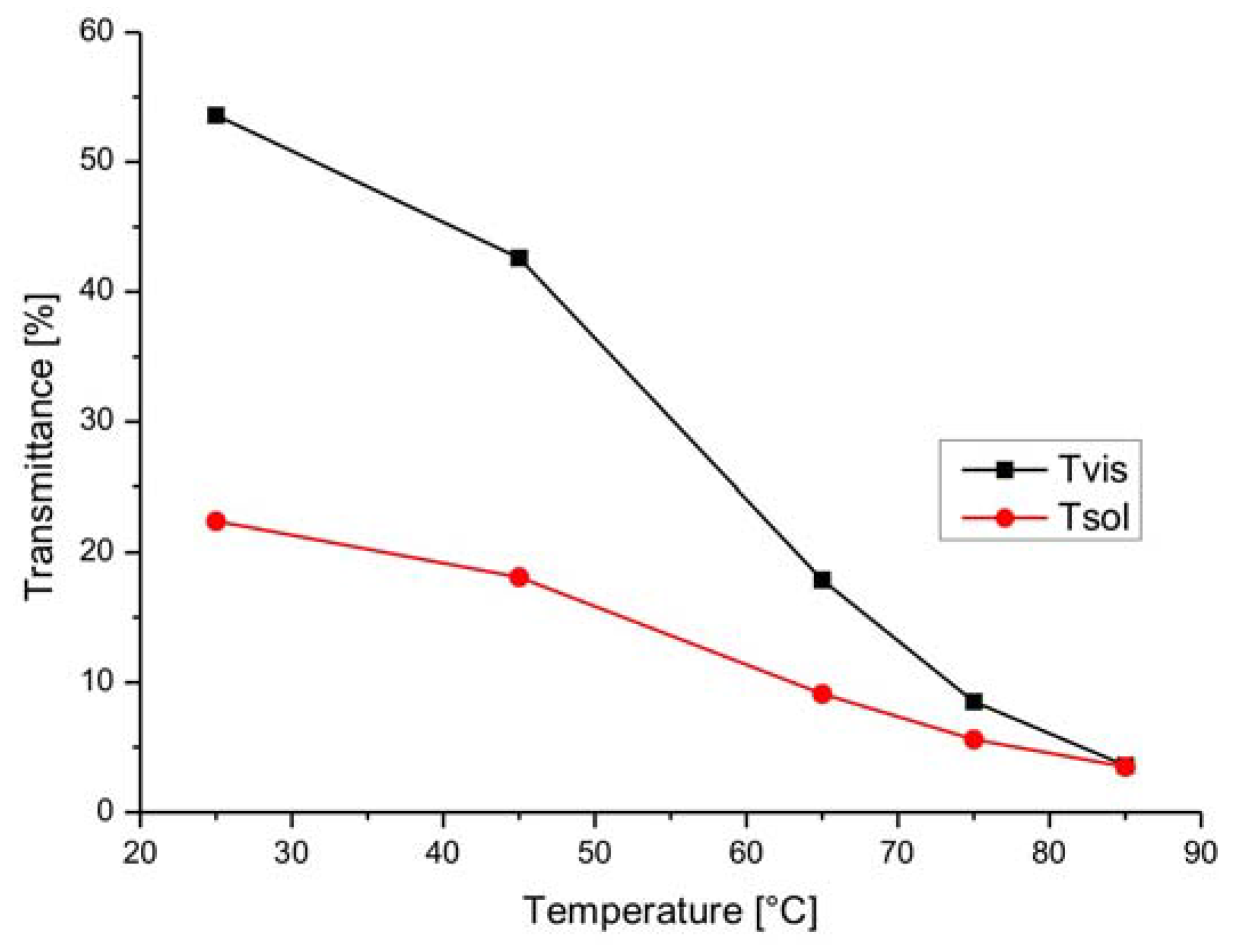
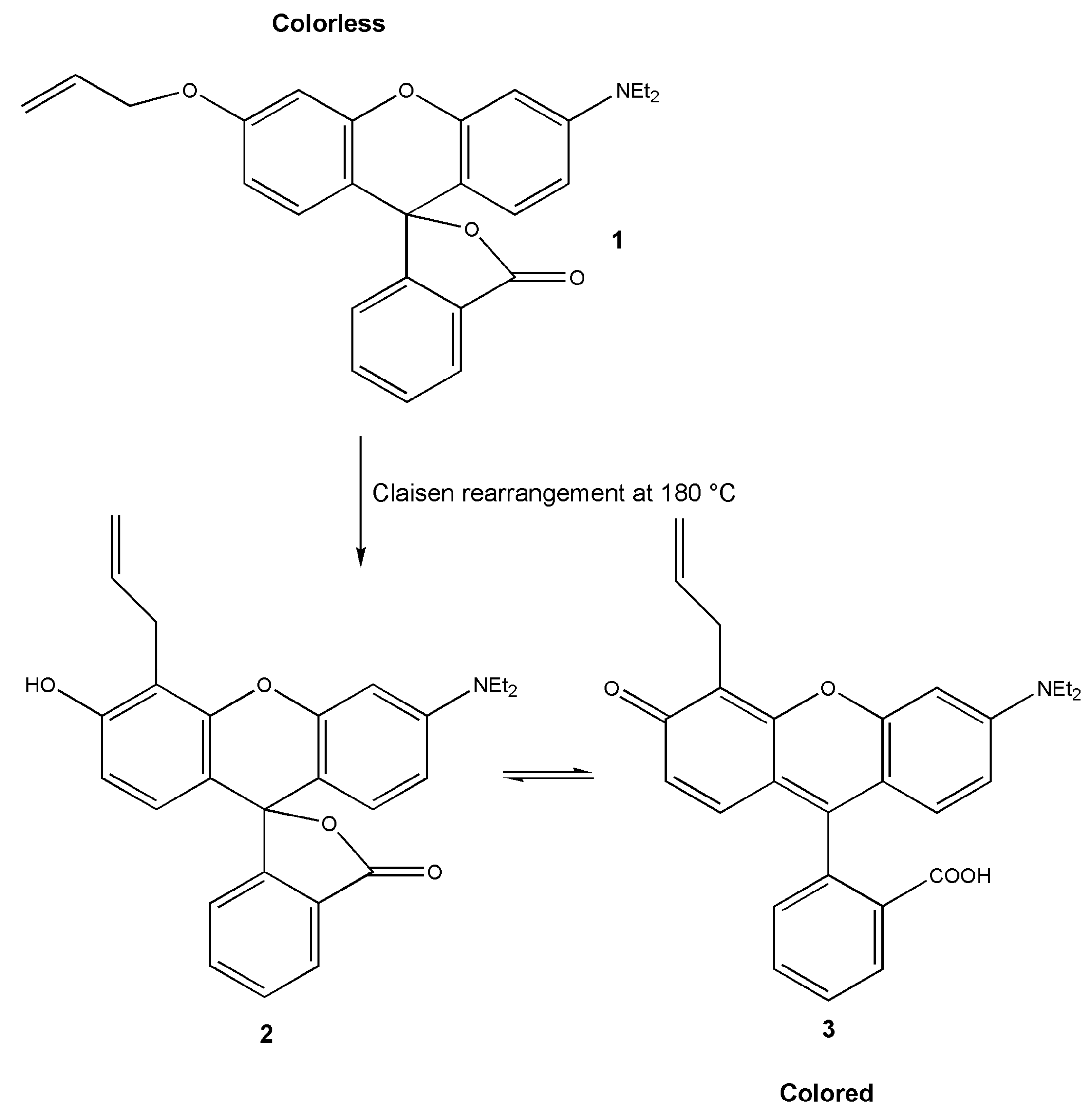
3.2. Leuco Dye-Developer-Solvent Systems
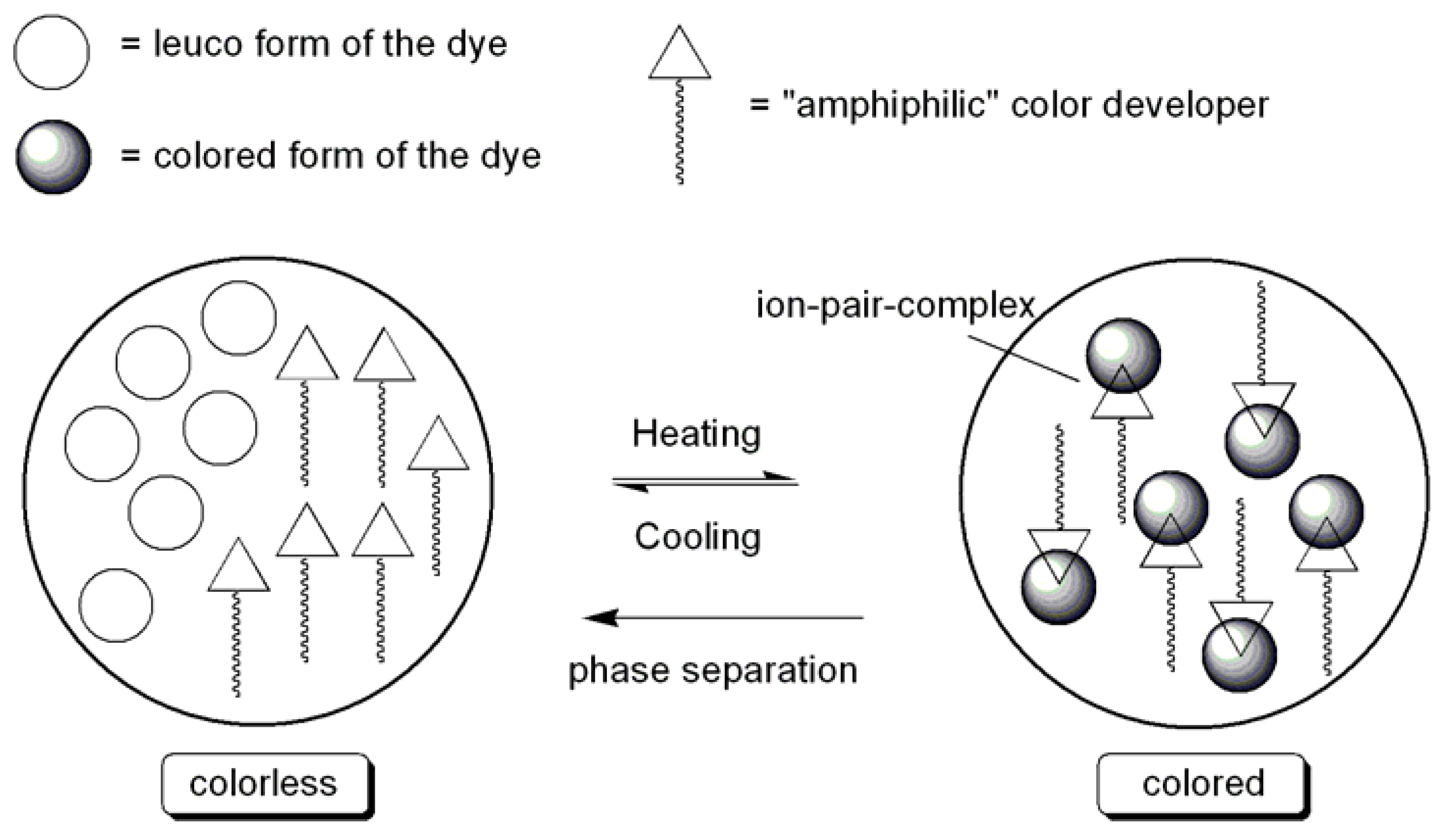

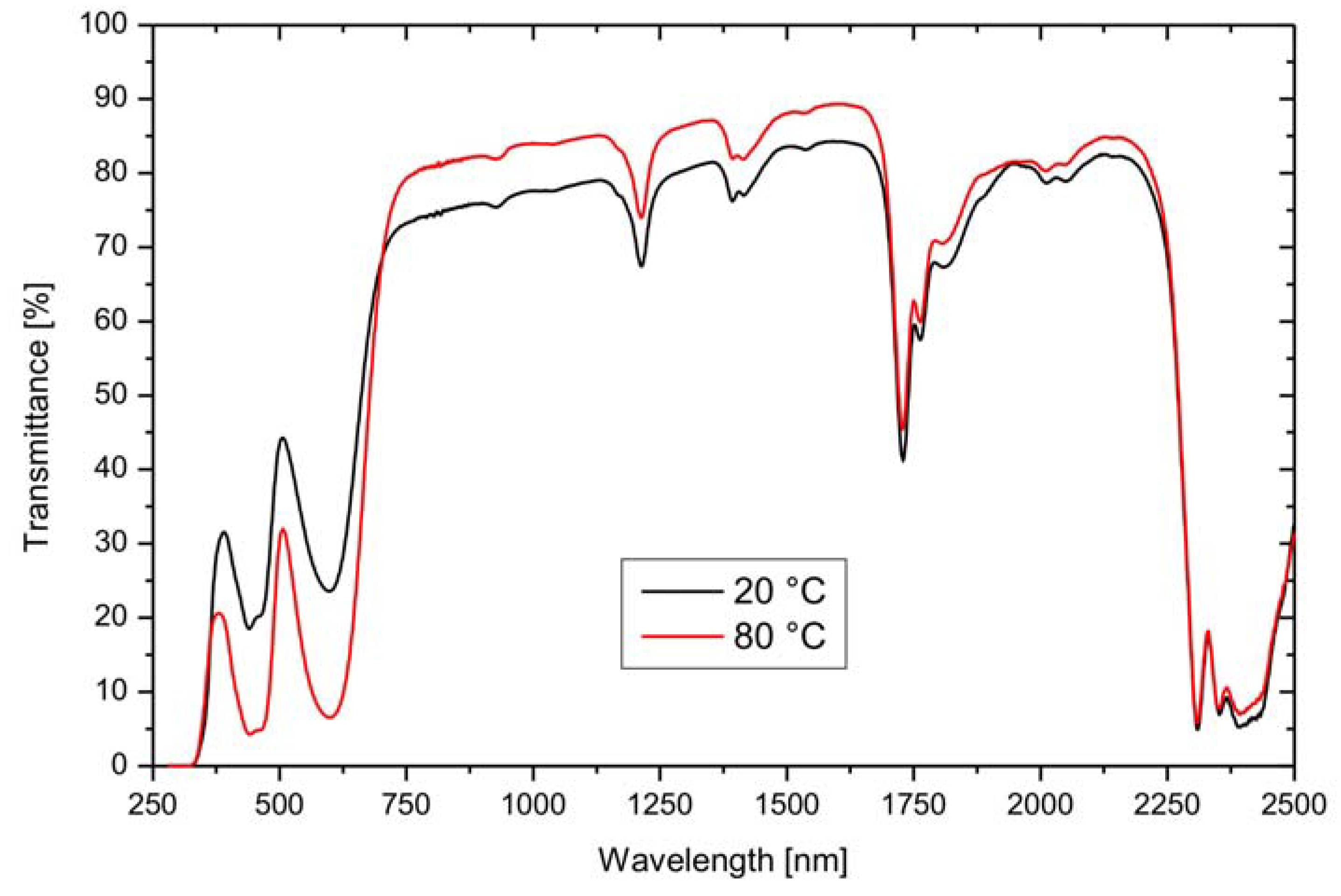
| Dye | Transmittance | |||||
|---|---|---|---|---|---|---|
| Tvis nh [%] | ΔTvis nh [%] | Tsol nh [%] | ΔTsol nh [%] | |||
| off | on | off | on | |||
| Green | 31 | 15 | 16 | 52 | 47 | 5 |
| Black | 56 | 49 | 7 | 62 | 56 | 6 |
| Blue | 61 | 34 | 27 | 68 | 62 | 6 |
4. Hybrid Thermotropic and Thermochromic Systems
- -
- Introduction of thermochromic properties into thermotropic hydrogels; and
- -
- Addition of a thermotropic character to thermochromic hydrogels.

5. Nanoparticles in Sun Protection
6. Conclusions
References and Notes
- Granqvist, C.G.; Lansåker, P.C.; Mlyuka, N.R.; Niklasson, G.A.; Avendaño, E. Progress in chromogenics: New results for electrochromic and thermochromic materials and devices. Solar Energ. Mater. Sol. Cell. 2009, 93, 2032–2039. [Google Scholar] [CrossRef]
- Lampert, C.M. Chromogenic smart materials. Mat. Today 2004, 7, 28–35. [Google Scholar] [CrossRef]
- Seeboth, A.; Schneider, J.; Patzak, A. Materials for intelligent sun protecting glazing. Solar Energ. Mater. Sol. Cell. 2000, 60, 263–277. [Google Scholar] [CrossRef]
- Sun protection without bubbles. Kunststoffe 2008, 1, 83.
- Selkowitz, S.; Aschehoug, O.; Lee, E.S. Advanced interactive facades—Critical elements for future green buildings? In Proceeding of GreenBuild, the Annual USGBC International Conference and Expo, Pittsburgh, PA, USA; 2003. Paper LBNL-53876, 13 pages. [Google Scholar]
- Seeboth, A.; Lötzsch, D. Thermochromic Phenomena in Polymers. Smithers Rapra Update; Smithers Rapra Technology Ltd.: Shropshire, UK, 2008. [Google Scholar]
- Nitz, P.; Ferber, J.; Stangl, R.; Wilson, H.R.; Wittwer, V. Simulation of multiply scattering media. Solar Energy Mater. Sol. Cells 1998, 54, 297–307. [Google Scholar] [CrossRef]
- Nitz, P. Optische Modellierung und Vermessung thermotroper Systeme. Ph.D. Thesis, Albert-Ludwigs-University Freiburg im Breisgau, Freiburg, Germany, 1999. [Google Scholar]
- Bohren, C.F.; Huffmann, D.R. Absorption and Scattering of Light by Small Particles, 1st ed.; Wiley-Interscience: New York, NY, USA, 1998. [Google Scholar]
- Novak, B.M. Hybrid nanocomposite materials—between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Resch, K.; Wallner, G.M. Thermotropic layers for flat-plate collectors—A review of various concepts for overheating protection with polymer materials. Solar Energ. Mater. Solar Cells 2009, 93, 119–128. [Google Scholar] [CrossRef]
- Resch, K.; Wallner, G.M.; Lang, R.W. Spectroscopic investigations of phase separated thermotropic layers based on UV cured acrylate resins. Macromol. Symp. 2008, 265, 49–60. [Google Scholar] [CrossRef]
- Resch, K.; Wallner, G.M.; Hausner, R. Phase separated thermotropic layers based on UV cured acrylate resins—Effect of material formulation on overheating protection properties and application in a solar collector. Solar Energ. 2009, 83, 1689–1697. [Google Scholar] [CrossRef]
- Wallner, G.M.; Resch, K.; Hausner, R. Property and performance requirements for thermotropic layers to prevent overheating in an all polymeric flat-plate collector. Solar Energ. Mater. Solar Cells 2008, 92, 614–620. [Google Scholar] [CrossRef]
- Muehling, O.; Seeboth, A.; Haeusler, T.; Ruhmann, R.; Potechius, E.; Vetter, R. Variable solar control using thermotropic core/shell particles. Solar Energ. Mater. Solar Cells 2009, 93, 1510–1517. [Google Scholar] [CrossRef]
- Ruhmann, R.; Muehling, O.; Seeboth, A. Development of Thermochromic Polymer Films for the Sun Protection in Buildings; Final report to the German Federal Ministry of Economics and Technology: Bonn, Germany, 2008. [Google Scholar]
- Siol, W.; Otto, H.J.; Terbrack, U. Glazing with temperature controlled light transparency. DE Patent 3436477, 10 April 1986. [Google Scholar]
- Eck, W.; Cantow, H.J.; Wittwer, V. Material with Temperature Dependent Light Transparency. DE Patent 4206317, 2 September 1993. [Google Scholar]
- Jahns, E. Crosslinked polymer systems. DE Patent 4408156, 14 September 1995. [Google Scholar]
- Wilson, H.R. Potential of thermotropic layers to prevent overheating: A review. Proc. SPIE 1994, 2255, 214. [Google Scholar]
- Wilson, H.R.; Ferber, J.; Platzer, W.J. Optical properties of thermotropic layers. Proc. SPIE 1994, 2255, 473. [Google Scholar]
- Raicu, A.; Wilson, H.R.; Nitz, P.; Platzer, W.; Wittwer, V.; Jahns, E. Façade systems with variable solar control using thermotropic polymer blends. Solar Energ. 2002, 72, 31–42. [Google Scholar] [CrossRef]
- Nitz, P.; Hartwig, H. Solar control with thermotropic layers. Solar Energ. 2005, 79, 573–582. [Google Scholar] [CrossRef]
- Seeboth, A.; Holzbauer, H.R. The Optical Behavior of Lyotropic Liquid Crystalline Polymer Gel Networks: Dependence on Temperature. Adv. Mater. 1996, 8, 408–411. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Progr. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Jones, M.S. Effect of pH on the lower critical solution temperatures of random copolymers of N-isopropylacrylamide and acrylic acid. Eur. Polym. J. 1999, 35, 795–801. [Google Scholar] [CrossRef]
- Yildiz, B.; Isik, B.; Kis, M. Synthesis and characterization of thermoresponsive isopropylacrylamide-acrylamide hydrogels. Eur. Polym. J. 2002, 38, 1343–1347. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, G.; Li, E.; Wang, E.; Zhu, R.; Qi, X. Thermosensitive aggregates self-assembled by an asymmetric block copolymer of dendritic polyether and poly(N-isopropylacrylamide). Eur. Polym. J. 2002, 38, 2503–2506. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Zrínyi, M.; Szilágyi, A.; Filipcsei, G.; Fehér, J.; Szalma, J.; Móczár, G. Smart gel-glass based on the responsive properties of polymer gels. Polym. Adv. Technol. 2001, 12, 501–505. [Google Scholar] [CrossRef]
- Chahroudi, D. Solar Control System. U.S. Patent 4,307,942, 29 December 1981. [Google Scholar]
- Chahroudi, D. Automatic light valves with polymeric layer containing network of bonds. U.S. Patent 5,404,245, 4 April 1995. [Google Scholar]
- Beck, A.; Körner, W.; Scheller, H.; Fricke, J.; Platzer, W.; Wittwer, V. Control of solar insolation via thermochromic light-switching gels. Solar Energ. Mater. Solar Cells 1995, 36, 339–347. [Google Scholar] [CrossRef]
- Seeboth, A.; Holzbauer, H.-R. Thermotropic materials for application in “intelligent windows”. Int. J. Restor. Bldg. Monuments 1998, 4, 507–520. [Google Scholar]
- Fischer, T.; Holzbauer, H.-R.; Seeboth, A. Influence of inorganic salts on optical transmission behavior of thermotropic hydrogels. Mat.-lwiss.u.Werkstofftech 1999, 30, 473–477. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D.; Potechius, E. Phase transitions and phase separations in aqueous polyether systems. Colloid Polym. Sci. 2001, 279, 696–704. [Google Scholar] [CrossRef]
- Seeboth, A.; Kriwanek, J.; Lötzsch, D.; Patzak, A. Chromogenic polymer gels for reversible transparency and color control with temperature at a constant volume. Polym. Adv. Technol. 2002, 13, 507–512. [Google Scholar] [CrossRef]
- Watanabe, H. Intelligent window using a hydrogel layer for energy efficiency. Solar Energ. Mater. Solar Cells 1998, 54, 203–211. [Google Scholar] [CrossRef]
- Watanabe, H. Thermotropic laminates having transparent parts or partitions in predetermined positions. JP 200849693, 3 March 2008. [Google Scholar]
- Schneider, J; Seeboth, A. Natural thermotropic materials for solar switching glazing. Mater. Sci. Eng. Technol. 2001, 32, 231–237. [Google Scholar]
- Chen, J.; Liu, M.; Zhang, N.; Dai, P.; Gao, C.; Ma, L.; Liu, H. Influence of the grafted chain length on responsive behaviors of the grafted poly(DEA-co-DMAEMA) hydrogel. Sens. Actuators B 2010, 149, 34–43. [Google Scholar] [CrossRef]
- Lutz, J.-F. Polymerization of Oligo(EthyleneGlycol) (Meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Fischer, T.; Lange, R.; Seeboth, A. Hybrid solar and electrically controlled transmission of light filters. Solar Energ. Mater. Solar Cells 2000, 64, 321–331. [Google Scholar] [CrossRef]
- Szilagyi, A.; Gyenes, T.; Filipcsei, G.; Zrinyi, M. Thermotropic polymer gels: Smart gel glass. Macromol. Symp. 2005, 227, 357–366. [Google Scholar] [CrossRef]
- Yuan, B.; Wicks, D.A. Thermotropic color changing nanoparticles prepared by encapsulating blue polystyrene particles with a poly-N-isopropylacrylamide gel. J. Appl. Polym. Sci. 2007, 105, 446–452. [Google Scholar] [CrossRef]
- Gong, X.; Li, J.; Chen, S.; Wen, W. Copolymer solution based “smart window”. Appl. Phys. Lett. 2009, 95, 251907. [Google Scholar] [CrossRef]
- Park, M.J.; Char, K. Two gel states of a PEO-PPO-PEO triblock copolymer formed by different mechanisms. Macromol. Rapid Commun. 2002, 23, 688–692. [Google Scholar] [CrossRef]
- Bühler, F.S.; Hewel, M. Reversible Thermotropic Composition, Its Preparation and Use. EP Patent 0985709, 15 March 2000. [Google Scholar]
- Resch, K. Overheating protection properties of thermotropic polyamide. IEA-SHC-T39-Newsletter 2009. [Google Scholar]
- DeArmitt, C.; Mc Kee, G.E. Body with temperature dependent transparency. EP Patent 1985663, 29 October 2008. [Google Scholar]
- Charoudi, D. Transparent Thermal Insulating System. U.S. Patent 3,953,110, 27 April 1976. [Google Scholar]
- Dabisch, W. Body with reversibly changeable temperature dependent extinction of light and method for the manufacture of this body. EP Patent 0000868, 6 July 1978. [Google Scholar]
- Meinhardt, S.; Schönfeld, U.; Gödeke, H. Thermo-Optical Polymer Material Providing Reversible Shading. DE Patent 4433090, 21 March 1996. [Google Scholar]
- Bicer, T.; Gödeke, H.; Werner, J.; Schönfeld, U. Method for Producing A Thermo Optical Variable Polymerized Material, and Use of Same. EP Patent 0946443, 6 October 1999. [Google Scholar]
- Bicer, T.; Schwitalla, C.; Gödecke, H. Method for Producing Thermotropic Casting Resin Systems and Utilization thereof. U.S. Patent 6,489,377, 3 December 2002. [Google Scholar]
- Bühler, F.S.; Hewel, M. Reversible Thermotropic Composition, Its Preparation and Use. EP Patent 0985709, 15 March 2000. [Google Scholar]
- Schwitalla, C.; Gödecke, H.; König, N. Thermotropic Foil and Method of Manufacturing the Same. EP Patent 1258504, 20 November 2002. [Google Scholar]
- Bicer, T.; Schwitalla, C.; Gödecke, H. Method for Producing Thermotropic Casting Resin Systems. DE Patent 19825984, 16 March 2000. [Google Scholar]
- Granqvist, C.G. Transparent conductors as solar energy materials: A panoramic review. Solar Energ. Mater. Solar Cells 2007, 91, 1529–1598. [Google Scholar] [CrossRef]
- How far along is the product development? Available online: http://www.pleotint.com (accessed on 3 December 2010).
- Millett, F.A.; Byker, H.J.; Bruikhuis, M.D. Thermochromic Window Structures. WO 2008/028099A2, 6 March 2008. [Google Scholar]
- Pleotint, L.L.C. Sunlight Resposive Thermochromic Window System; Final Report on Award DE-FG36-04GO14336: West Olive, MI, USA, 18 October 2006. [Google Scholar]
- Arutjunjan, R.E.; Markova, T.S.; Halopenen, I.Y.; Maksimov, I.K.; Tutunnikov, A.I.; Yanush, O.V. Thermochromic Glazing for “Zero Net Energy” House; Proc. Glass Processing Days: Tampere, Finland, 2003; pp. 299–301. [Google Scholar]
- Inouye, M.; Tsuchiya, K.; Kitao, T. New thermo-response dyes: Coloration by the claisen rearrangement and intramolecular acid-base reaction. Angew. Chem. Int. Ed. 1992, 31, 204–205. [Google Scholar] [CrossRef]
- Naito, K. Rewritable color recording media consisting of leuco dye and biphenyl developer with a long alkyl chain. J. Mater. Chem. 1998, 8, 1379–1384. [Google Scholar] [CrossRef]
- Tsutsui, K.; Yamaguchi, T.; Sato, K. Thermochromic properties of mixture systems of octadecylphosphonic acid and fluoran dye. Jpn. J. Appl. Phys. 1994, 33, 5925–5928. [Google Scholar] [CrossRef]
- MacLaren, D.C.; White, M.A. Dye-developer interactions in the crystal violet lactone-lauryl gallate binary system: implications for thermochromism. J. Mater. Chem. 2003, 13, 1695–1700. [Google Scholar] [CrossRef]
- MacLaren, D.C.; White, M.A. Competition between dye-developer and solvent-developer interactions in a reversible thermochromic system. J. Mater. Chem. 2003, 13, 1701–1704. [Google Scholar] [CrossRef]
- MacLaren, D.C.; White, M.A. Design rules for reversible thermochromic mixtures. J. Mater. Sci. 2005, 40, 669–676. [Google Scholar] [CrossRef]
- Seeboth, A.; Muehling, O.; Ruhmann, R.; Vetter, R. Composite having Inverse Thermochromic Properties, Composite Material Comprising the Same and Its Use. WO Patent 2008/125350, 23 October 2008. [Google Scholar]
- Seeboth, A.; Kriwanek, J.; Vetter, R. Novel chromogenic polymer gel networks for hybrid transparency and color control with temperature. Adv. Mater. 2000, 12, 1424–1426. [Google Scholar] [CrossRef]
- Seeboth, A.; Kriwanek, J.; Vetter, R. The first example of thermochromism of dyes embedded in transparent polymer gel networks. J. Mater. Chem. 1999, 9, 2277–2278. [Google Scholar] [CrossRef]
- Kriwanek, J.; Vetter, R.; Lötzsch, D.; Seeboth, A. Influence of a zwitterionic surfactant on the chromogenic behavior of a dye-containing aqueous PVA-polyether gel network. Polym. Adv. Technol. 2003, 14, 79–82. [Google Scholar] [CrossRef]
- Smith, G.B. Materials and systems for efficient lighting and delivery of daylight. Solar Energ. Mater. Solar Cells 2004, 84, 395–409. [Google Scholar] [CrossRef]
- Schelm, S.; Smith, G.B. Dilute LaB6 nanoparticles in polymer as optimized clear solar control glazing. Appl. Phys. Lett. 2003, 82, 4346–4348. [Google Scholar] [CrossRef]
- Chowdhury, H.; Xu, X.; Cortie, M.B. Radiative heat transfer across glass coated with gold nano-particles. J. Sol. Energy Eng. 2005, 127, 70–75. [Google Scholar] [CrossRef]
- Smith, G.B.; Deller, C.A.; Swift, P.D.; Gentle, A.; Garrett, P.D.; Fisher, W.K. Nanoparticle-doped polymer foils for use in solar control glazing. J. Nanopart. Res. 2002, 4, 157–165. [Google Scholar] [CrossRef]
- Smith, G.B.; Dligatch, S.; Sullivan, R.; Hutchins, M.G. Thin film angular selective glazing. Solar Energ. 1998, 62, 229–244. [Google Scholar] [CrossRef]
- Schelm, S.; Smith, G.B.; Garrett, P.D.; Fisher, W.K. Tuning the surface-plasmon resonance in nanoparticles for glazing applications. J. Appl. Phys. 2005, 97, 124314. [Google Scholar] [CrossRef]
- Cortie, M.B.; Xu, X.; Ford, M.J. Effect of composition and packing configuration on the dichroic optical properties of coinage metal nanorods. Phys. Chem. Chem. Phys. 2006, 8, 3520–3527. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, L.; Jin, X.; Wang, D.; Chen, G.Z. Solar-thermochromism of pseudocrystalline nanodroplets of ionic liquid-NiII complexes immobilized inside translucent microporous PVDF films. Adv. Mater. 2009, 21, 776–780. [Google Scholar] [CrossRef]
- Carotenuto, G.; La Peruta, G.; Nicolais, L. Thermo-chromic materials based on polymer-embedded silver clusters. Sens. Actuators B 2005, 114, 1092–1095. [Google Scholar] [CrossRef]
- SOLARDIM®ECO. Available online: http://www.tilse.com (accessed on 28 September 2010).
- Kulčar, R.; Friškovec, M.; Hauptman, N.; Vesel, A.; Klanjšek, G.M. Colorimetric properties of reversible thermochromic printing inks. Dyes Pigm. 2010, 86, 271–277. [Google Scholar] [CrossRef]
- Kunzelman, J.; Crenshaw, B.R.; Weder, C. Self-assembly of chromogenic dyes—A new mechanism for humidity sensors. J. Mater. Chem. 2007, 17, 2989–2991. [Google Scholar] [CrossRef]
- Sussman, J.; Snoswell, D.; Kontogeorgos, A.; Baumberg, J.J.; Spahn, P. Thermochromic polymer opals. Appl. Phys. Lett. 2009, 95, 173116. [Google Scholar] [CrossRef]
- Seeboth, A.; Ruhmann, R.; Vetter, R. Piezochromic Material, Piezochromic Composite Material and Piezochromic Sensor. DE Patent application 102009035363, 29 April 2009. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Seeboth, A.; Ruhmann, R.; Mühling, O. Thermotropic and Thermochromic Polymer Based Materials for Adaptive Solar Control. Materials 2010, 3, 5143-5168. https://doi.org/10.3390/ma3125143
Seeboth A, Ruhmann R, Mühling O. Thermotropic and Thermochromic Polymer Based Materials for Adaptive Solar Control. Materials. 2010; 3(12):5143-5168. https://doi.org/10.3390/ma3125143
Chicago/Turabian StyleSeeboth, Arno, Ralf Ruhmann, and Olaf Mühling. 2010. "Thermotropic and Thermochromic Polymer Based Materials for Adaptive Solar Control" Materials 3, no. 12: 5143-5168. https://doi.org/10.3390/ma3125143




