A New Approach for the Modification of Paper Surface Properties Using Polyoxometalates
Abstract
:1. Introduction

2. Results and Discussion
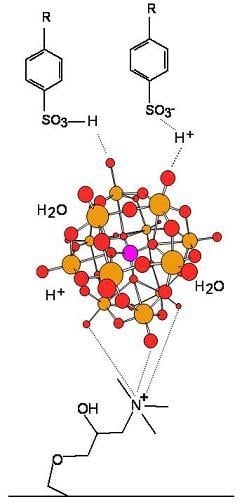
2.1. Treatment of the Paper Surface with Cationic Starch and Polyoxometalates
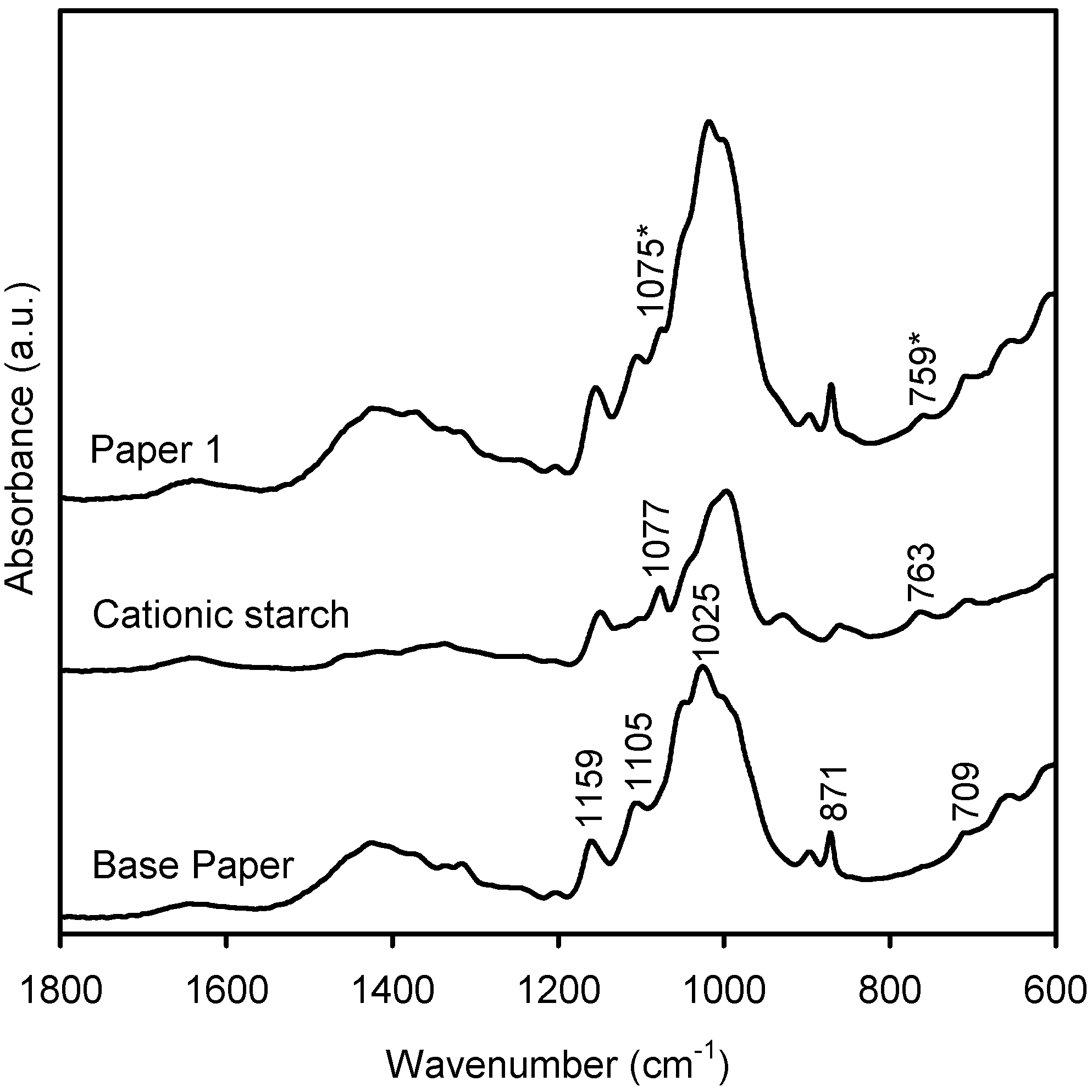
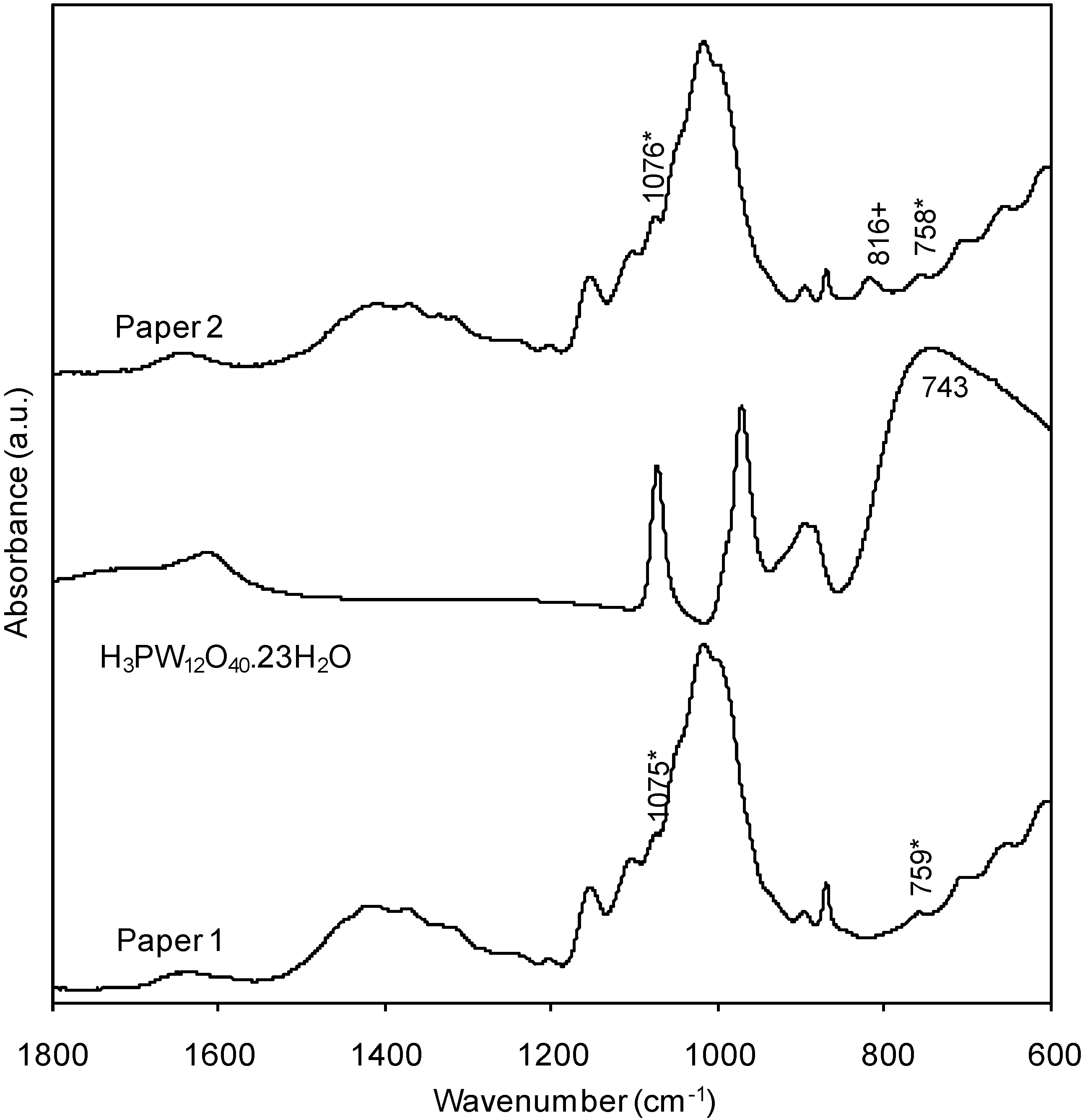
2.2. Characterization of the New Paper Samples by FTIR-ATR
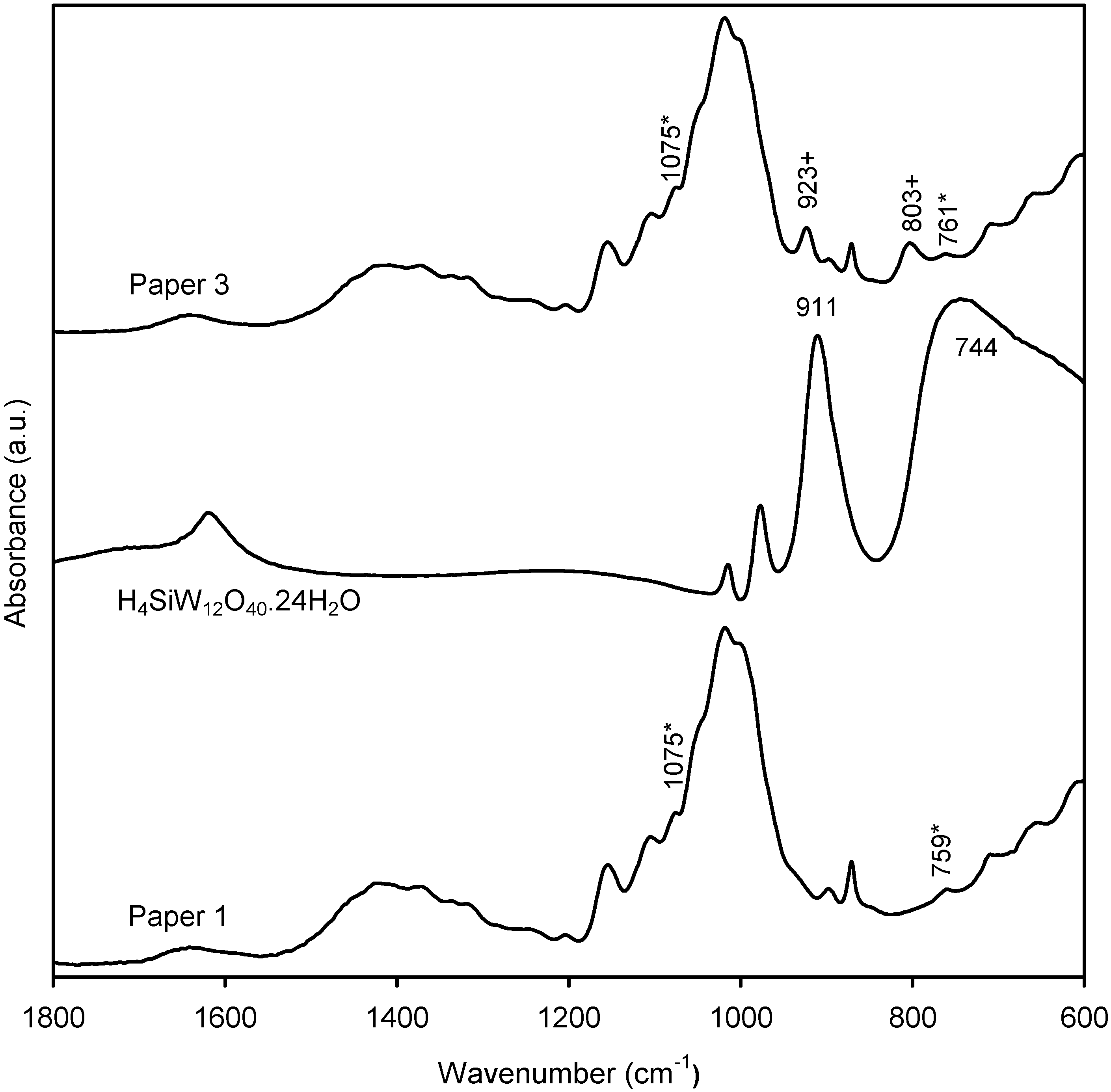
2.3. Surface Energy Parameters
| Paper samples | Water contact angle (°) | Surface energy, σs (mN/m) | Polar component, σsp (mN/m) | Dispersive component, σsd (mN/m) | Correlation (R2) |
|---|---|---|---|---|---|
| Base paper | 101.7 ± 2.2 | 47.4 | 0.1 | 47.3 | 0.660 |
| 1 | 38.2 ± 1.8 | 73.8 | 72.7 | 1.1 | 0.992 |
| 2 | 49.7 ± 2.3 | 59.6 | 56.9 | 2.7 | 0.991 |
| 3 | 42.2 ± 3.9 | 73.7 | 73.2 | 0.48 | 0.994 |
| 4 | 29.7 ± 2.4 | 87.4 | 87.2 | 0.21 | 0.988 |
2.4. Printability

| Paper samples | Cyan | Magenta | Yellow | Black 0 |
|---|---|---|---|---|
| Base paper | 0.935 | 0.91 | 0.968 | 1.376 |
| 1 | 0.897 | 0.922 | 0.958 | 1.482 |
| 2 | 0.951 | 0.924 | 1.029 | 1.459 |
| 3 | 0.976 | 0.948 | 1.005 | 1.425 |
| 4 | 0.914 | 0.939 | 0.980 | 1.462 |
| Paper samples | Print-through [0.0.0] | Line width, (black, μm) | Intercolor bleed | Dot diameter (black, μm) | Dot diameter (magenta, μm) |
|---|---|---|---|---|---|
| Base paper | 1.31 | 474 | 50.1 | 507 | 409 |
| 1 | 0.53 | 465 | 37.4 | 527 | 419 |
| 2 | 0.70 | 480 | 39.4 | 529 | 413 |
| 3 | 0.42 | 519 | 43.3 | 551 | 426 |
| 4 | 0.64 | 480 | 36.4 | 528 | 416 |
2.5. Relation between Printability and Chemical and Surface Properties of the New Paper Samples
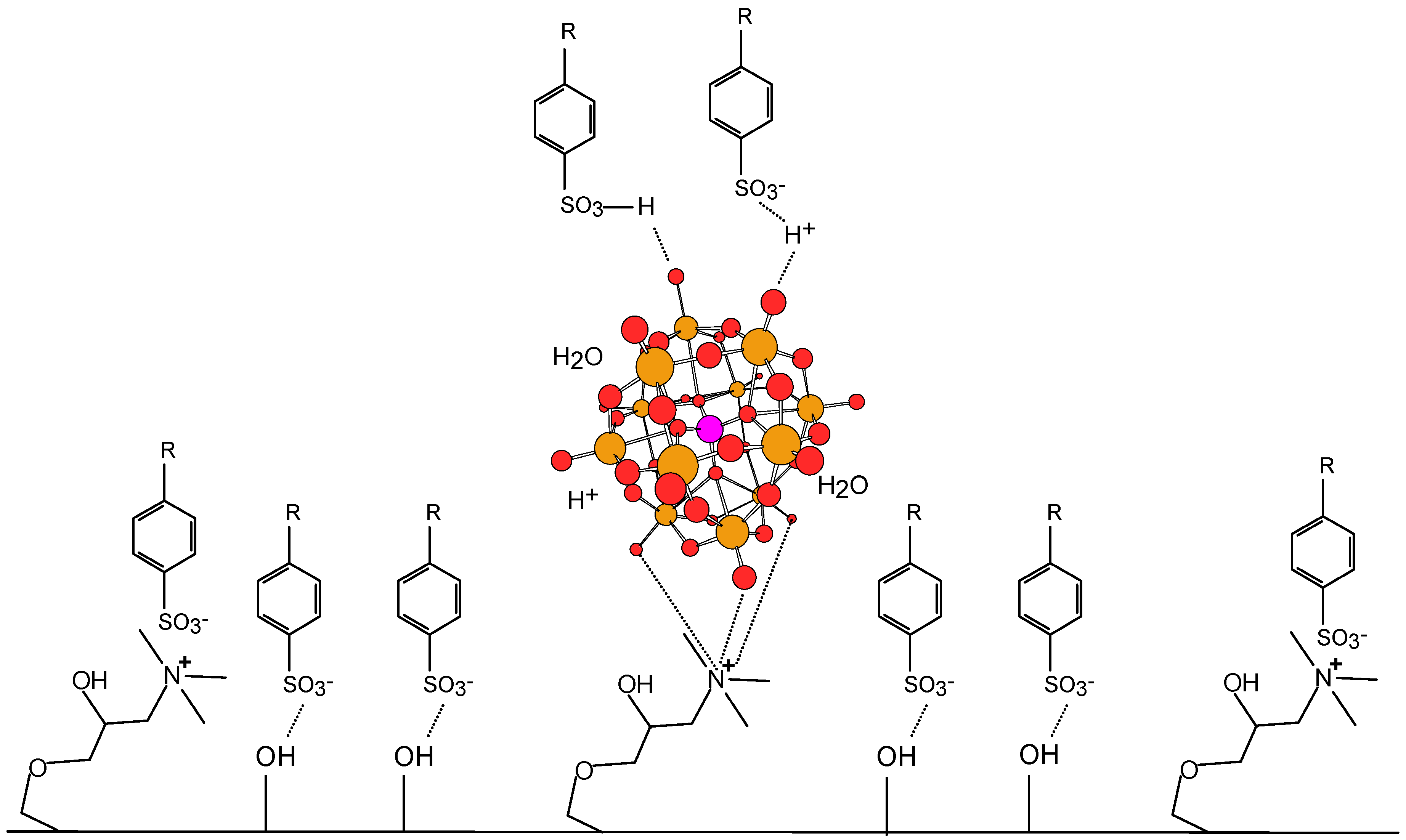
3. Experimental Section
3.1. Materials
3.2 Preparation of the Cationic Starch/Polyoxometalate Mixtures for the Paper Surface Treatment
| Paper samples | Mixtures used in the surface treatment | Average pick-up (g/m2)a | |
|---|---|---|---|
| Relative amounts (w/w) of solid compounds | pH (T~50 °C) | ||
| 1 | 100% Cationic starch | 5.9 | 2.73 |
| 2 | 80% cationic starch/ 20% H3PW12O40 | 2.3 | 2.92 |
| 3 | 80% cationic starch/ 20% H4SiW12O40 | 2.1 | 2.51 |
| 4 | 80% cationic starch/ 20% K7PW11O39 | 6.0 | 2.88 |
3.3. Treatment of the Paper Surface
3.4. Evaluation of the Paper Surface Properties
4. Conclusions
References
- Prinz, M.; Schultz, W.-S. Sizing agents for surface and wet end application. Prof. Papermaking 2007, 1, 44–49. [Google Scholar]
- Moutinho, I.M.T.; Ferreira, P.J.T.; Figueiredo, M.M.L. Impact of surface sizing on inkjet printing quality. Ind. Eng. Chem. Res. 2007, 46, 6183–6188. [Google Scholar] [CrossRef]
- Lehtinen, E. Coating pigments general. In Pigment Coating and Surface Sizing of Paper; Lehtinen, E., Ed.; Fapet Oy: Helsinki, Finland, 2000; pp. 61–67. [Google Scholar]
- Donigian, D.W.; Wernett, P.C.; McFadden, M.G.; McKay, J.J. Ink-jet dye fixation and coating pigments. Tappi J. 1999, 82, 175–180. [Google Scholar]
- Hladnik, A.; Muck, T. Characterization of pigments in coating formulations for high-end ink-jet papers. Dyes Pigments 2002, 54, 253–263. [Google Scholar] [CrossRef]
- Vikman, K. Fastness properties of ink jet prints on coated papers-part 1: Effect of coating polymer system on light fastness. J. Imaging Sci. Techn. 2003, 47, 30–37. [Google Scholar]
- Vikman, K. Fastness properties of ink jet prints on coated papers-part 2: Effect of coating polymer system on water fastness. J. Imaging Sci. Techn. 2003, 47, 38–43. [Google Scholar]
- Lee, H.; Joyce, M.K.; Fleming, P.D.; Cawthorne, J.E. Influence of silica and alumina oxide on coating structure and print quality of ink-jet papers. Tappi J. 2005, 4, 11–16. [Google Scholar]
- Sreekumar, J.; Sain, M.; Farnood, R.; Dougherty, W. Styrene maleic anhydride imide resin (SMAI): A novel cationic additive in paper coating for ink-jet printing. Pulp Pap. Can. 2005, 106, 38–41. [Google Scholar]
- Sreekumar, J.; Sain, M.; Farnood, R.; Dougherty, W. Influence of styrene maleic anhydride imide on inkjet print quality and coating structure. Nord. Pulp Pap. Res. J. 2007, 22, 307–313. [Google Scholar] [CrossRef]
- Nilsson, H.; Fogden, A. Inkjet print quality on model paper coatings. Appita J. 2008, 61, 120–127. [Google Scholar]
- Perng, Y.; Wang, E.I.; Lu, C.; Kuo, L. Application of sericite to LWC coatings. Tappi J. 2008, 7, 21–26. [Google Scholar]
- Andersson, C. New ways to enhance the functionality of paperboard by surface treatment—A review. Packag. Technol. Sci. 2008, 21, 339–373. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer Verlag: Berlin, Germany, 1983. [Google Scholar]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition-metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Baker, L.C.W.; Glick, D.C. Present general status of understanding of heteropoly electrolytes and a tracing of some major highlights in the history of their elucidation. Chem. Rev. 1998, 98, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Pope, M.T.; Muller, A. (Eds.) Polyoxometalate Chemistry: From Topology via Self Assembly to Applications; Kluwer: Dordrecht, The Netherlands, 2001.
- Borrás-Almenar, J.J.; Coronado, E.; Muller, A.; Pope, M.T. (Eds.) Polyoxometalate Molecular Science; Kluwer: Dordrecht, The Netherlands, 2004.
- Gaspar, A.R.; Gamelas, J.A.F.; Evtuguin, D.V.; Pascoal Neto, C. Alternatives for lignocellulosic pulp delignification using polyoxometalates and oxygen: A review. Green Chem. 2007, 9, 717–730. [Google Scholar] [CrossRef]
- Attanasio, D.; Bonamico, M.; Fares, V.; Imperatori, P.; Suber, L. Weak charge-transfer polyoxoanion salts: The reaction of quinolin-8-ol (Hquin) with phosphotungstic acid and the crystal and molecular structure of [H2quin]3[PW12O40].4EtOH.2H2O. J. Chem. Soc., Dalton Trans. 1990, 3221–3228. [Google Scholar]
- Gamelas, J.A.F.; Cavaleiro, A.M.V.; Matos Gomes, E.; Belsley, M.; Herdtweck, E. Synthesis, properties and photochromism of novel charge transfer compounds with Keggin anions and protonated 2,2’- biquinoline. Polyhedron 2002, 21, 2537–2545. [Google Scholar] [CrossRef]
- Kato, C.N.; Tanabe, A.; Negishi, S.; Goto, K.; Nomiya, K. An efficient PMo11VVO404−/silica material having cationic ammonium moiety: Synthesis, characterization, and catalytic performance for oxidation of alcohols with dioxygen. Chem. Lett. 2005, 34, 238–239. [Google Scholar] [CrossRef]
- Gamelas, J.A.F.; Santos, F.M.; Felix, V.; Cavaleiro, A.M.V.; Matos Gomes, E.; Belsley, M.; Drew, M.G.B. Novel charge transfer supramolecular assemblies with Keggin anions and 2-amino-5-nitropyridine. Dalton Trans. 2006, 1197–1203. [Google Scholar] [CrossRef]
- Gamelas, J.A.F.; Evtuguin, D.V.; Esculcas, A.P. Transition metal substituted polyoxometalates supported on amine-functionalized silica. Trans. Met. Chem. 2007, 32, 1061–1067. [Google Scholar] [CrossRef]
- Makinen, M. Coating color preparation. In Pigment Coating and Surface Sizing of Paper; Lehtinen, E., Ed.; Fapet Oy: Helsinki, Finland, 2000; pp. 319–387. [Google Scholar]
- Nyquist, R.A.; Putzig, C.L.; Leugers, M.A. The Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts; Academic press: San Diego, CA, USA, 1997; Volumes 1 and 4. [Google Scholar]
- Kacurakova, M.; Smith, A.C.; Gidley, M.J.; Wilson, R.H. Molecular interactions in bacterial cellulose composites studied by 1D FT-IR and dynamic 2D FT-IR spectroscopy. Carbohydr. Res. 2002, 337, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.J.; Gamelas, J.A.; Moutinho, I.M.; Ferreira, A.G.; Gómez, N.; Molleda, C.; Figueiredo, M.M. Application of FT-IR-ATR spectroscopy to evaluate the penetration of surface sizing agents into the paper structure. Ind. Eng. Chem. Res. 2009, 48, 3867–3872. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FT-IR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mal, D.; Singh, R.P. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Matsushita, Y.; Suzuki, A.; Sekiguchi, T.; Saito, K.; Imai, T.; Fukushima, K. Mapping of the cationic starch adsorbed on pulp fibers by ToF-SIMS. Appl. Surf. Sci. 2008, 255, 1022–1024. [Google Scholar] [CrossRef]
- Brown, G.M.; Noe-Spirlet, M.-R.; Busing, W.R.; Levy, H.A. Dodecatungstophosphoric acid hexahydrate, (H5O2+)3(PW12O403-). The true structure of Keggin’s “pentahydrate” from single-crystal X-ray and neutron diffraction data. Acta Crystallogr. B 1977, 33, 1038–1046. [Google Scholar] [CrossRef]
- Kallio, T.; Kekkonen, J.; Stenius, P. Acid/base properties and adsorption of an azo dye on coating pigments. J. Disper. Sci. Technol. 2006, 27, 825–834. [Google Scholar] [CrossRef]
- Hladnik, A.; Cernic, M.; Bukosek, V. Role of paper coating pigments and additives in darkfastness of ink jet prints. J. Imaging Sci. Techn. 2008, 52, 010507:1–010507:7. [Google Scholar] [CrossRef]
- Haraguchi, N.; Okaue, Y.; Isobe, T.; Matsuda, Y. Stabilization of tetravalent cerium upon coordination of unsaturated heteropolytungstate anions. Inorg. Chem. 1994, 33, 1015–1020. [Google Scholar] [CrossRef]
- Oittinen, P. Papermaking Science and Technology Series, Book 13; Fapet Oy: Helsinki, Finland, 1999; pp. 33, 75–77. [Google Scholar]
- Levlin, J.; Soderhjelm, L. Papermaking Science and Technology Series, Book 17; Fapet Oy: Helsinki, Finland, 1999; p. 171. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saraiva, M.S.; Gamelas, J.A.F.; Mendes de Sousa, A.P.; Reis, B.M.; Amaral, J.L.; Ferreira, P.J. A New Approach for the Modification of Paper Surface Properties Using Polyoxometalates. Materials 2010, 3, 201-215. https://doi.org/10.3390/ma3010201
Saraiva MS, Gamelas JAF, Mendes de Sousa AP, Reis BM, Amaral JL, Ferreira PJ. A New Approach for the Modification of Paper Surface Properties Using Polyoxometalates. Materials. 2010; 3(1):201-215. https://doi.org/10.3390/ma3010201
Chicago/Turabian StyleSaraiva, Mikhail S., José A. F. Gamelas, António P. Mendes de Sousa, Bruno M. Reis, José L. Amaral, and Paulo J. Ferreira. 2010. "A New Approach for the Modification of Paper Surface Properties Using Polyoxometalates" Materials 3, no. 1: 201-215. https://doi.org/10.3390/ma3010201