Interactions between CO and Poly(p-phenylene vinylene) as Induced by Ion-Exchanged Zeolites
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
3.1. Characterization of Poly(p-phenylene vinylene)
| Functional groups | Wavenumber (cm–1) | References | ||
|---|---|---|---|---|
| PPV Precursor | PPV | doped PPV | ||
| Phenylene out of plane ring bending | 560 | 557 | 557 | [32] |
| [550 ± 10] | [550 ± 10] | [550 ± 10] | ||
| C-S stretching | 637 | _ | _ | [33] |
| [632 ± 10] | ||||
| S-O stretching | _ | _ | 640 | [33] |
| [650 ± 10] | ||||
| para-phenylene ring C-H out of plane bending | 840 | 837 | 837 | [32] |
| [830 ± 10] | [830 ± 10] | [830 ± 10] | ||
| C-H out of plane bending | 950 | 963 | 963 | [32] |
| [960 ± 10] | [960 ± 10] | [960 ± 10] | ||
| S=O symmetric stretching | _ | _ | 1,056 | [33] |
| [1,050 ± 10] | ||||
| Quinoid ring C=C stretching | _ | _ | 1,177 | [33] |
| [1,170 ± 10] | ||||
| S=O asymmetric stretching | _ | _ | 1,204 | [33] |
| [1,200 ± 10] | ||||
| C-C ring stretching | 1,513 | 1,517 | 1,517 | [32] |
| [1,517 ± 10] | [1,517 ± 10] | [1,517 ± 10] | ||
| CH3 symmetric stretching | 2,880 | 2,882 | 2,880 | [32] |
| [2,872 ± 10] | [2,872 ± 10] | [2,872 ± 10] | ||
| CH3 asymmetric stretching | 2,950 | 2,961 | 2,962 | [32] |
| [2,960 ± 10] | [2,960 ± 10] | [2,960 ± 10] | ||
| trans-vinylene C-H strectching | _ | 3,023 | 3,023 | [32] |
| [3,022 ± 10] | [3,022 ± 10] | |||
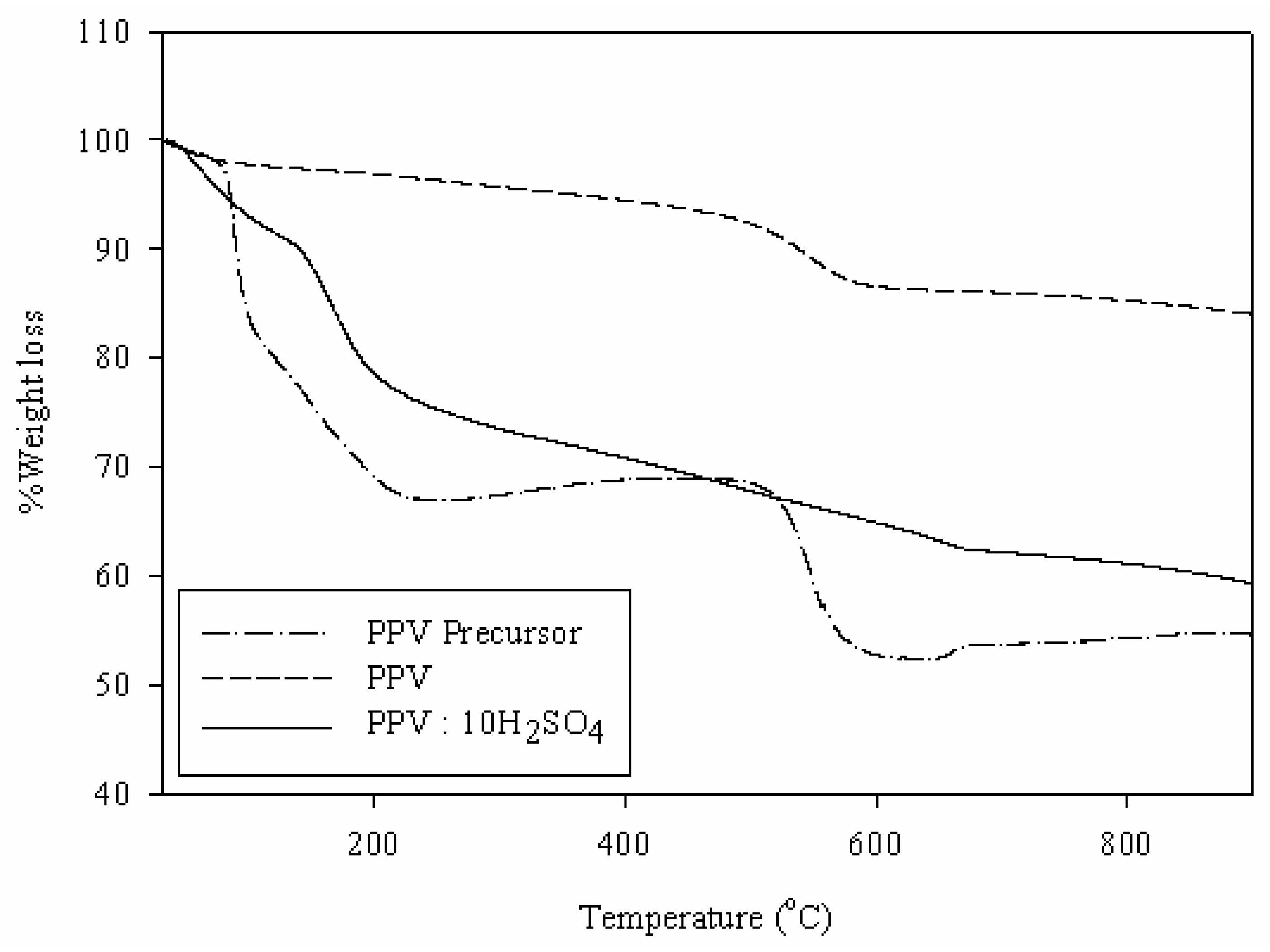
3.2. Characterization of Zeolite
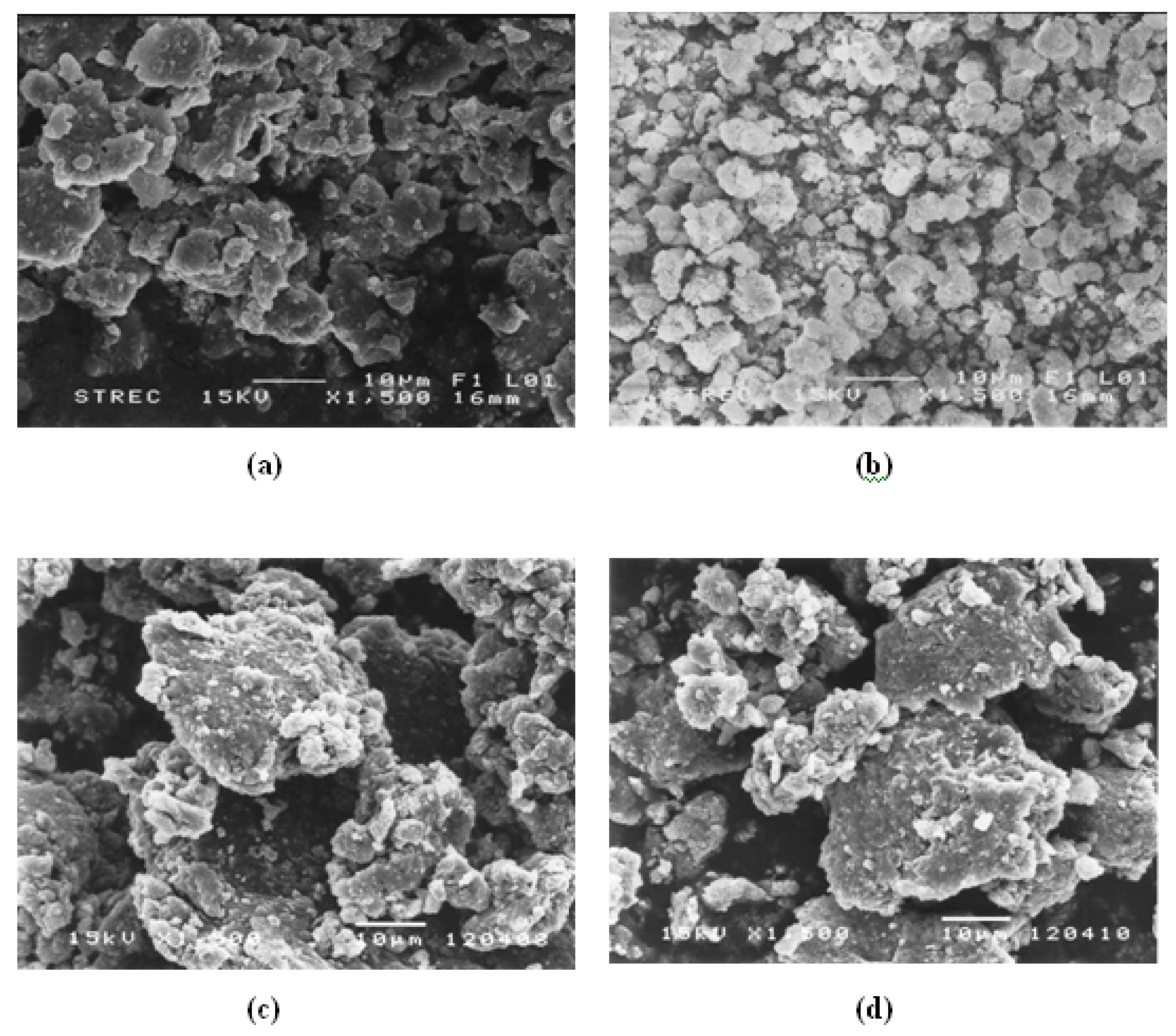
3.3. Ion-Exchange of Zeolites
3.4. Conductivity Measurements
3.4.1. Electrical Conductivity: Steady State Sensitivity to CO
| Sample | Electrical conductivity sensitivity (Δσ/σN2) | Response time (tr, min) |
|---|---|---|
| PPV | 6.01E−02 | 10.26 |
| Doped PPV | 5.44E−01 | 146 |
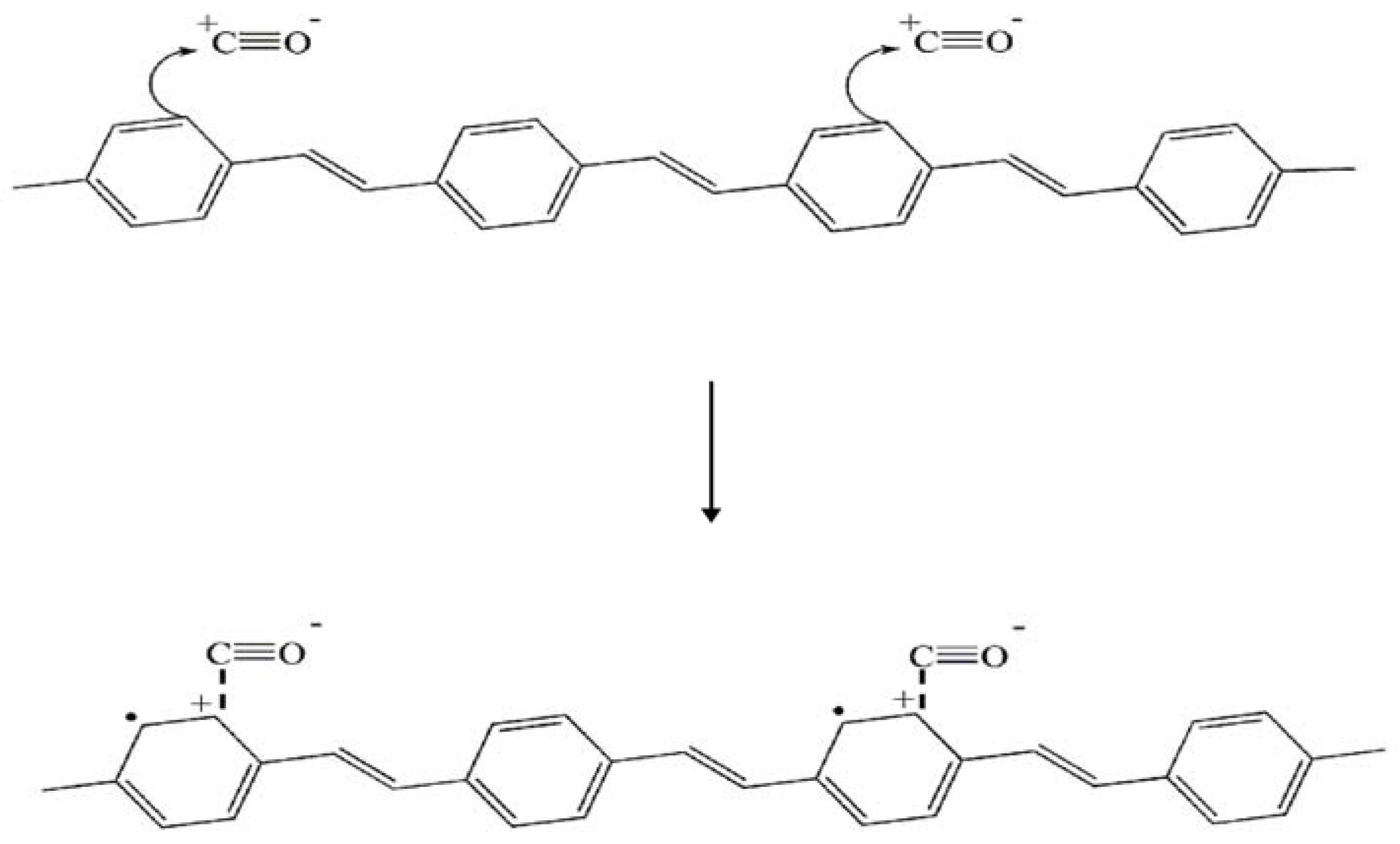
3.4.2. Effect of Zeolite Type on Electrical Sensitivity
| Sample | The sensitivity (∆σ/σN2) | The temporal response (tr, min) | Ion-exchanged level (%) |
|---|---|---|---|
| dPPV/13X_Na | 1.02E+00 | 42 | 100 |
| dPPV/fer_Na | 9.16E-01 | 123 | 17 |
| dPPV/ZSM5(23)_Na | 1.48E+00 | 56 | 28 |
| dPPV/ZSM5(80)_Na | 1.17E+00 | 153 | 17 |
| dPPV/ZSM5(23)_Cu | -1.54E-01 | 24 | 44 |
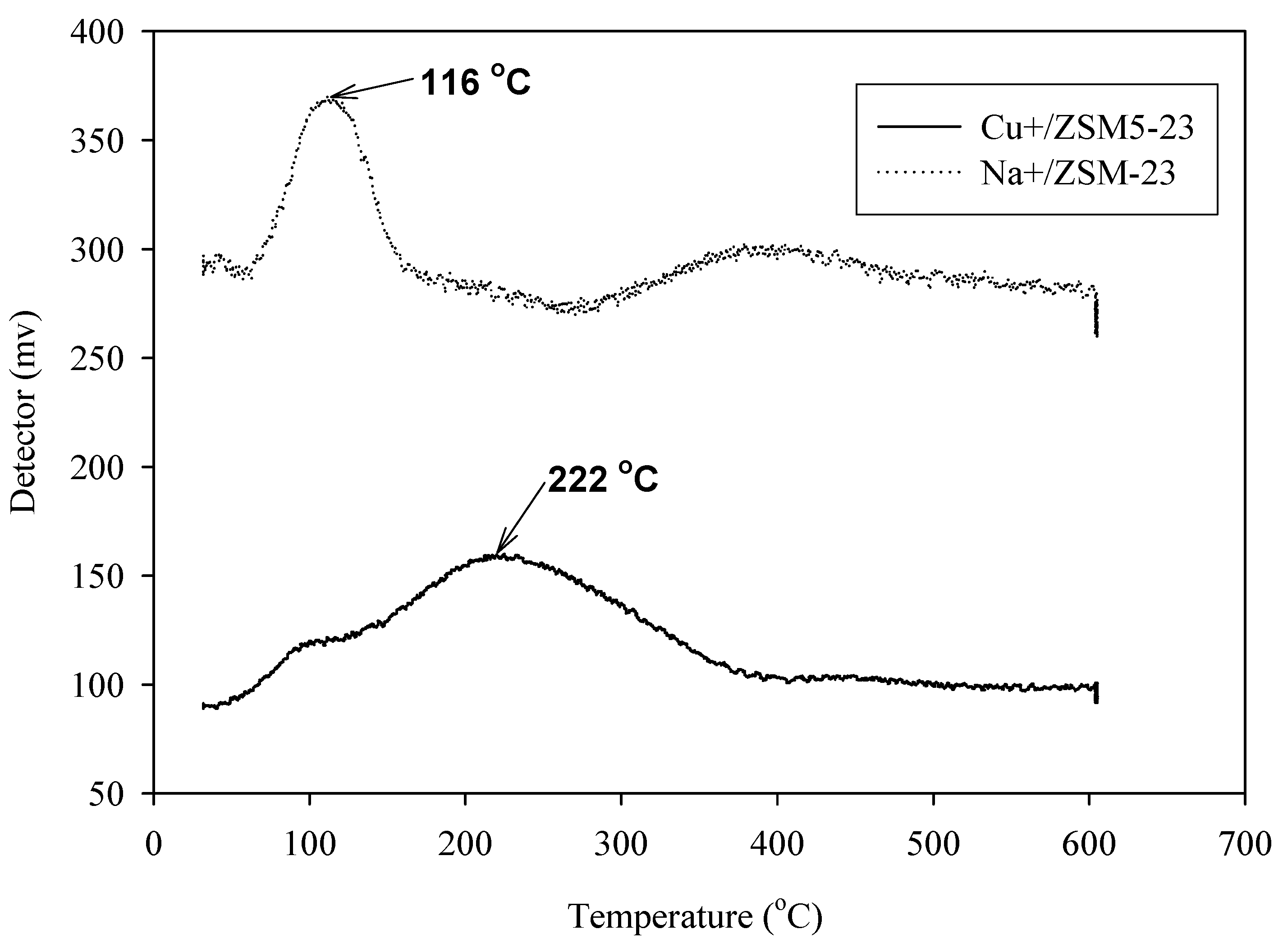
3.4.3. Effect of Cation Type on Electrical Sensitivity
| Sample | The temperatures at the maximum desorptions of CO (°C) |
|---|---|
| Na+-ZSM5(Si/Al = 23) | 116 |
| Cu+-ZSM 5(Si/Al = 23) | 222 |
3.5. FTIR Investigation of Interactions of Adsorbed CO
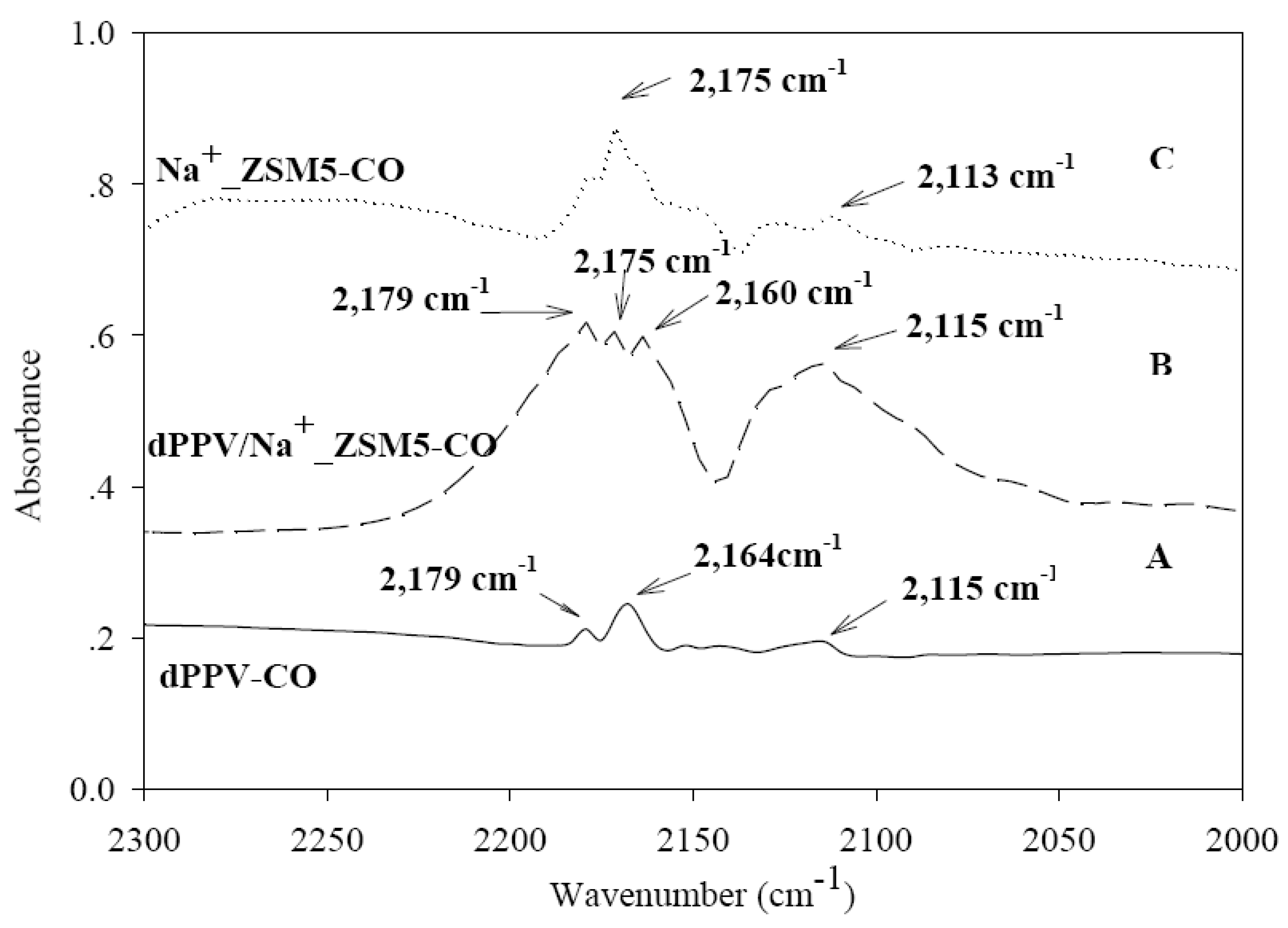
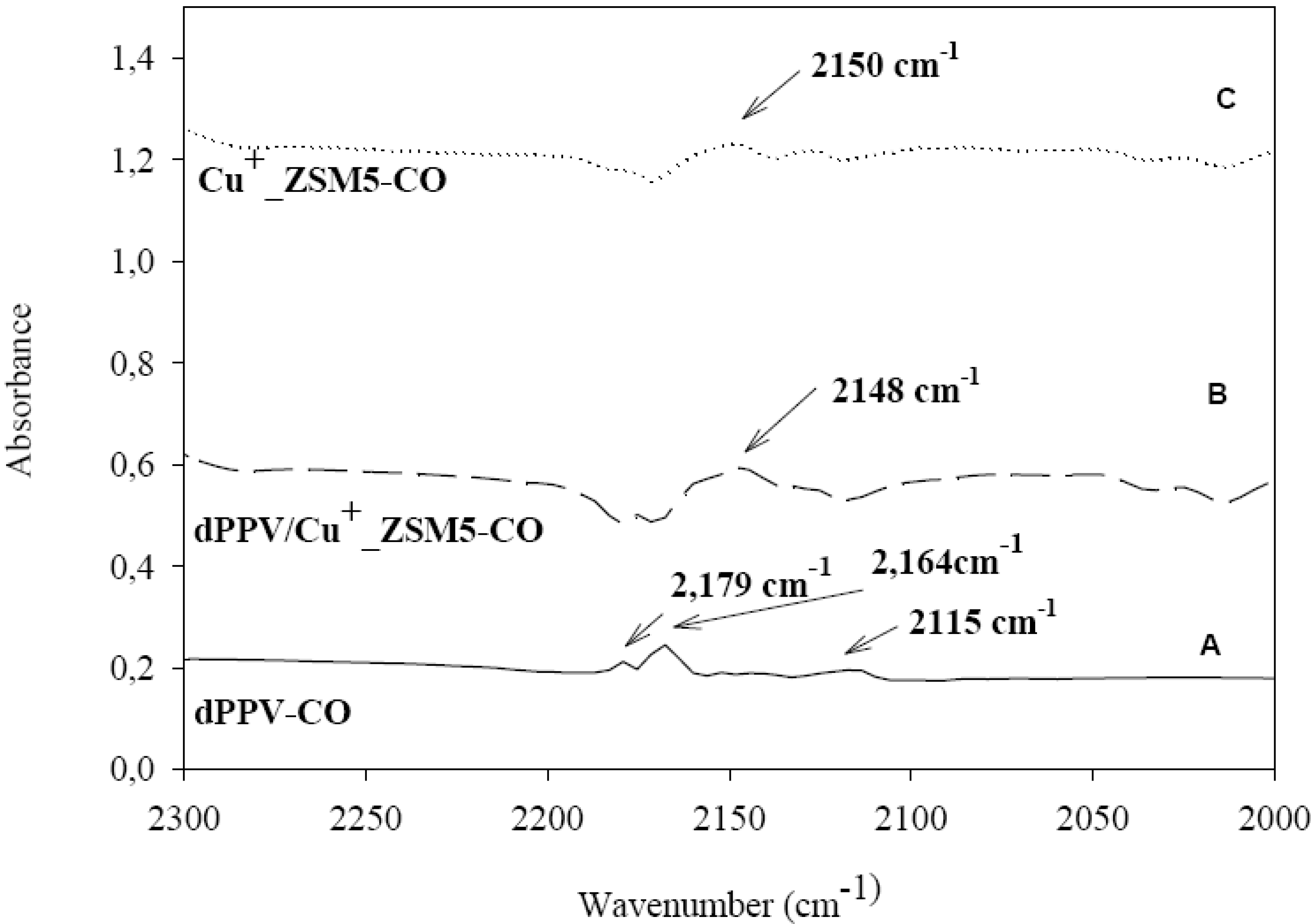
4. Conclusions
Acknowledgments
References
- Ram, M. K.; Yavuz, O.; Lahsangah, V.; Aldissi, M. CO gas sensing from ultrathin nano-composite conducting polymer film. Sens. Actuat. B 2005, 106, 750–757. [Google Scholar] [CrossRef]
- Becker, T.; Mühlberger, S.; Braunmühl, B.; Müller, G.; Ziemann, Th.; Hechtenberg, K.V. Air pollution monitoring using tin-oxide-based microreactor systems. Sens. Actuat. B 2000, 69, 108–119. [Google Scholar] [CrossRef]
- Lenz, R.W.; Han, C.C.; Stenger-Smith, J.; Karasz, F.E. Preparation of poly(phenylene vinylene) from cycloalkylene sulfonium salt monomers and polymers. J. Polym. Sci. Polym. Chem. 1988, 26, 3241–3249. [Google Scholar] [CrossRef]
- Wessling, R.A. The polymerization of xylyene bisdialkyl sulfonium salts. J. Polym. Sci. Polym. Symp. 1986, 72, 55–66. [Google Scholar] [CrossRef]
- Antoun, S.; Gagnon, D.R.; Karasz, F.E.; Lenz, R.W. Synthesis and electrical conductivity of AsF5-doped poly(arylene vinylenes). Polym. Bull. 1986, 15, 181–184. [Google Scholar] [CrossRef]
- Gagnon, D.R.; Karasz, F.E.; Lenz, R.W. Molecular orientation and conductivity in highly drawn poly(p-phenylene vinylene). Synth. Meth. 1987, 20, 85–95. [Google Scholar] [CrossRef]
- Karasz, F.E.; Capistran, J.D.; Gagnon, D.R.; Lenz, R.W. High molecular weight polyphenylene vinylene. Mol. Cryst. Liq. Cryst. 1985, 118, 327–332. [Google Scholar] [CrossRef]
- Gagnon, D.R.; Capistran, J.D.; Karasz, F.E.; Lenz, R.W. Molecular orientation and conductivity in highly drawn poly(p-phenylene vinylene). Polym. Bull. 1984, 12, 293–298. [Google Scholar] [CrossRef]
- Granier, T.; Thomas, E.L.; Gagnon, D.R.; Karasz, F.E.; Lenz, R.W. Structure investigation of poly(p-phenylene vinylene). J. Polym. Sci. Phys. 1986, 24, 2793–2804. [Google Scholar] [CrossRef]
- Wessling, R.A.; Zimmerman, R.G. Polyelectrolytes from bis-sulfonium salts. U.S. Patent 3,401, 152, 1968. [Google Scholar]
- Murase, I.; Ohnishi, T.; Noguchi, T.; Hiroka, M.; Murakaami, S. Highly conducting poly(p-phenylene vinylene) prepared from sulfonium salt. Mol. Cryst. Liq. Cryst. 1985, 118, 333–336. [Google Scholar] [CrossRef]
- Murase, I.; Ohnishi, T.; Noguchi, T.; Hiroka, M. Highly conducting poly(2,5-thienylene vinylene) via a precursor route. Polym. Commun. 1984, 25, 327–329. [Google Scholar]
- Montaudo, G.; Vitalini, D.; Lenz, R.W. Mechanism of thermal generation of poly(p-phenylenevinylene) from poly(p-xylene-alpha-dimethylsulfonium halides). Polymer 1987, 28, 837–842. [Google Scholar] [CrossRef]
- Angell, C.L.; Schaffer, P.C. Infrared spectroscopic investigations of zeolites and adsorbed molecules, II: Adsorbed carbon monoxide. J. Phys. Chem. 1966, 70, 1413–1418. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Lamberti, C.; Spoto, G.; Carnelli, L.; Otero, A.C. low temperature fourier transform infrared study of the interaction of co with cations in alkali-metal exchanged ZSM-5 zeolites. J. Phys. Chem. 1994, 98, 9577–9582. [Google Scholar] [CrossRef]
- Bordiga, S.; Lamberti, C.; Geobaldo, F.; Zecchina, A.; Turnes, P.G.; Otero, A.C. fourier-transform infrared study of co adsorbed at 77 k on h-mordenite and alkali- metal-exchanged mordenites. Langmuir 1995, 11, 527–533. [Google Scholar] [CrossRef]
- Bordiga, S.; Garrone, E.; Lamberti, C.; Zecchina, A.; Otero, A.C.; Kazansky, V.B.; Kustov, L.M. Brønsted acid strength in US-Y: FTIR study of CO adsorption. J. Chem. Soc. Faraday Trans. 1994, 90, 3367–3372. [Google Scholar] [CrossRef]
- Katoh, M.; Yamazaki, T.; Ozawa, S. IR spectroscopic study of adsorption of binary gases over ion-exchanged ZSM-5 zeolites. J. Colloid. Interf. Sci. 1998, 203, 447–455. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Neyman, K.M.; Rösch, N. reliability of small cluster models for cu-exchanged zeolites. J. Phys. Chem. 1997, 101, 9292–9298. [Google Scholar] [CrossRef]
- Sauer, J.; Ugliengo, P.; Garrone, E.; Saunders, V.R. Theoretical study of van der waals complexes at surface sites in comparison with experiment. Chem. Rev. 1994, 94, 2095–2106. [Google Scholar] [CrossRef]
- Colbourn, E.A. Computer simulation of defects and reacrtions at oxides surfacees. Surf. Sci. Rep. 1992, 15, 281–319. [Google Scholar] [CrossRef]
- Kuroda, Y.; Yoshikawa, Y.; Ryotaro, K.; Mahiko, N. analysis of active sites on copper ion-exchanged ZSM-5 for CO adsorption through ir and adsorption heat measurements. J. Phys. Chem. 1997, 101, 6497–6503. [Google Scholar] [CrossRef]
- Sodupe, M.; Baushchlicher, C.W.; Lee, T. The calculation of the vibrational frequencies of CuCO+, NiCO and CuCH3. Chem. Phys. Lett. 1992, 189, 266–272. [Google Scholar] [CrossRef]
- Meyer, F.; Chen, Y.M.; Armentrout, P.B. Sequential bond energies of Cu(CO)x+ and Ag(CO)x+ (x = 1–4). J. Am. Chem. Soc. 1995, 117, 4071–4081. [Google Scholar] [CrossRef]
- Ack, J.J.; Webb, J.D.; Strauss, S.H. [Cu(CO)n]+ complex ions in the solid state (n = 1, 2, 3). Inorg. Chem. 1996, 35, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Hush, N.S.; Williams, M.L. Carbon monoxide bond length, force constant and infrared intensity variations in strong electric fields: Valence-shell calculations with applications to properties of adsorbed and complexed CO. J. Mol. Spectrosc. 1974, 50, 349–368. [Google Scholar] [CrossRef]
- Larsson, R.; Lykvist, R.; Rebenstof, B.Z.; Rebenstorf, B. On the IR frequency shift of carbon monoxide adsorbed on positive surface ions Z. Phys. Chem. Leipzig. 1982, 263, 1089–1104. [Google Scholar]
- Zecchina, A.; Escalona, P.E.; Otero, A.C. Low temperature CO adsorption on alum-derived active alumina: an infrared investigation. J. Catal. 1987, 107, 244–247. [Google Scholar] [CrossRef]
- Zaki, M.I.; Knozinger, H.J. Carbon monoxide adsorption on oxide surfaces—an infrared study of the þ-chromia surface. J. Catal. 1989, 119, 311–321. [Google Scholar] [CrossRef]
- Pacchioni, G.; Cogliandro, G.; Bagus, P.S. Molecular orbital cluster model study of bonding and vibrations of CO adsorbed on MgO surface. J. Quant. Chem. 1992, 42, 1115–1139. [Google Scholar] [CrossRef]
- Scarano, D.; Spoto, G.; Bordiga, S.; Coluccia, S.; Zecchina, A. CO adsorption at 77 K on CoO/MgO and NiO/MgO solid solutions: A Fourier-transform infrared study. J. Chem. Soc. Faraday Trans. 1992, 88, 291–296. [Google Scholar] [CrossRef]
- Ćirpan, A.; Küçükyavuz, Z.; Küçükyavuz, S. Synthesis, characterization and electrical conductivity of poly (paraphenylene vinylene). Turk. J. Chem. 2003, 27, 135–143. [Google Scholar]
- Fernandes, M.R.; Garcia, J.R.; Schultz, M.S.; Nart, F.C. Polaron and bipolaron transitions in doped poly(p-phenylene vinylene) films. Thin. Solid. Films 2005, 474, 279–284. [Google Scholar] [CrossRef]
- Break, D.W. Zeolite Molecular Sieves; Robert, E., Ed.; Krieger Publishing: Malabar, FL, USA, 1973. [Google Scholar]
- Feast, W.J.; Tsibouklis, J.; Pouwer, K.L.; Groenendaalb, L.; Meijer, E.W. Synthesis, processing and material properties of conjugated polymers. Polymer 1996, 22, 5017–5047. [Google Scholar] [CrossRef]
- Patrick, C.; Larsen, S.C. EPR study of copper-exchanged zeolites: effects of correlated g- and A-Strain, Si/Al Ratio, and Parent zeolite. J. Phys. Chem. 2000, 104, 6568–6575. [Google Scholar] [CrossRef]
- Ewing, G.E.; Pimentel, G.C. Infrared spectrum of solid carbon monoxide. J. Chem. Phys. 1961, 35, 925–930. [Google Scholar] [CrossRef]
- Otero, A.C.; Turnes, P.G.; Zecchina, A.; Spoto, G.; Bordiga, B.; Roy, P. Cation carbon stretching vibration of adducts formed upon CO adsorption on alkaline zeolites. Phys. Chem. Chem. Phys. 1999, 1, 4139–4140. [Google Scholar] [CrossRef]
- Ewing, G.E. Infrared spectra of liquid and solid carbon monoxide. J. Chem. Phys. 1962, 37, 2250–2256. [Google Scholar] [CrossRef]
- Gary, W. Inorganic Chemistry; Maple-Vail Book Manufacturing Group: Sausalito, CA, USA, 1944. [Google Scholar]
- Kamble, V.S.; Gupta, N.M. Effect of charge balancing cations on the entrapment of CO in Y-zeolite: FTIR spectroscopy study. J. Phys. Chem. B 2000, 104, 4588–4592. [Google Scholar] [CrossRef]
- Gunzler, H.; Gremlich, H.U. IR Spectroscopy; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Kuroda, Y.; Yoshikawa, Y.; Emura, S.; Kumashiro, R.; Nagao, M. Characterization of specific N2-adsorption site existing on CuZSM-5 type zeolite: Effect of ion-exchange level on adsorption properties. J. Phys. Chem. B 1999, 103, 2155–2164. [Google Scholar] [CrossRef]
- Hadjivanov, K.; Knozinger, H. FTIR study of the low-temperature CO adsorption on Cu+-ZSM-5: evidence of formation of Cu2+(CO)2 species. J. Catal. 2000, 191, 480–485. [Google Scholar] [CrossRef]
- Chuapradit, C.; Ruangchuay, L.; Chotpattananot, D.; Hiamtup, P.; Sirivat, A.; Schwank, J. Polyaniline/zeolite composites and electrical conductivity response towards CO-N2. Polymer 2005, 46, 947–956. [Google Scholar] [CrossRef]
- Densakulprasert, N.; Wannatong, L.; Chotpattananont, D.; Hiamtup, P.; Sirivat, A.; Schwank, J. Electrical conductivity of polyaniline/zeolite composites and synergetic interaction with CO. Mat. Sci. Engr. B 2005, 117, 276–282. [Google Scholar] [CrossRef]
- Soontornworajit, B.; Wannatong, L.; Hiamtup, P.; Naimlang, S.; Chotpattananont, D.; Sirivat, A.; Schwank, J. Induced interaction between polypyrrole and SO2 via molecular sieve 13X. Mat. Sci. Engr. B 2007, 136, 78–86. [Google Scholar] [CrossRef]
- Thuwachawsoan, K.; Chotpattananont, D.; Sirivat, A.; Rujiravanit, R.; Schwank, J. Electrical conductivity responses and interactions of poly(3-thiopheneacetic acid)/zeolites L, mordenite, beta and H2. Mat. Sci. Engr. B 2007, 140, 23–30. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thongchai, N.; Kunanuruksapong, R.; Niamlang, S.; Wannatong, L.; Sirivat, A.; Wongkasemjit, S. Interactions between CO and Poly(p-phenylene vinylene) as Induced by Ion-Exchanged Zeolites. Materials 2009, 2, 2259-2275. https://doi.org/10.3390/ma2042259
Thongchai N, Kunanuruksapong R, Niamlang S, Wannatong L, Sirivat A, Wongkasemjit S. Interactions between CO and Poly(p-phenylene vinylene) as Induced by Ion-Exchanged Zeolites. Materials. 2009; 2(4):2259-2275. https://doi.org/10.3390/ma2042259
Chicago/Turabian StyleThongchai, Nareerat, Ruksapong Kunanuruksapong, Sumonman Niamlang, Ladawan Wannatong, Anuvat Sirivat, and Sujitra Wongkasemjit. 2009. "Interactions between CO and Poly(p-phenylene vinylene) as Induced by Ion-Exchanged Zeolites" Materials 2, no. 4: 2259-2275. https://doi.org/10.3390/ma2042259




