Anisotropic Porous Biodegradable Scaffolds for Musculoskeletal Tissue Engineering
Abstract
:1. Introduction
2. Solid Free-Form Fabrication
2.1. 3D Fiber Deposition/Fused Deposition Modeling
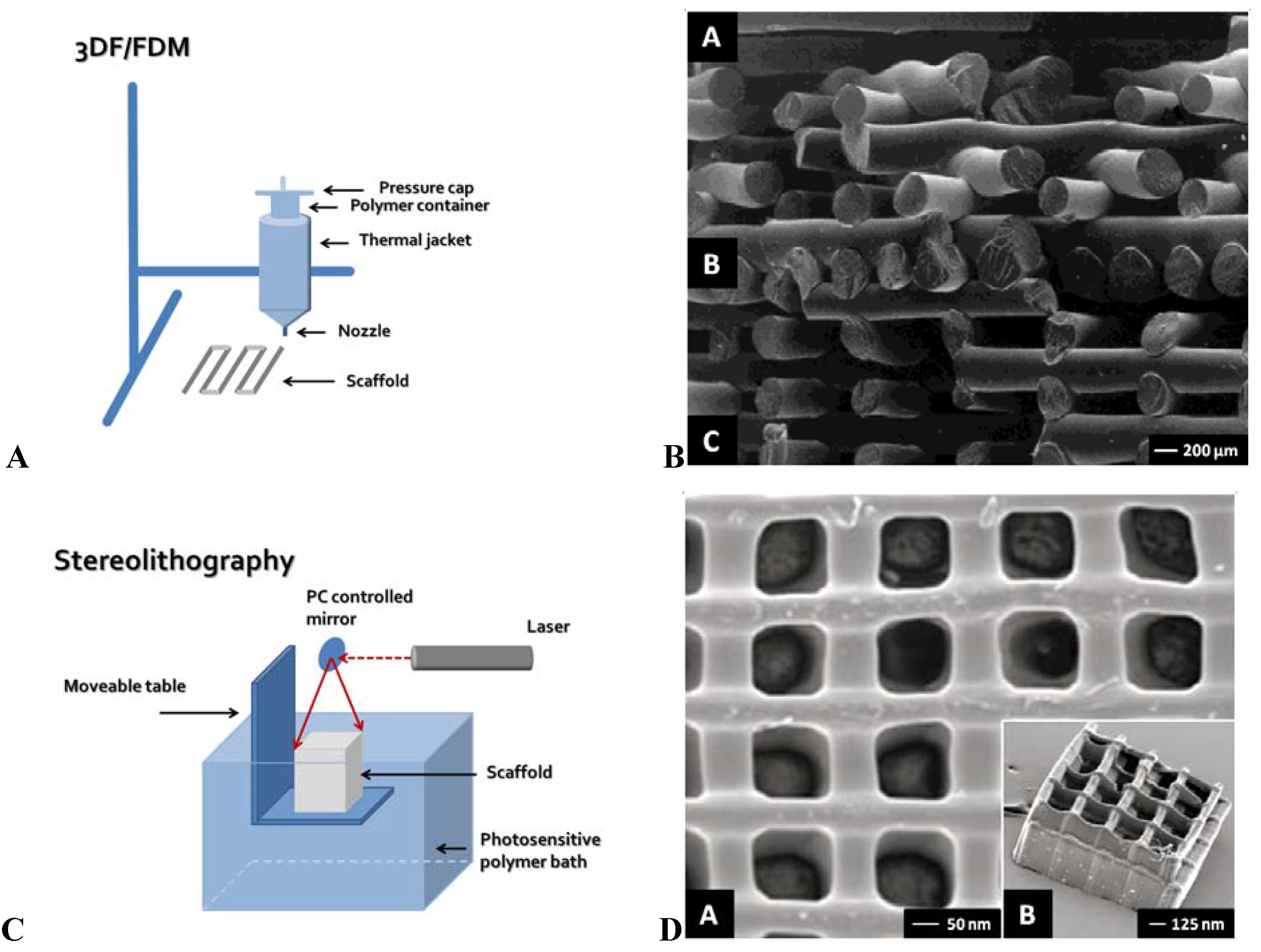
2.2. Bioplotter
2.3. Stereolithography
3. Modified Conventional Techniques
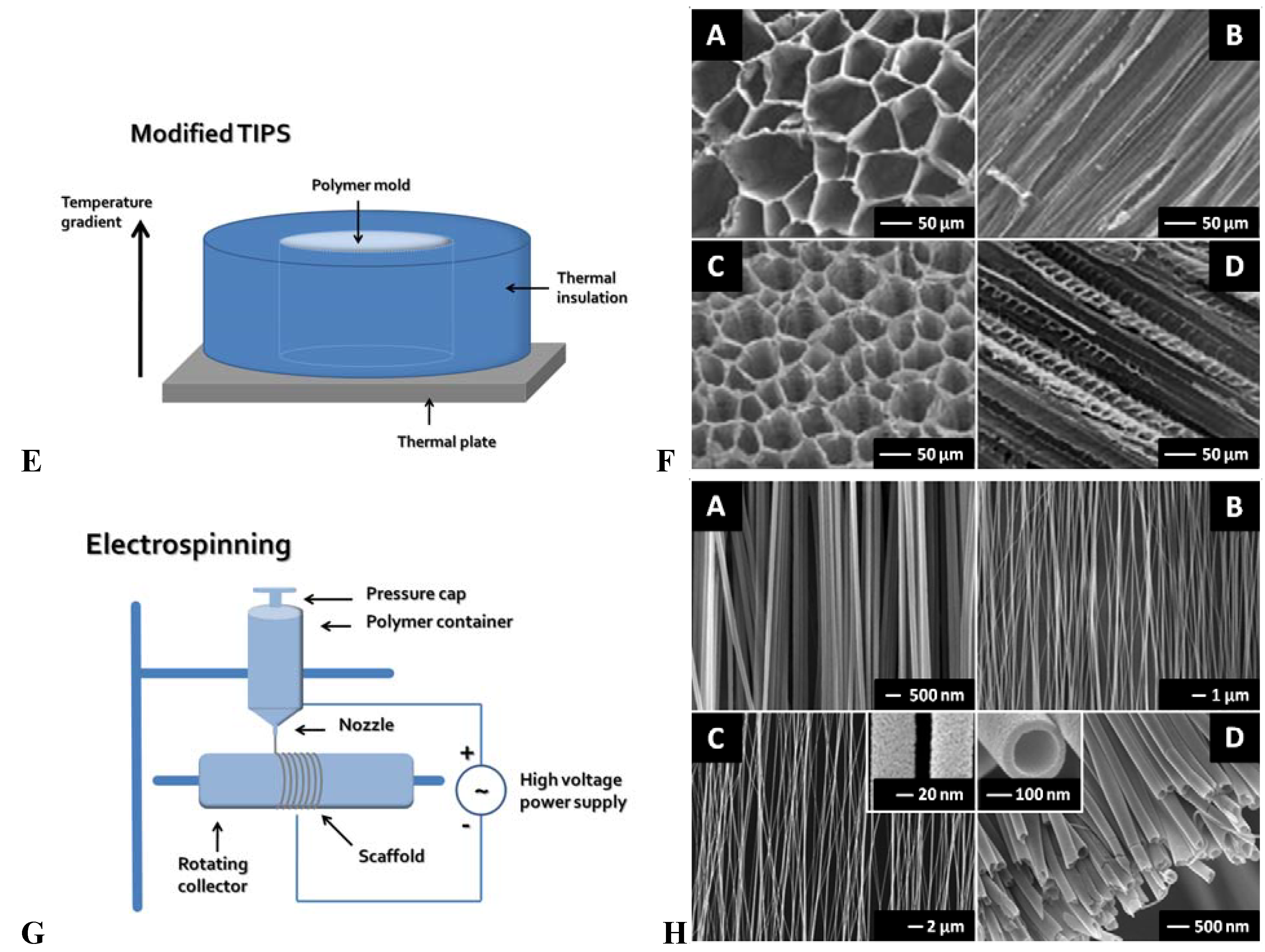
3.1. Modified Thermal Induced Phase Separation (TIPS)
3.2. Electrospinning
4. Alternative Methods to Create Anisotropic Scaffolds
4.1. Direct Methods
4.2. Post-Processing Methods
5. Discussion
6. Conclusions
References and Notes
- Jurgens, W.J.; Oedayrajsingh-Varma, M.J.; Helder, M.N.; Zandiehdoulabi, B.; Schouten, T.E.; Kuik, D.J.; Ritt, M.J.; van Milligen, F.J. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Buma, P.; Ramrattan, N.N.; van Tienen, T.G.; Veth, R.P. Tissue engineering of the meniscus. Biomaterials 2004, 25, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert. Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Buma, P.; Pieper, J.S.; van, T.T.; van Susante, J.L.; van der Kraan, P.M.; Veerkamp, J.H.; van den Berg, W.B.; Veth, R.P.; van Kuppevelt, T.H. Cross-linked type I and type II collagenous matrices for the repair of full-thickness articular cartilage defects—A study in rabbits. Biomaterials 2003, 24, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Heijkants, R.G.; van Calck, R.V.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; van Tienen, T.G.; Ramrattan, N.; Buma, P.; Veth, R.P. Design, synthesis and properties of a degradable polyurethane scaffold for meniscus regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, J.; Rollo, J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. Biomaterials and scaffold design: key to tissue-engineering cartilage. Biotechnol. Appl. Biochem. 2007, 46, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Preparation of interconnected highly porous polymeric structures by a replication and freeze-drying process. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Jayasuriya, A.C.; Kohn, D.H. Effect of ionic activity products on the structure and composition of mineral self assembled on three-dimensional poly(lactide-co-glycolide) scaffolds. J. Biomed. Mater. Res. A 2007, 83, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef] [PubMed]
- van de Witte, P.; Dijkstra, P.J.; van den Berg, J.W.A.; Feijen, J. Phase behavior of polylactides in solvent-nonsolvent mixtures. J. Polym. Sci. Pol. Phys. 1996, 34, 2553–2568. [Google Scholar] [CrossRef]
- Budyanto, L.; Goh, Y.Q.; Ooi, C.P. Fabrication of porous poly(L-lactide) (PLLA) scaffolds for tissue engineering using liquid-liquid phase separation and freeze extraction. J. Mater. Sci. Mater. Med. 2009, 20, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bashur, C.A.; Shaffer, R.D.; Dahlgren, L.A.; Guelcher, S.A.; Goldstein, A.S. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng. Part A 2009, 15, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; Dennis, R.G.; Kileny, J.L.; Mooney, D.J. Salt fusion: An approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Heijkants, R.G.; van Calck, R.V.; van Tienen, T.G.; de Groot, J.H.; Pennings, A.J.; Buma, P.; Veth, R.P.; Schouten, A.J. Polyurethane scaffold formation via a combination of salt leaching and thermally induced phase separation. J. Biomed. Mater. Res. A 2008, 87, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.Y.; Hung, L.H.; Chu, I.M.; Ko, C.S.; Lee, Y.D. The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. A 2009. [Google Scholar] [CrossRef]
- Chang, K.Y.; Cheng, L.W.; Ho, G.H.; Huang, Y.P.; Lee, Y.D. Fabrication and characterization of poly(gamma-glutamic acid)-graft-chondroitin sulfate/polycaprolactone porous scaffolds for cartilage tissue engineering. Acta Biomater. 2009, 5, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef] [PubMed]
- Welsing, R.T.; van Tienen, T.G.; Ramrattan, N.; Heijkants, R.; Schouten, A.J.; Veth, R.P.; Buma, P. Effect on tissue differentiation and articular cartilage degradation of a polymer meniscus implant: A 2-year follow-up study in dogs. Am. J. Sports Med. 2008, 36, 1978–1989. [Google Scholar] [CrossRef] [PubMed]
- Coward, T.J.; Watson, R.M.; Wilkinson, I.C. Fabrication of a wax ear by rapid-process modeling using stereolithography. Int. J. Prosthodont. 1999, 12, 20–27. [Google Scholar] [PubMed]
- Meakin, J.R.; Shepherd, D.E.; Hukins, D.W. Short communication: fused deposition models from CT scans. Br. J. Radiol. 2004, 77, 504–507. [Google Scholar] [CrossRef] [PubMed]
- D'Urso, P.S.; Earwaker, W.J.; Barker, T.M.; Redmond, M.J.; Thompson, R.G.; Effeney, D.J.; Tomlinson, F.H. Custom cranioplasty using stereolithography and acrylic. Br. J. Plast. Surg. 2000, 53, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, B.; Amis, A.; McGurk, M. Preliminary study of potential for rapid prototype and surface scanned radiotherapy facemask production technique. J. Med. Eng Technol. 2002, 26, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Phua, K.K.; Chua, C.K.; Du, Z.H.; Teo, K.O. Fabrication of porous polymeric matrix drug delivery devices using the selective laser sintering technique. Proc. Inst. Mech. Eng. H. 2001, 215, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.M.; Leong, K.F.; Chua, C.K.; Low, K.H.; Quek, H.S. Characterization of microfeatures in selective laser sintered drug delivery devices. Proc. Inst. Mech. Eng H. 2002, 216, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Wiria, F.E.; Chua, C.K.; Li, S.H. Characterization of a poly-epsilon-caprolactone polymeric drug delivery device built by selective laser sintering. Biomed. Mater. Eng 2007, 17, 147–157. [Google Scholar] [PubMed]
- Moroni, L.; Schotel, R.; Sohier, J.; de Wijn, J.R.; van Blitterswijk, C.A. Polymer hollow fiber three-dimensional matrices with controllable cavity and shell thickness. Biomaterials 2006, 27, 5918–5926. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C.A. 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, T.B.; Malda, J.; de Wijn, J.R.; Peters, F.; Riesle, J.; van Blitterswijk, C.A. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004, 25, 4149–4161. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; de Wijn, J.R.; Verbout, A.J.; Alblas, J.; Dhert, W.J. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng. Part A 2008, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Curti, M.; Welti, M.; Korom, S.; Weder, W.; de Wijn, J.R.; van Blitterswijk, C.A. Anatomical 3D fiber-deposited scaffolds for tissue engineering: designing a neotrachea. Tissue Eng. 2007, 13, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Hendriks, J.A.; Schotel, R.; de Wijn, J.R.; van Blitterswijk, C.A. Design of biphasic polymeric 3-dimensional fiber deposited scaffolds for cartilage tissue engineering applications. Tissue Eng. 2007, 13, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C.A. Integrating novel technologies to fabricate smart scaffolds. J. Biomater. Sci. Polym. Ed. 2008, 19, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. The effect of scaffold architecture on properties of direct 3D fiber deposition of porous Ti6Al4V for orthopedic implants. J. Biomed. Mater. Res. A 2009. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Lambers, F.M.; Wilson, W.; van Donkelaar, C.C.; de Wijn, J.; Huiskes, R.; van Blitterswijk, C.A. Finite element analysis of meniscal anatomical 3D scaffolds: Implications for tissue engineering. Open. Biomed. Eng J. 2007, 1, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L. A Mechanistic Approach to Design Smart Scaffolds for Tissue Engineering. PhD Thesis, University of Twente, Enschede, The Netherlands, 2006. [Google Scholar]
- Den Buijs, J.O.; Dragomir-Daescu, D.; Ritman, E.L. Cyclic deformation-induced solute transport in tissue scaffolds with computer designed, interconnected, pore networks: Experiments and simulations. Ann. Biomed. Eng. 2009, 37, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.C., Jr.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 272, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Malone, E.; Lipson, H.; Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006, 12, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and organ printing 2: Fusion of cell aggregates in three–dimensional gels. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 272, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Peltola, S.M.; Melchels, F.P.; Grijpma, D.W.; Kellomaki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.; Melchels, F.P.; Grijpma, D.W.; Feijen, J. Fumaric acid monoethyl ester-functionalized poly(D,L-lactide)/N-vinyl-2-pyrrolidone resins for the preparation of tissue engineering scaffolds by stereolithography. Biomacromolecules 2009, 10, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell Mater. 2003, 5, 29–39. [Google Scholar] [PubMed]
- Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mageswaran, P.; Mente, P.; Modi, R.; Auyeung, R.; Chrisey, D.B.; Ovsianikov, A.; Chichkov, B. Two photon induced polymerization of organic-inorganic hybrid biomaterials for microstructured medical devices. Acta Biomater. 2006, 2, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hidai, H.; Jeon, H.; Hwang, D.J.; Grigoropoulos, C.P. Self-standing aligned fiber scaffold fabrication by two photon photopolymerization. Biomed. Microdevices 2009, 11, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Schlie, S.; Ngezahayo, A.; Haverich, A.; Chichkov, B.N. Two-photon polymerization technique for microfabrication of CAD-designed 3D scaffolds from commercially available photosensitive materials. J. Tissue Eng Regen. Med. 2007, 1, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Schlie, S.; Ngezahayo, A.; Ovsianikov, A.; Fabian, T.; Kolb, H.A.; Haferkamp, H.; Chichkov, B.N. Three-dimensional cell growth on structures fabricated from ORMOCER by two-photon polymerization technique. J. Biomater. Appl. 2007, 22, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Arcaute, K.; Mann, B.K.; Wicker, R.B. Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann. Biomed. Eng 2006, 34, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.N.; Fisher, J.P.; Dean, D.; Rimnac, C.; Mikos, A.G. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 64, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Vehof, J.W.; Dean, D.; van der Waerden, J.P.; Holland, T.A.; Mikos, A.G.; Jansen, J.A. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J. Biomed. Mater. Res. 2002, 59, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Mapili, G.; Lu, Y.; Chen, S.; Roy, K. Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Mizutani, M. Liquid acrylate-endcapped biodegradable poly(epsilon-caprolactone-co-trimethylene carbonate). II. Computer-aided stereolithographic microarchitectural surface photoconstructs. J. Biomed. Mater. Res. 2002, 62, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Naumann, A.; Aigner, J.; Staudenmaier, R.; Seemann, M.; Bruening, R.; Englmeier, K.H.; Kadegge, G.; Pavesio, A.; Kastenbauer, E.; Berghaus, A. Clinical aspects and strategy for biomaterial engineering of an auricle based on three-dimensional stereolithography. Eur. Arch. Otorhinolaryngol. 2003, 260, 568–575. [Google Scholar] [PubMed]
- Sodian, R.; Fu, P.; Lueders, C.; Szymanski, D.; Fritsche, C.; Gutberlet, M.; Hoerstrup, S.P.; Hausmann, H.; Lueth, T.; Hetzer, R. Tissue engineering of vascular conduits: fabrication of custom-made scaffolds using rapid prototyping techniques. Thorac. Cardiovasc. Surg. 2005, 53, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Loebe, M.; Hein, A.; Martin, D.P.; Hoerstrup, S.P.; Potapov, E.V.; Hausmann, H.; Lueth, T.; Hetzer, R. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO J. 2002, 48, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.X.; Lee, J.W.; Seol, Y.J.; Cho, D.W. Development of 3D PPF/DEF scaffolds using micro-stereolithography and surface modification. J. Mater. Sci. Mater. Med. 2009, 20, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A poly(D,L-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials 2009, 30, 3801–3809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Lan, P.X.; Kim, B.; Lim, G.; Cho, D.W. Fabrication and characteristic analysis of a poly(propylene fumarate) scaffold using micro-stereolithography technology. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Engelmayr, G.C., Jr.; Papworth, G.D.; Watkins, S.C.; Mayer, J.E., Jr.; Sacks, M.S. Guidance of engineered tissue collagen orientation by large-scale scaffold microstructures. J. Biomech. 2006, 39, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.M.; Halloran, J.W.; Hollister, S.J.; Feinberg, S.E. Hydroxyapatite implants with designed internal architecture. J. Mater. Sci. Mater. Med. 2001, 12, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yeong, W.Y.; Chua, C.K.; Leong, K.F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, P.P.; Wang, J.P.; Chen, G.Q.; Wu, Q. Guided growth of smooth muscle cell on poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds with uniaxial microtubular structures. J. Biomed. Mater. Res. A 2008, 86, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Rodriguez-Lorenzo, L.M. Biodegradable composite scaffolds with an inter-connected spherical network for bone tissue engineering. Biomaterials 2004, 25, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Kuo, P.Y.; Hsieh, H.J.; Hsien, T.Y.; Hou, L.T.; Lai, J.Y.; Wang, D.M. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Mooney, D.J. Engineering smooth muscle tissue with a predefined structure. J. Biomed. Mater. Res. 1998, 41, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.Q.; Ooi, C.P. Fabrication and characterization of porous poly(L-lactide) scaffolds using solid-liquid phase separation. J. Mater. Sci. Mater. Med. 2008, 19, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Schugens, C.; Maquet, V.; Grandfils, C.; Jerome, R.; Teyssie, P. Polylactide macroporous biodegradable implants for cell transplantation. II. Preparation of polylactide foams by liquid-liquid phase separation. J. Biomed. Mater. Res. 1996, 30, 449–461. [Google Scholar] [CrossRef] [PubMed]
- van Minnen, B.; van Leeuwen, M.B.; Stegenga, B.; Zuidema, J.; Hissink, C.E.; van Kooten, T.G.; Bos, R.R. Short-term in vitro and in vivo biocompatibility of a biodegradable polyurethane foam based on 1,4-butanediisocyanate. J. Mater. Sci. Mater. Med. 2005, 16, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, P.X. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Zhang, R. Microtubular architecture of biodegradable polymer scaffolds. J. Biomed. Mater. Res. 2001, 56, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Fujimoto, K.L.; Wagner, W.R. Elastase-sensitive elastomeric scaffolds with variable anisotropy for soft tissue engineering. Pharm. Res. 2008, 25, 2400–2412. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qu, X.; Cui, W.; Bei, J.; Yu, F.; Lu, S.; Wang, S. Manufacturing and morphology structure of polylactide-type microtubules orientation-structured scaffolds. Biomaterials 2006, 27, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, H.; Yang, F.; Bei, J.; Wang, S. Preparation and cell affinity of microtubular orientation-structured PLGA(70/30) blood vessel scaffold. Biomaterials 2008, 29, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Braescu, L. Shape of menisci in terrestrial dewetted Bridgman growth. J. Colloid Interface Sci. 2008, 319, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.; Korber, C.; Rau, G.; Hubel, A.; Cravalho, E.G. Redefining cooling rate in terms of ice front velocity and thermal gradient: First evidence of relevance to freezing injury of lymphocytes. Cryobiology 1990, 27, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Faraj, K.A.; van Kuppevelt, T.H.; Daamen, W.F. Construction of collagen scaffolds that mimic the three-dimensional architecture of specific tissues. Tissue Eng. 2007, 13, 2387–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O'Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chang, J. Electrospinning of three-dimensional nanofibrous tubes with controllable architectures. Nano. Lett. 2008, 8, 3283–3287. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.C.; Atkin, N.; Gunning, P.A.; Granville, N.; Wilson, K.; Wilson, D.; Southgate, J. Characterisation of electrospun polystyrene scaffolds for three-dimensional in vitro biological studies. Biomaterials 2006, 27, 3136–3146. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Feng, Z.; Gu, Z.; Liu, C. Growth of outgrowth endothelial cells on aligned PLLA nanofibrous scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Kidoaki, S.; Kwon, I.K.; Matsuda, T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 2005, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shah, J.D.; Wang, H. Nanofiber enabled layer-by-layer approach toward three-dimensional tissue formation. Tissue Eng. Part A 2009, 15, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.C.; Chew, S.Y.; Leong, K.W. Aligned core-shell nanofibers delivering bioactive proteins. Nanomed. 2006, 1, 465–471. [Google Scholar] [CrossRef]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J. Biomed. Mater. Res. A 2006, 79, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y.N. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Bagnaninchi, P.O.; Yang, Y.; Zghoul, N.; Maffulli, N.; Wang, R.K.; Haj, A.J. Chitosan microchannel scaffolds for tendon tissue engineering characterized using optical coherence tomography. Tissue Eng. 2007, 13, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Cyster, L.A.; Barry, J.J.; Yang, X.B.; Oreffo, R.O.; Grant, D.M.; Scotchford, C.A.; Howdle, S.M.; Shakesheff, K.M.; Rose, F.R. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 2006, 27, 5909–5917. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.D.; Bennett, J.M.; Waddell, R.L.; Doctor, J.S.; Marra, K.G. Multi-channeled biodegradable polymer/CultiSpher composite nerve guides. Biomaterials 2004, 25, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Nazhat, S.N.; Neel, E.A.; Kidane, A.; Ahmed, I.; Hope, C.; Kershaw, M.; Lee, P.D.; Stride, E.; Saffari, N.; Knowles, J.C.; Brown, R.A. Controlled microchannelling in dense collagen scaffolds by soluble phosphate glass fibers. Biomacromolecules 2007, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.; Salih, V.; Mudera, V.; Knowles, J.C.; Lewis, M.P. Soluble phosphate glasses: In vitro studies using human cells of hard and soft tissue origin. Biomaterials 2004, 25, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.C. Phosphate based glasses for biomedical applications. J. Mater. Chem. 2003, 13, 2395–2401. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, E.J.; Jun, I.K.; Kim, H.E.; Knowles, J.C. Degradation and drug release of phosphate glass/polycaprolactone biological composites for hard-tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, R.L.; Brocchini, S.; Knowles, J.C. Effect of glass composition on the degradation properties and ion release characteristics of phosphate glass—Polycaprolactone composites. Biomaterials 2005, 26, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, G.; Mathieu, L.; Pioletti, D.P.; Bourban, P.E.; Manson, J.A.; Knowles, J.C.; Nazhat, S.N. Polylactic acid-phosphate glass composite foams as scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Ginebra, M.P.; Planell, J.A.; Zeppetelli, S.; Ambrosio, L. Development and cell response of a new biodegradable composite scaffold for guided bone regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Veth, R.P.; Jansen, H.W.; Leenslag, J.W.; Pennings, A.J.; Hartel, R.M.; Nielsen, H.K. Experimental meniscal lesions reconstructed with a carbon fiber-polyurethane-poly(L-lactide) graft. Clin. Orthop. Relat Res. 1986, 202, 286–293. [Google Scholar] [PubMed]
- Izquierdo, R.; Garcia-Giralt, N.; Rodriguez, M.T.; Caceres, E.; Garcia, S.J.; Gomez Ribelles, J.L.; Monleon, M.; Monllau, J.C.; Suay, J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J. Biomed. Mater. Res. A 2008, 85, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yaszemski, M.J.; Lu, L. Three-dimensional porous biodegradable polymeric scaffolds fabricated with biodegradable hydrogel porogens. Tissue Eng. C Methods 2009. [Google Scholar] [CrossRef]
- Tyson, T.; Finne-Wistrand, A.; Albertsson, A.C. Degradable porous scaffolds from various L-lactide and trimethylene carbonate copolymers obtained by a simple and effective method. Biomacromolecules 2009, 10, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Fan, Y. Preparation of poly(D,L-lactic acid) scaffolds using alginate particles. J. Biomater. Sci. Polym. Ed. 2008, 19, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Capes, J.S.; Ando, H.Y.; Cameron, R.E. Fabrication of polymeric scaffolds with a controlled distribution of pores. J. Mater. Sci. Mater. Med. 2005, 16, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.A.; Lu, Y.; Mao, S.; Chen, S. Direct micro-patterning of biodegradable polymers using ultraviolet and femtosecond lasers. Biomaterials 2005, 26, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Brayfield, C.A.; Marra, K.G.; Leonard, J.P.; Tracy, C.X.; Gerlach, J.C. Excimer laser channel creation in polyethersulfone hollow fibers for compartmentalized in vitro neuronal cell culture scaffolds. Acta Biomater. 2008, 4, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Nishi, S.; Ishibashi-Ueda, H.; Matsuda, T. Surface microarchitectural design in biomedical applications: in vivo analysis of tissue ingrowth in excimer laser-directed micropored scaffold for cardiovascular tissue engineering. J. Biomed. Mater. Res. 2000, 51, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Tiaw, K.S.; Goh, S.W.; Hong, M.; Wang, Z.; Lan, B.; Teoh, S.H. Laser surface modification of poly(epsilon-caprolactone) (PCL) membrane for tissue engineering applications. Biomaterials 2005, 26, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Vishnubhatla, K.C.; Bellini, N.; Ramponi, R.; Cerullo, G.; Osellame, R. Shape control of microchannels fabricated in fused silica by femtosecond laser irradiation and chemical etching. Opt. Express 2009, 17, 8685–8695. [Google Scholar] [CrossRef] [PubMed]
- Papenburg, B.J.; Vogelaar, L.; Bolhuis-Versteeg, L.A.; Lammertink, R.G.; Stamatialis, D.; Wessling, M. One-step fabrication of porous micropatterned scaffolds to control cell behavior. Biomaterials 2007, 28, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Courtney, T.; Sacks, M.S.; Stankus, J.; Guan, J.; Wagner, W.R. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 2006, 27, 3631–3638. [Google Scholar] [PubMed]
- Li, W.J.; Mauck, R.L.; Cooper, J.A.; Yuan, X.; Tuan, R.S. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J. Biomech. 2007, 40, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.A.; Johannessen, W.; Wu, J.P.; Jawa, A.; Elliott, D.M. Effect of fiber orientation and strain rate on the nonlinear uniaxial tensile material properties of tendon. J. Biomech. Eng. 2003, 125, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Stankiewicz, A.; Ateshian, G.A.; Mow, V.C. Anisotropy, inhomogeneity, and tension-compression nonlinearity of human glenohumeral cartilage in finite deformation. J. Biomech. 2005, 38, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Guo, X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Tillmann, B. Collagenous fibril texture of the human knee joint menisci. Anat. Embryol. (Berl.) 1998, 197, 317–324. [Google Scholar] [CrossRef]
- Fithian, D.C.; Kelly, M.A.; Mow, V.C. Material properties and structure-function relationships in the menisci. Clin. Orthop. Relat Res. 1990, 252, 19–31. [Google Scholar] [PubMed]
- Proctor, C.S.; Schmidt, M.B.; Whipple, R.R.; Kelly, M.A.; Mow, V.C. Material properties of the normal medial bovine meniscus. J. Orthop. Res. 1989, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Setton, L.A.; Guilak, F.; Hsu, E.W.; Vail, T.P. Biomechanical factors in tissue engineered meniscal repair. Clin. Orthop. Relat Res. 1999, 367 (Suppl.), S254–S272. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Mauck, R.L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 2007, 28, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Jia, F.; Gilbert, T.W.; Woo, S.L. Cell orientation determines the alignment of cell-produced collagenous matrix. J. Biomech. 2003, 36, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Martin, I.; Vunjak-Novakovic, G. Frontiers in tissue engineering. In vitro modulation of chondrogenesis. Clin. Orthop. Relat Res. 1999, 367 (Suppl.), S46–S58. [Google Scholar] [CrossRef] [PubMed]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; Sales, K.; Butler, P.; Seifalian, A.M. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: A review. Biomaterials 2005, 26, 1857–1875. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Mikos, A.G. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J. Biomater. Sci. Polym. Ed. 2004, 15, 701–726. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, G.N.; Sikavitsas, V.I.; van den, D.J.; Sheffield, T.L.; Ambrose, C.G.; Jansen, J.A.; Mikos, A.G. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc. Natl. Acad. Sci. USA 2002, 99, 12600–12605. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Pazzano, D.; Mercier, K.A.; Moran, J.M.; Fong, S.S.; DiBiasio, D.D.; Rulfs, J.X.; Kohles, S.S.; Bonassar, L.J. Comparison of chondrogensis in static and perfused bioreactor culture. Biotechnol. Prog. 2000, 16, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Marini, R.P.; Schaefer, D.; Aronson, J.; Langer, R.; Shastri, V.P. In vivo engineering of organs: the bone bioreactor. Proc. Natl. Acad. Sci. USA 2005, 102, 11450–11455. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.R.; Hou, Q.; Oreffo, R.O. Delivery systems for bone growth factors - the new players in skeletal regeneration. J. Pharm. Pharmacol. 2004, 56, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Maravelakis, E.; David, K.; Antoniadis, A.; Manios, A.; Bilalis, N.; Papaharilaou, Y. Reverse engineering techniques for cranioplasty: a case study. J. Med. Eng Technol. 2008, 32, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Solaro, P.; Pierangeli, E.; Pizzoni, C.; Boffi, P.; Scalese, G. From computerized tomography data processing to rapid manufacturing of custom-made prostheses for cranioplasty. Case report. J. Neurosurg. Sci. 2008, 52, 113–116. [Google Scholar] [PubMed]
- Chim, H.; Schantz, J.T. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast. Reconstr. Surg. 2005, 116, 1726–1741. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, K.; Hirsch, J.M. Malignant tumors of the maxilla: virtual planning and real-time rehabilitation with custom-made R-zygoma fixtures and carbon-graphite fiber-reinforced polymer prosthesis. Clin. Implant. Dent. Relat Res. 2008, 10, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.M.; Orton, D.G.; Hollister, S.J.; Feinberg, S.E.; Halloran, J.W. Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials 2002, 23, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Taboas, J.M.; Maddox, R.D.; Krebsbach, P.H.; Hollister, S.J. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 2003, 24, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.E.; de Bruijn, J.D.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J. Design and fabrication of standardized hydroxyapatite scaffolds with a defined macro-architecture by rapid prototyping for bone-tissue-engineering research. J. Biomed. Mater. Res. A 2004, 68, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Reis, N.; Ainsley, C.; Derby, B.; Czernuszka, J.T. Novel collagen scaffolds with predefined internal morphology made by solid freeform fabrication. Biomaterials 2003, 24, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Mulder, E.L.W.; Buma, P.; Hannink, G. Anisotropic Porous Biodegradable Scaffolds for Musculoskeletal Tissue Engineering. Materials 2009, 2, 1674-1696. https://doi.org/10.3390/ma2041674
De Mulder ELW, Buma P, Hannink G. Anisotropic Porous Biodegradable Scaffolds for Musculoskeletal Tissue Engineering. Materials. 2009; 2(4):1674-1696. https://doi.org/10.3390/ma2041674
Chicago/Turabian StyleDe Mulder, Eric L. W., Pieter Buma, and Gerjon Hannink. 2009. "Anisotropic Porous Biodegradable Scaffolds for Musculoskeletal Tissue Engineering" Materials 2, no. 4: 1674-1696. https://doi.org/10.3390/ma2041674



