3.2. Chemicals
Unless otherwise noted, materials obtained from commercial suppliers were used without further purification. THF was purchased from Kanto Chemical Co., stored under argon, and used as-is. Dioxane was obtained from Wako Pure Chemicals Co., and stored over slices of sodium. Palladium acetate, cesium carbonate, ceric ammonium nitrate, and copper(I) iodide were obtained from Wako Pure Chemicals Co. PEPPSI and 2nd generation Grubbs catalyst were purchased from Aldrich. Arylzinc iodide-lithium chloride complexes were prepared according to the literature [
13] and stored under argon. [CpFe(CO)
2I] was prepared according to the literature [
11].
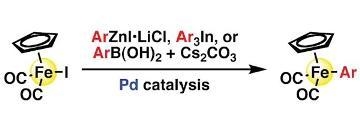
Typical Procedure for Arylation with Arylzinc Reagents (Table 2, entry 1): THF (1.0 mL) was placed in a 20-mL reaction flask under argon. [CpFe(CO)
2I] (
1, 152 mg, 0.50 mmol), palladium acetate (0.050 M THF solution, 0.025 mL, 0.0013 mmol),
trans-
N,
N,
N’,
N’-tetramethyl-1,2-cyclohexane-diamine (0.050 M THF solution, 0.050 mL, 0.0025 mmol), and phenylzinc iodide–lithium chloride complex (0.66 M THF solution, 1.14 mL, 0.75 mmol) were sequentially added at 0 °C. After the mixture was stirred for 15 min, a saturated ammonium chloride solution (1 mL) was added, and the product was extracted with ethyl acetate (10 mL × 3). Combined organic layer was passed through a pad of anhydrous sodium sulfate/Florisil and concentrated.
1H-NMR analysis of the crude product by using 1,1,2,2-tetrabromoethane as an internal standard indicated that
2a was quantitatively formed. The crude oil was purified in air on silica gel by using carbon disulfide as an eluent to yield
2a (114 mg, 0.45 mmol, 90%).
Procedure for Phenylation with Triphenylindium (Table 3, entry 1): Indium trichloride (1.55 g, 7.0 mmol) and THF (2 mL) were placed in a 20-mL reaction flask under argon. Phenylmagnesium bromide (1.03 M THF solution, 20.4 mL, 21 mmol) was added at 0 °C. The resulting mixture was stirred at ambient temperature overnight to prepare a THF solution of triphenylindium (0.31 M). THF (0.60 mL) was placed in another 20-mL reaction flask under argon. [CpFe(CO)
2I] (
1, 91 mg, 0.30 mmol), palladium acetate (3.4 mg, 0.015 mmol),
trans-
N,
N,
N’,
N’-tetramethyl-1,2-cyclohexanediamine (3.6 mg, 0.018 mmol), and a THF solution of triphenylindium (0.31 M THF solution, 1.4 mL, 0.45 mmol) were sequentially added at 0 °C. After the mixture was stirred for 1 h, a saturated ammonium chloride solution (0.6 mL) was added, and the product was extracted with ethyl acetate (10 mL × 3). Combined organic layer was passed through a pad of anhydrous sodium sulfate/Florisil and concentrated.
1H- NMR analysis of the crude product by using 1,1,2,2-tetrabromoethane as an internal standard indicated that
2a was obtained in 92% yield. Chromatographic purification using carbon disulfide as an eluent yielded
2a (66 mg, 0.26 mmol, 87%).
Typical Procedure for Arylation with Arylboronic Acids (Table 4, entry 1): [CpFe(CO)
2I] (
1, 152 mg, 0.50 mmol), phenylboronic acid (122 mg, 1.0 mmol), PEPPSI (17 mg, 0.025 mmol), and cesium carbonate (489 mg, 1.50 mmol) were placed in a 20-mL reaction flask under an atmosphere of argon. 1,4-Dioxane (1.5 mL) was then added, and the resulting mixture was stirred for 4 h at 60 °C. The reaction was quenched with a saturated ammonium chloride solution (1 mL). Extractive workup followed by silica gel column purification (eluent: carbon disulfide) afforded
2a (104 mg, 0.41 mmol) in 88% yield.
Procedure for Oxidative Methoxycarbonylation Reactions of [CpFe(CO)2Ar] with Ce(NH4)2(NO3)6 (Table 5, entry 4): Diammonium cerium(IV) nitrate (658 mg, 1.2 mmol) and methanol (6.0 mL) were added in a 50-mL reaction flask under argon. The mixture was cooled to –78 °C and then dicarbonylcyclopentadienyl(4-ethoxycarbonylphenyl)iron (
2i, 98 mg, 0.30 mmol) in methanol (4.0 mL) was added slowly over 1 min. After being stirred for 2 h at –78 °C, the reaction mixture was quenched with aqueous solution of sodium thiosulfate and sodium bicarbonate. The products were extracted with diethyl ether (20 mL × 3). The combined organic layer was passed through pads of Florisil and sodium sulfate and concentrated. Silica gel column purification (eluent: hexane/ethyl acetate = 10:1) of the crude product provided ethyl methyl terephthalate (
4d, 48 mg, 0.27 mmol, 91% yield).
Procedure for Photo-induced Allylation Reactions of [CpFe(CO)2Ar] (Table 6, entry 4): Dicarbonylcyclopentadienyl(4-ethoxycarbonylphenyl)iron (
2i, 98 mg, 0.30 mmol), 3-bromo-2-methyl-propene (203 mg, 1.5 mmol), and THF (1.5 mL) were sequentially added in a quartz tube under argon. The reaction mixture was irradiated by a UV lamp at 25 °C and stirred for 2 h with irradiation. The distance between the reaction flask and the UV lamp was 2 cm. After irradiation, the mixture was filtered through a pad of Florisil, and the filtrate was concentrated. Silica gel column purification (eluent: hexane/ethyl acetate = 10:1) provided ethyl 4-(2-methyl-2-propenyl)benzoate (
5d, 44 mg, 0.21 mmol, 71% yield).
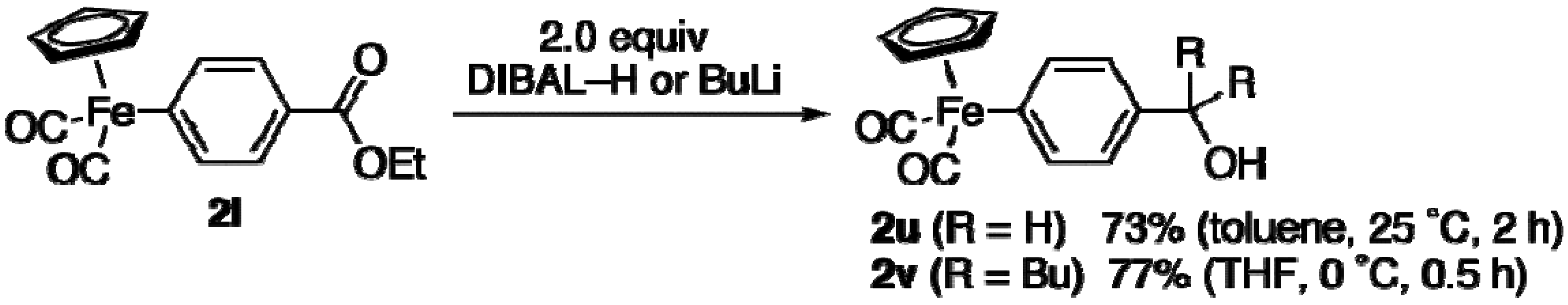
DIBAL-Reduction of 2i: Aryliron 2i (98 mg, 0.30 mmol) was placed in a reaction flask under argon. Toluene (1.5 mL) and DIBAL-H (1.0 M in hexane, 0.60 mL, 0.60 mmol) were sequentially added at 25 °C. After the mixture was stirred for 2 h, a 30% Rochelle salt solution (5 mL) was added slowly at 0 °C. The resulting mixture was stirred overnight. Extractive workup followed by silica gel column purification (eluent: CS2/CHCl3 = 1:1 to CHCl3 only) provided 2u (62 mg, 0.22 mmol, 73%).
Reaction of 2i with Butyllithium: Aryliron 2i (98 mg, 0.30 mmol) and THF (1.0 mL) were placed in a reaction flask under argon. Butyllithium (1.66 M in hexane, 0.36 mL, 0.60 mmol) was added dropwise at 0 °C. After the mixture was stirred for 30 min, a saturated ammonium chloride solution (5 mL) was added slowly at 0 °C. Extractive workup followed by silica gel column purification (eluent: CS2/CHCl3 = 1:1) provided 2v (92 mg, 0.23 mmol, 77%).
Metathesis Reactions of 2r: (Vinylphenyl)iron 2r (84 mg, 0.30 mmol), dichloromethane (1.0 mL), and ethyl acrylate (0.098 mL, 0.90 mmol) were sequentially added in a reaction flask under argon. 2nd Generation Grubbs catalyst (13 mg, 0.015 mmol) was then added. After the mixture was stirred for 6 h at 40 °C, the mixture was passed through a pad of Florisil. The filtrate was concentrated in vacuo. Silica gel column purification (eluent: CS2/CHCl3 = 2:1) provided 2x (85 mg, 0.24 mmol, 79%).
Characterization data for arylirons
2 [
11,
12],
4a [
30],
4b [
31],
4c [
31],
4d [
32],
5a [
33],
5b [
34],
5c [
35], and
5d [
36] were available in the literature. That of other compounds is given below.
Dicarbonylcyclopentadienyl(3-trifluoromethylphenyl)iron (2e): IR (neat) 2016, 1966, 1311, 1183, 1160, 1121, 1096, 1081, 1047, 790, 703, 676, 637, 621 cm-1; 1H-NMR δ = 4.89 (s, 5H), 7.05 (t, J = 7.5 Hz, 1H), 7.17 (d, J = 7.5 Hz, 1H), 7.64 (d, J = 7.5 Hz, 1H), 7.68 (s, 1H); 13C-NMR δ = 86.00, 120.05 (q, J = 3.8 Hz), 124.86 (q, J = 273.8 Hz), 126.98, 129.17 (q, J = 30.2 Hz), 140.37 (q, J = 3.8 Hz), 148.28, 148.41 (q, J = 1.5 Hz), 215.66; Found: C, 52.48; H, 2.91%. Calcd. for C14H9F3FeO2: C, 52.21; H, 2.82%.
Dicarbonylcyclopentadienyl(2-fluorophenyl)iron (2f): IR (nujol) 2028, 1977, 1454, 1193, 1016, 834, 764, 710, 629 cm-1; 1H-NMR δ = 4.91 (s, 5H), 6.80–6.88 (m, 2H), 6.95–6.99 (m, 1H), 7.49–7.52 (m, 1H); 13C-NMR δ = 85.57, 113.79 (d, J = 30.7 Hz), 123.86, 125.26 (d, J = 7.7 Hz), 127.99, 128.30, 146.73 (d, J = 13.0 Hz), 169.62 (d, J = 230.5 Hz) , 215.49; Found: C, 57.61; H, 3.41%. Calcd. for C13H9FFeO2: C, 57.39; H, 3.33%; m.p.: 57–58 °C.
Dicarbonyl(4-cyanophenyl)cyclopentadienyliron (2h): IR (nujol) 2225, 2014, 1964, 1942, 1920, 1575, 1462, 1418, 1042, 1010, 848, 818, 717 cm-1; 1H-NMR δ = 4.89 (s, 5H), 7.16 (d, J = 8.0 Hz, 2H), 7.61 (d, J = 8.0 Hz, 2H); 13C-NMR δ = 86.05, 106.54, 120.32, 129.13, 145.47, 161.07, 215.21; Found: C, 60.22; H, 3.24%. Calcd. for C14H9FeNO2: C, 60.25; H, 3.25%; m.p.: 100–101 °C.
Dicarbonylcyclopentadienyl(4-ethoxycarbonylphenyl)iron (2i): IR (nujol) 2023, 1949, 1708, 1576, 1454, 1283, 1124, 1008, 758 cm-1; 1H-NMR δ = 1.36 (t, J = 7.0 Hz, 3H), 4.33 (q, J = 7.0 Hz, 2H), 4.88 (s, 5H), 7.58 (s, 4H); 13C-NMR δ = 14.59, 60.55, 86.04, 125.76, 127.24, 144.96, 159.28, 167.97, 215.61; Found: C, 58.99; H, 4.43%. Calcd. for C16H14FeO4: C, 58.93; H, 4.33%; m.p.: 104–106 °C.
Dicarbonylcyclopentadienyl(3-ethoxycarbonylphenyl)iron (2j): IR (nujol) 2015, 1962, 1944, 1703, 1456, 1367, 1253, 1110, 751 cm-1; 1H-NMR δ =1.38 (t, J = 7.5 Hz, 3H), 4.35 (q, J = 7.5 Hz, 2H), 4.89 (s, 5H), 7.03 (t, J = 7.5 Hz, 1H), 7.58–7.60 (m, 1H), 7.65–7.67 (m, 1H), 8.12–8.13 (m, 1H); 13C-NMR δ = 14.56, 60.80, 86.00, 124.53, 127.03, 129.16, 145.36, 146.63, 149.66, 167.71, 215.82; Found: C, 58.79; H, 4.45%. Calcd. for C16H14FeO4: C, 58.93; H, 4.33%; m.p.: 67–68 °C.
Dicarbonylcyclopentadienyl(2-ethoxycarbonylphenyl)iron (2k): IR (nujol) 2020, 1969, 1945, 1707, 1456, 1377 cm-1; 1H-NMR δ = 1.37 (t, J = 7.0 Hz, 3H), 4.32 (q, J = 7.0 Hz, 2H), 4.94 (s, 5H), 6.97–6.99 (m, 2H), 7.44–7.46 (m, 1H), 7.72–7.74 (m, 1H); 13C-NMR δ = 14.48, 60.81, 86.85, 122.95, 128.10, 129.56, 146.55, 147.63, 147.70, 172.04, 215.62; Found: C, 58.78; H, 4.39%. Calcd. for C16H14FeO4: C, 58.93; H, 4.33%. m.p.: 64–65 °C.
Dicarbonylcyclopentadienyl(2-thienyl)iron (2m): IR (neat) 3104, 2854, 2026, 1966, 1420, 1391, 1317, 905, 826, 808, 682 cm-1; 1H-NMR δ = 4.98 (s, 5H), 6.83 (d, J = 3.0 Hz, 1H), 7.08 (dd, J = 3.0, 4.5 Hz, 1H), 7.45 (d, J = 4.5 Hz, 1H); 13C-NMR δ = 85.89, 128.89, 131.31, 134.68, 138.23, 214.72; Found: C, 50.55; H, 3.23%. Calcd. for C11H8FeO2S: C, 50.80; H, 3.10%.
Dicarbonylcyclopentadienyl(4-trifluoromethylphenyl)iron (2o): IR (nujol) 2007, 1957, 1588, 1325, 1158, 1072, 1008, 820, 631, 605 cm–1; 1H-NMR δ = 4.88 (s, 5H), 7.17 (d, J = 7.5 Hz, 2H), 7.59 (d, J = 7.5 Hz, 2H); 13C NMR δ = 86.01, 123.08 (q, J = 3.8 Hz), 125.01 (q, J = 272.3 Hz), 125.71 (q, J = 32.3 Hz), 144.94, 155.28 (q, J = 1.4 Hz), 215.63; Found: C, 52.07; H, 2.97%. Calcd. for C14H9F3FeO2: C, 52.21; H, 2.82%; m.p.: 72–73 °C.
Dicarbonylcyclopentadienyl(4-methoxymethylphenyl)iron (2q): IR (neat) 2920, 2012, 1955, 1480, 1187, 1096, 1042, 1007, 832, 797, 632 cm-1; 1H-NMR δ = 3.36 (s, 3H), 4.34 (s, 2H), 4.86 (s, 5H), 6.96 (d, J = 7.2 Hz, 2H), 7.43 (d, J = 7.2 Hz, 2H); 13C-NMR δ = 58.18, 75.02, 85.95, 127.50, 132.88, 145.04, 145.24, 216.19; Found: C, 60.69; H, 4.91%. Calcd. for C15H14FeO3: C, 60.43; H, 4.73%.
Dicarbonylcyclopentadienyl(4-hydroxymethylphenyl)iron (2u):IR (nujol) 3450, 2007, 1952, 1005 cm‑1; 1H-NMR δ = 1.48 (bs, 1H), 4.57 (d, J = 5.4 Hz, 2H), 4.87 (s, 5H), 7.00 (d, J = 7.5 Hz, 2H), 7.46 (d, J = 7.5 Hz, 2H); 13C-NMR δ = 65.68, 85.96, 126.75, 135.78, 145.24, 145.61, 216.12; Found: C, 58.97; H, 4.29%. Calcd. for C14H12FeO3: C, 59.19; H, 4.26%; m.p.: 92–93 °C.
Dicarbonylcyclopentadienyl[4-(1-butyl-1-hydroxypentyl)phenyl]iron (2v): IR (neat) 3480, 2935, 2862, 2016, 1961, 1684, 1456, 1003, 815 cm-1; 1H-NMR δ = 0.85 (t, J = 7.2 Hz, 6H), 1.10–1.29 (m, 8H), 1.61 (s, 1H), 1.72–1.77 (m, 4H), 4.86 (s, 5H), 6.96 (d, J = 8.1 Hz, 2H), 7.36 (d, J = 8.1 Hz, 2H); 13C-NMR δ = 14.27, 23.35, 25.95, 42.47, 76.72, 85.95, 124.78, 141.52, 141.93, 144.51, 216.40; Found: C, 66.91; H, 7.07%. Calcd. for C22H28FeO3: C, 66.68; H, 7.12%.