Cytotoxic Evaluation of Elastomeric Dental Impression Materials on a Permanent Mouse Cell Line and on a Primary Human Gingival Fibroblast Culture
Abstract
:1. Introduction
2. Results and Discussion
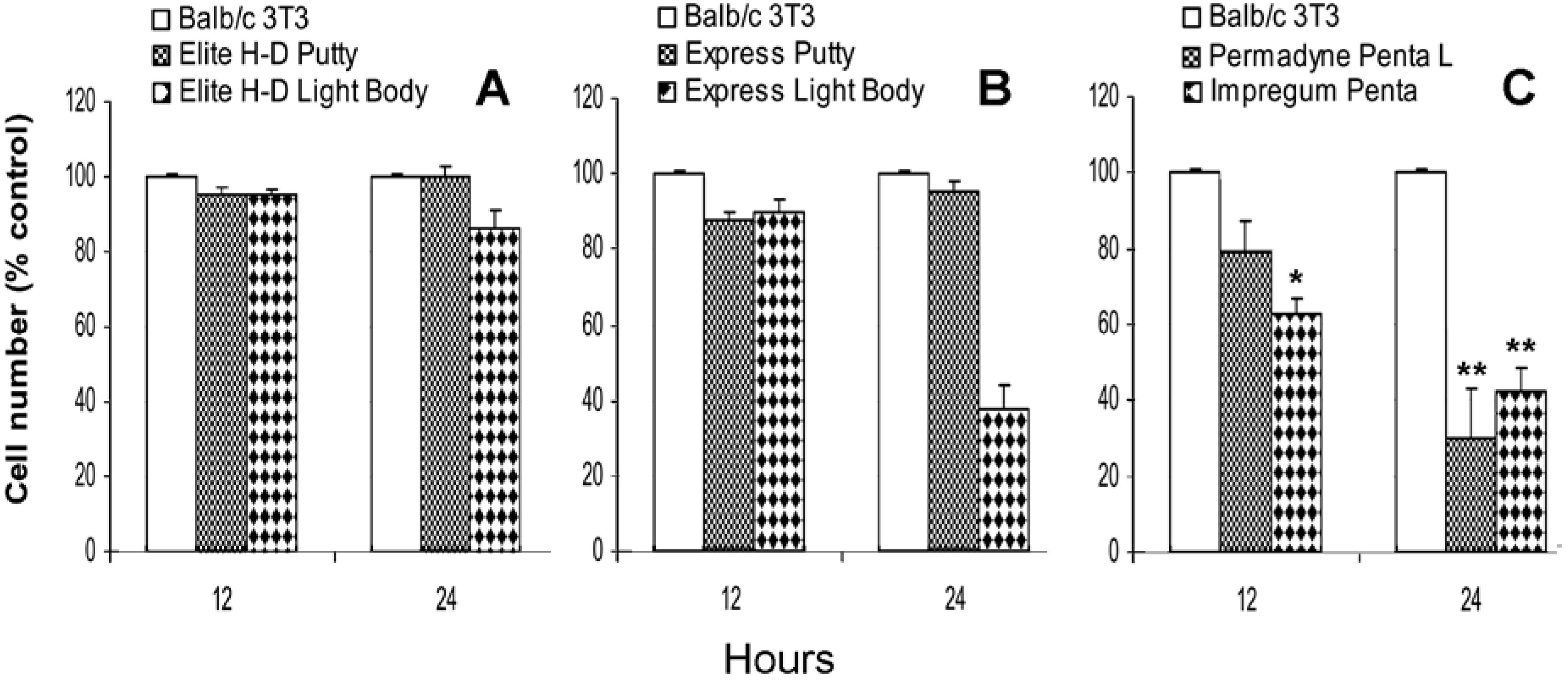
2.1. Direct effect of vinylpolysiloxane and polyether on Balb/c 3T3 proliferation
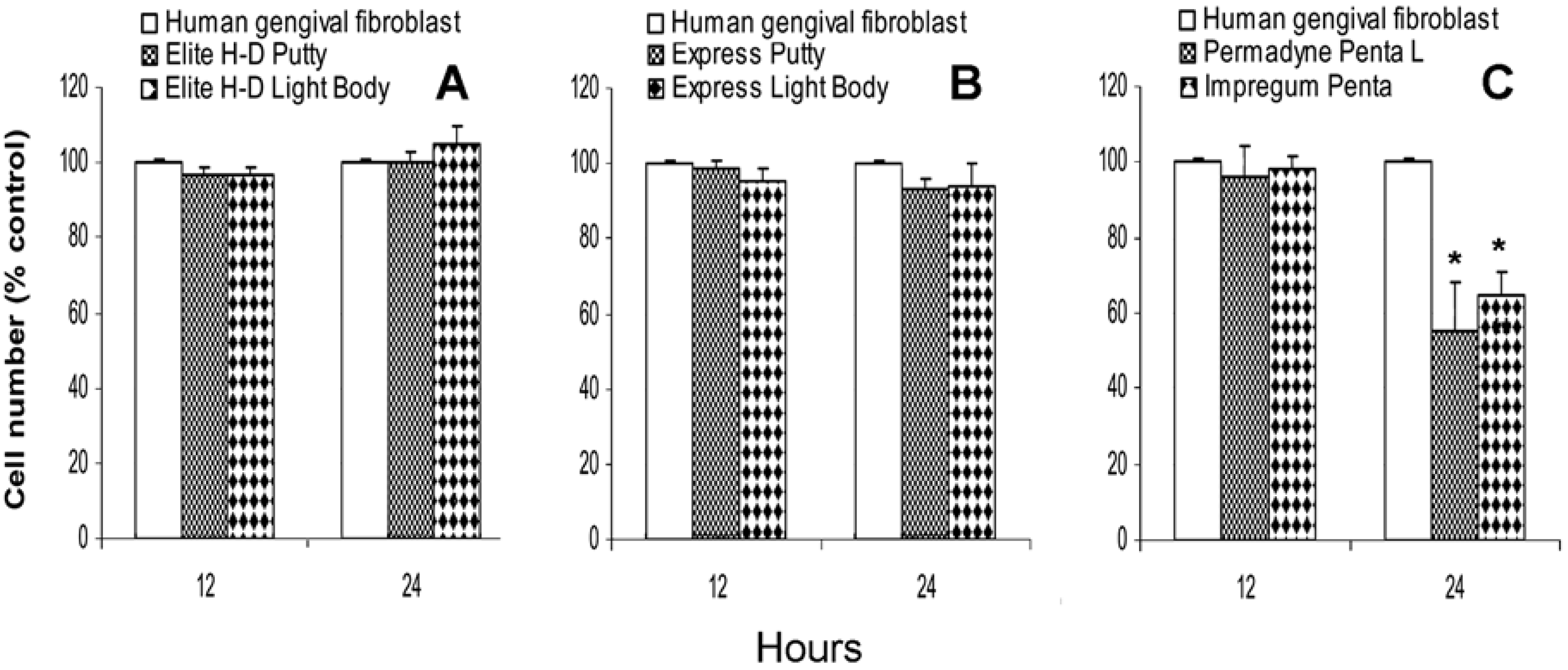
2.2. Direct effect of vinylpolysiloxane and polyether on human gingival fibroblasts proliferation
2.3. Indirect effect of impression material on Balb/c 3T3 viability

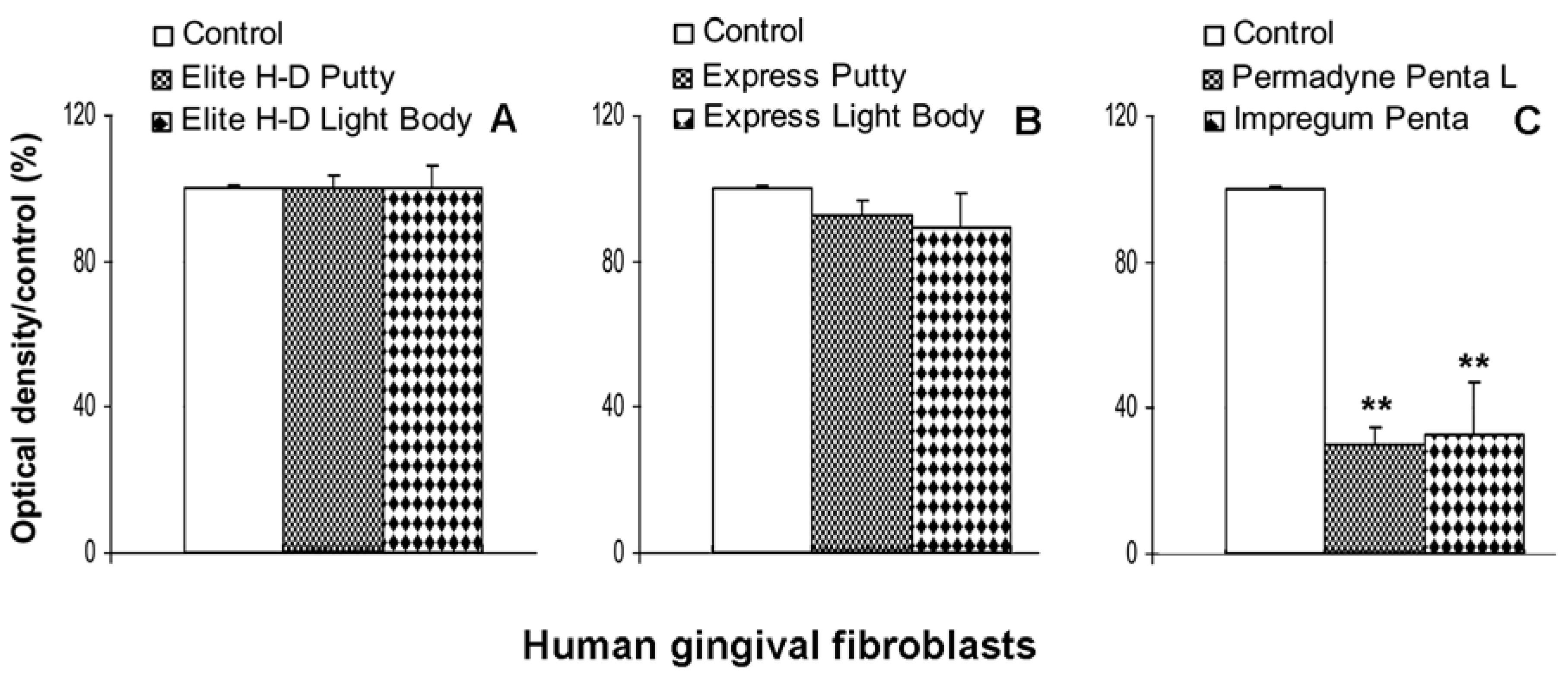
2.4. Indirect effect of impression material on human gingival fibroblast viability
3. Experimental Section
3.1. Impression materials
| Vinylpolysiloxanes: | Elite H-D Putty (Zhermack, Badia Polesine, Rovigo, Italy) |
| Elite H-D Light Body (Zhermack, Badia Polesine, Rovigo, Italy) | |
| Express Putty (3M ESPE AG Seefeld, Germany) | |
| Express Light Body (3M ESPE AG Seefeld, Germany) | |
| Polyethers: | Impregum Penta (3M ESPE AG Seefeld, Germany) |
| Permadyne Penta L (3M ESPE AG Seefeld, Germany) |
3.2. Cell culture
3.3. Cytotoxicity measurements
3.3.1. Direct test
3.3.2. Indirect test
3.3.3. MTT test
4. Conclusions
- a)
- in vitro cell culture technique can be used to rate the cytotoxic effect of dental impression materials;
- b)
- Balb/c 3T3, a continuous cell line, is more sensitive to cytoxic effects of dental materials than human gingival fibroblasts, a finite cell line;
- c)
- the direct in vitro tests are able to evaluate the toxic effects of the materials on adjacent cells. In the indirect test, the effect of “eluates” derived from the incubation of dental materials in culture medium, might be precisely quantified;
- c)
- both direct and indirect tests demonstrate that the vinylpolysiloxanes materials are less toxic than polyethers in our experimental conditions;
- d)
- among the vinylpolysiloxanes materials, Elite H-D Putty and Express Putty do not reduce Balb/c 3T3 and human gingival fibroblasts proliferation. The Express Light Body is found more toxic than Elite H-D Light Body;
- e)
- continuous cell line such Blab/c 3T3 are recommended as cells of choice for preliminary screening dental materials in vitro
- f)
- the biotoxicity studies ideally should employ human diploid gingival fibroblasts because the cells retain specialized features.
Acknowledgements
References
- Shen, C. Phillips’ Science of Dental Materials, 11th ed.; Anusavice K.J. Saunders: Toronto, Canada, 2003. [Google Scholar]
- Wataha, J.C. Biocompatibility of dental materials. In Phillips’ Science of Dental Materials, 11th ed.; Anusavice, K.J. Saunders: Toronto, Canada, 2003; Chapter 8; pp. 171–202. [Google Scholar]
- Rubel, B.S. Impression materials: A comparative review of impression materials most commonly used in restorative dentistry. Dent. Clin. North Am. 2007, 51, 629–642. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization, UNI EN ISO 10993-1:2004, Biological evaluation of medical devices-Part 1: Evaluation and testing.
- International Organization for Standardization, UNI EN ISO 10993-5:2000, Biological evaluation of medical devices-Part 5: Tests for in vitro cytotoxicity.
- Wataha, J.C.; Craig, R.G.; Hanks, C.T. Precision and new methods for testing in vitro alloy cytotoxicity. Dent. Mater. 1992, 8, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kostoryz, E.L.; Tong, P.Y.; Chappelow, C.C.; Eick, J.D.; Glaros, A.G.; Yourtee, D.M. In vitro cytotoxicity of solid epoxy-based dental resins and their components. Dent. Mater. 1999, 15, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, N.M.; Craig, R.G.; Hanks, C.T. Cytotoxicity of urethane dimethacrylate composites before and after aging and leaching. J. Biomed. Mater. Res. 1998, 39, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kopac, I.; Batista, U.; Cvetko, E.; Marion, L. Viability of fibroblasts in cell culture after treatment with different chemical retraction agents. J. Oral Rehabil. 2002, 29, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Mertens, T. The use of cultures of human osteoblastlike cells as an in vitro test system for dental materials. J. Oral Maxillofac. Surg. 1990, 48, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Hanks, C.T.; Sun, Z. Effect of cell line on in vitro metal ion cytotoxicity. Dent. Mater. 1994, 10, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Sydiskis, R.J.; Gerhardt, D.E. Cytotoxicity of impression materials. J. Prosthet. Dent. 1993, 69, 431–435. [Google Scholar] [CrossRef]
- Ciapetti, G.; Granchi, D.; Stea, S.; Savarino, L.; Verri, E.; Gori, A.; Salvioli, F.; Montanaro, L. Cytotoxicity testing of materials with limited in vivo exposure is affected by the duration of cell-material contact. J. Biomed. Mater. Res. 1998, 42, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chen, C.C.; Kuo, H.W. Cytotoxicity of dental impression materials. Bull. Environ. Contam. Toxicol. 2002, 69, 350–355. [Google Scholar] [CrossRef]
- Tiozzo, R.; Magagna, F.; Boraldi, F.; Croce, M.A; Bortolini, S.; Consolo, U. Study of the potential cytotoxicity of dental impression materials. Toxicol. In Vitro 2003, 17, 657–662. [Google Scholar] [CrossRef]
- Liu, C.M.; Huang, F.M.; Yang, L.C.; Chou, L.S.; Chou, M.Y.; Chang, Y.C. Cytotoxicity effects of gingival retraction cords on human gingival fibroblasts in vitro. J. Oral. Rehabil. 2004, 31, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Coppi, C.; Paolinelli Devincenzi, C.; Bortolini, S.; Consolo, U.; Tiozzo, R. A new generation of sterile and radiopaque impression materials: an in vitro cytotoxicity study. J. Biomater. Appl. 2007, 1, 83–95. [Google Scholar] [CrossRef]
- Brunton, P.A.; Christensen, G.J.; Cheung, S.W.; Burke, F.J.; Wilson, N.H. Contemporary dental practice in the UK: Indirect restorations and fixed prosthodontics. Br. Dent. J. 2005, 198, 99–103. [Google Scholar] [CrossRef]
- Nally, F.F.; Storrs, J. Hypersensitivity to a dental material. A case report. Br. Dent. J. 1973, 134, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.G. Characteristics and clinical and tissue reactions of impression materials. In Biocompatibility of Dental Materials; Smith, D.C., Williams, D.F., Eds.; CRC Press: Boca Raton, FL, USA, 1982; pp. 277–289. [Google Scholar]
- Marshak, B.L.; Cardash, H.S.; Ben-Ur, Z. Incidence of impression material found in the gingival sulcus after impression procedure for fixed partial dentures. J. Prosthet. Dent. 1987, 57, 306–308. [Google Scholar] [CrossRef]
- Prince, C.; Whitehead, I.H. Impression materials as foreign bodies. Br. Dent. J. 1972, 133, 9–14. [Google Scholar] [CrossRef]
- Garey, R.C.; Narang, R. An unusual foreign body in the buccal vestibule. Report a case. Oral Med. Oral Surg. Oral Pathol. 1976, 42, 314–315. [Google Scholar] [CrossRef]
- Gullet, C.E.; Caulder, S.L. Residual fragment of rubber base material. Operative Dent. 1978, 3, 250–252. [Google Scholar]
- Ree, M.H. An unusual swelling following endodontic and prosthodontic treatment of a mandibular molar due to a foreign body reaction. Int. Endod. J. 2001, 34, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Mertens, T.H. The use of cultures of human osteoblastlike cells as an in vitro test system for dental materials. J. Oral Maxillofac. Surg. 1990, 48, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Felder, S.M.; Gettleman, L.; Alchediak, T. A model culture system with human gingival fibroblasts for evaluating the cytotoxicity of dental materials. In Vitro Cell. Dev. Biol. Plant 1982, 18, 650–660. [Google Scholar] [CrossRef]
- Costa, R.; Baccarani Contri, M.; Cingi, M.R.; Pasquali Ronchetti, I.; Salvini, R.; Rindi, S.; De Luca, G. Pseudoxanthoma elaticum (PXE): Ultrastructural and biochemical study on proteglycan and proteoglycan-associated materials produced by skin fibroblasts in vitro. Coll. Relat. Res. 1988, 8, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, D.; Boraldi, F.; Barbieri, D.; Croce, M.A.; Tiozzo, R.; Pasquali-Ronchetti, I. Abnormal phenotype of in vitro dermal fibroblasts from patients with Pseudoxanthoma elasticum. Biochim. Biophys. Acta 2000, 1501, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimatric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemiosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boraldi, F.; Coppi, C.; Bortolini, S.; Consolo, U.; Tiozzo, R. Cytotoxic Evaluation of Elastomeric Dental Impression Materials on a Permanent Mouse Cell Line and on a Primary Human Gingival Fibroblast Culture. Materials 2009, 2, 934-944. https://doi.org/10.3390/ma2030934
Boraldi F, Coppi C, Bortolini S, Consolo U, Tiozzo R. Cytotoxic Evaluation of Elastomeric Dental Impression Materials on a Permanent Mouse Cell Line and on a Primary Human Gingival Fibroblast Culture. Materials. 2009; 2(3):934-944. https://doi.org/10.3390/ma2030934
Chicago/Turabian StyleBoraldi, Federica, Chiara Coppi, Sergio Bortolini, Ugo Consolo, and Roberta Tiozzo. 2009. "Cytotoxic Evaluation of Elastomeric Dental Impression Materials on a Permanent Mouse Cell Line and on a Primary Human Gingival Fibroblast Culture" Materials 2, no. 3: 934-944. https://doi.org/10.3390/ma2030934





