1. Introduction
Lithium cobalt oxide (LiCoO
2) remains the most exploited cathode material for commercial Li-ion batteries [
1]. LiCoO
2 cathode exhibits high output-voltage, high specific-energy, long cycle-life and low self-discharge features that are central to batteries powering portable-electronic devices [
1,
2,
3,
4]. Electrochemical performance of LiCoO
2 greatly depends on its crystallographic structure, as it exists in two different modifications, namely the high-temperature (HT) phase, crystallizing in an ideal layered-structure isomorphic to α-NaFeO
2 (space group: R
3m) with ordered cobalt and lithium ions resulting in hexagonal sheets of Li
+- and Co
3+- ions in alternate layers of (111) planes [
5], and the low-temperature (LT) phase with spinel-like structure (space group: Fd3m) with about 6 % of Co
3+ ions located at lithium sites [
6]. Unlike LT-LiCoO
2, HT-LiCoO
2 exhibits excellent electrochemical stability on prolonged cycling [
7,
8]. The stability of HT phase originates from the structural durability of the material with the layered cation-ordering that remains well preserved even after repeated insertion and de-insertion of Li
+-ions during the charge-discharge processes of the lithium-ion cell.
The structure and degree of cation ordering in LiCoO
2 vary with the synthetic conditions that affect its electrochemical activity. Accordingly, the optimization of synthetic procedure for LiCoO
2 is seminal for attaining its improved electrochemical behavior. To this end, various synthetic methods, such as ceramic method [
9], oxalate method [
10], hydroxide precipitation method [
11], sol-gel method [
12], molten salt method [
13], hydrothermal method [
14], template method [
15], spray pyrolysis [
16], polymer pyrolysis method [
17], Pechini method [
18] and combustion method [
19], have been attempted in the literature for realizing electrochemically active LiCoO
2.
This communication reports a rapid synthesis of LiCoO
2 cathode material with high specific-capacity for Li-ion batteries. The method involves heating stoichiometric amounts of metal-nitrate precursors that on decomposing yield a crystalline oxide. Since the metal nitrates have low melting-point, their mixtures transform to a eutectic melt on heating to the eutectic temperature that on further heating yields nano-crystalline LiCoO
2 with perfect layered-structure suitable for use as active cathode material in a lithium-ion rechargeable battery. The method is also useful for synthesizing Mg-/Al-ions doped LiCoO
2 and related intercalation oxides. It is noteworthy that the present method is altogether novel and differs substantially from other nitrate-decomposition methods [
18,
19,
20]. The method is simple and cost-effective for bulk synthesis of HT-LiCoO
2.
3. Results and Discussion
Figures 1(a-d) show the powder X-ray diffraction patterns for the products formed by heating the mixed-metal nitrates of lithium and cobalt in a 1.1:1 molar ratio at varying temperatures between 600 ºC and 900 ºC for 4 h. The diffraction pattern for the product obtained at 600 ºC could be indexed as rhombohedral R
3m LiCoO
2 along with small amounts of lithium-deficient LiCoO
2 crystallizing in cubic Fm3m structure. X-ray powder diffraction pattern for the product obtained by heating at 700 ºC shows single-phase LiCoO
2 as indexed on the basis of α-NaFeO
2 structure (space group: R
3m).
Figure 1.
Powder X-ray diffraction patterns for LiCoO2 obtained by nitrate melt decomposition at varying temperatures (a) 600 ºC (b) 700 ºC (c) 800 ºC (d) 900 ºC. The peaks marked as (#) correspond to Co3O4 and (*) correspond to lithium-deficient phase, Li0.115Co0.885O (ICSD collection code: 029229).
Figure 1.
Powder X-ray diffraction patterns for LiCoO2 obtained by nitrate melt decomposition at varying temperatures (a) 600 ºC (b) 700 ºC (c) 800 ºC (d) 900 ºC. The peaks marked as (#) correspond to Co3O4 and (*) correspond to lithium-deficient phase, Li0.115Co0.885O (ICSD collection code: 029229).
The lattice parameters obtained by Rietveld refinement of LiCoO
2-700 sample agree well with those prepared by conventional solid-state method [
21] and are given in
Table 1. All diffraction patterns show clear (006)/(102) peaks and (018)/(110) split peaks indicating a perfect layered-structure for LiCoO
2 [
21]. The diffraction patterns of samples prepared at 800 ºC and 900 ºC comprise a major LiCoO
2 phase crystallizing in rhombohedral structure with a small Co
3O
4 spinel phase marked with (*) in
Figure 1 (c) and (d); the latter arising due to lithium evaporation from the parent compound at high temperatures. I
(003)/I
(104) intensity ratio decreases with increasing calcination temperature and is sensitive to the degree of cation mixing [
22] that influences electrochemical properties substantially.
Table 1.
Lattice parameters, c/a ratio and I(003)/I(104) intensity ratio for HT-LiCoO2 related phases.
Table 1.
Lattice parameters, c/a ratio and I(003)/I(104) intensity ratio for HT-LiCoO2 related phases.
| Sample | a (Å) | c (Å) | c/a | I(003)/I(104) |
|---|
| LiCoO2-700 | 2.8132(8) | 14.0630(3) | 4.998 | 1.58 |
| LiCoO2-SS | 2.8164(6) | 14.0568(7) | 4.991 | 1.79 |
| LiCo0.95Mg0.05O2 | 2.8172(6) | 14.0770(5) | 4.996 | 1.27 |
| LiCo0.95Al0.05O2 | 2.8116(8) | 14.0702(6) | 5.004 | 1.14 |
It is clear from the aforesaid X-ray diffraction studies that 700 ºC is the optimal temperature for obtaining pure LiCoO
2 in ordered rock-salt superstructure. In order to determine the optimal duration, the synthesis was carried out with varying heating durations between 15 min and 4 h at 700 ºC. The X-ray diffraction patterns for the products formed at different time intervals are presented in
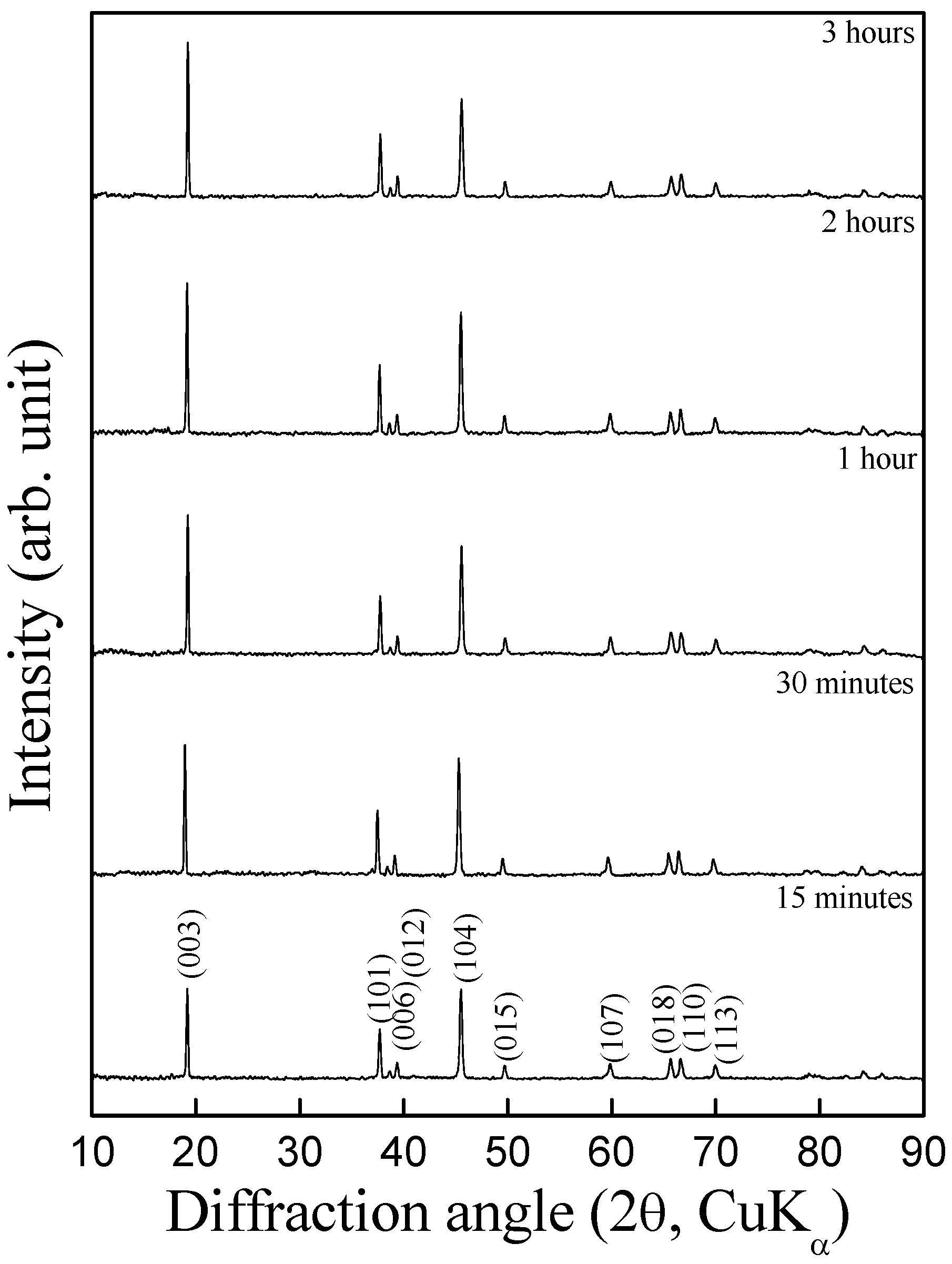
Figure 2. It is seen from the diffractograms that the sample prepared at 700 ºC for as low as 15 min matches well with HT-LiCoO
2 pattern with R
3m space group; the intensity ratio of I
(003)/I
(104) <1.2 indicating a considerable extent of cation mixing in the crystal lattice. By contrast, the samples synthesized at 700 ºC for 1 h (or more) exhibit I
(003)/I
(104) >1.2 indicating the absence of cation mixing. On increasing the heating duration beyond 1 h, little change is observed in the diffraction pattern with increase in overall peak intensity owing to the improved crystallinity of the sample.
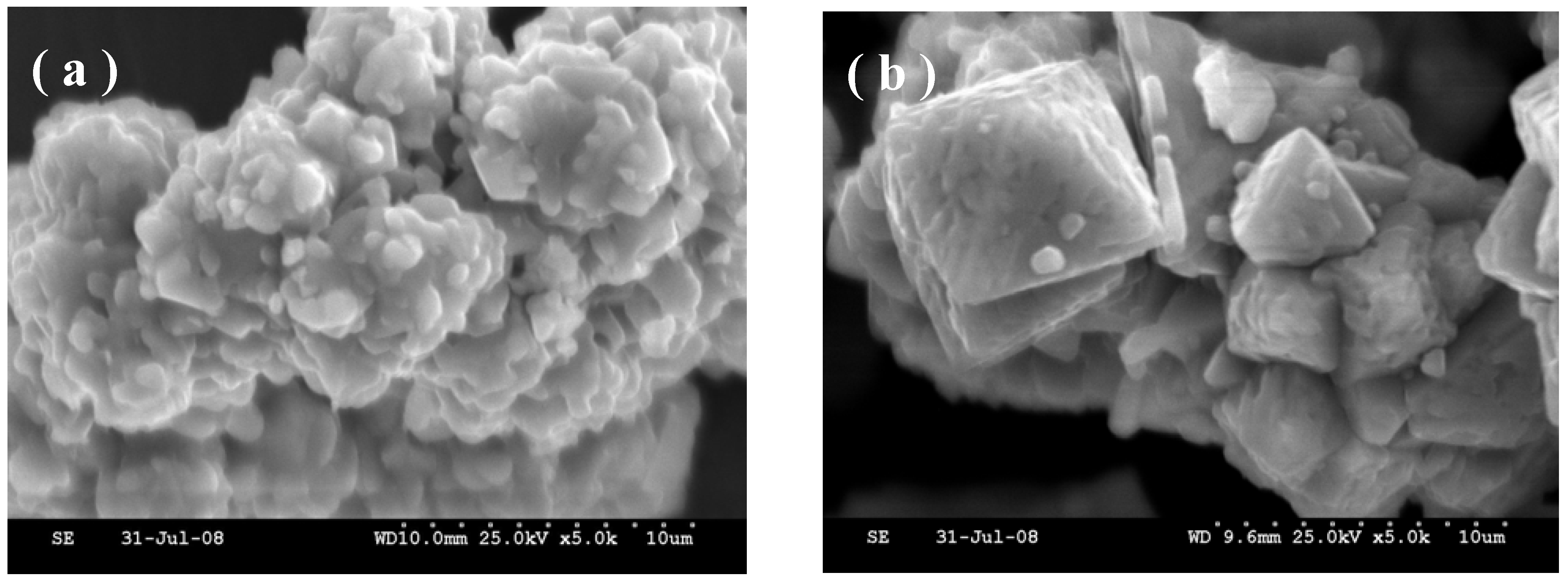
The increase in crystallinity of the sample with longer heating duration is further corroborated from morphological studies by scanning electron microscopy (SEM). The SEM image for the samples heated at 700 ºC for 1 h shown in
Figure 3(a) depicts agglomerates of smaller crystallites of ~1 μm. The sample obtained by heating at 700 ºC for 4 h shows well defined crystals of platelet-like morphology of ~ 50 μm [
Figure 3(b)]. The chemical compositions of the prepared samples obtained from AAS analysis of Li and Co suggest cation stoichiometry to be strongly dependent on the synthesis temperature. LiCoO
2 prepared at 600 ºC shows a slight excess of lithium stoichiometry corresponding to Li
1.09CoO
2 and closure to the initial stoichiometry of nitrates taken, suggesting little loss of Li during heating. It is noteworthy that impurity peaks corresponding to lithium salts are absent in the X-ray diffractogram. The sample prepared at 700 ºC has its nominal composition as LiCoO
2 while the samples prepared at 800 ºC and above reflect Co
3O
4 as impurity in the diffractogram albeit the use of 10 mol % excess Li salt in the synthesis. This suggests substantial amount of Li loss at high temperatures. Chemical analyses of these samples were not possible owing to the insolubility of Co
3O
4 in acids. From the fore going, it is clear that the synthetic conditions have a seminal role in controlling structural as well as compositional aspects of LiCoO
2 that affect its electrochemical activity.
Figure 2.
Powder X-ray diffraction patterns for LiCoO2 obtained by heating nitrates at 700 °C for varying durations.
Figure 2.
Powder X-ray diffraction patterns for LiCoO2 obtained by heating nitrates at 700 °C for varying durations.
Figure 3.
Scanning Electron Micrographs for HT-LiCoO2 obtained by heating corresponding metal nitrates at 700 °C in air for (a) 1 h and (b) 4 h.
Figure 3.
Scanning Electron Micrographs for HT-LiCoO2 obtained by heating corresponding metal nitrates at 700 °C in air for (a) 1 h and (b) 4 h.
The metal-nitrates-melt decomposition method has added advantage in relation to solution methods as the former results in homogeneously mixed metal ions leading to their effective substitution in the end product. It has been possible to synthesize about 5-6 atom % of Mg- or Al- doped LiCoO
2.
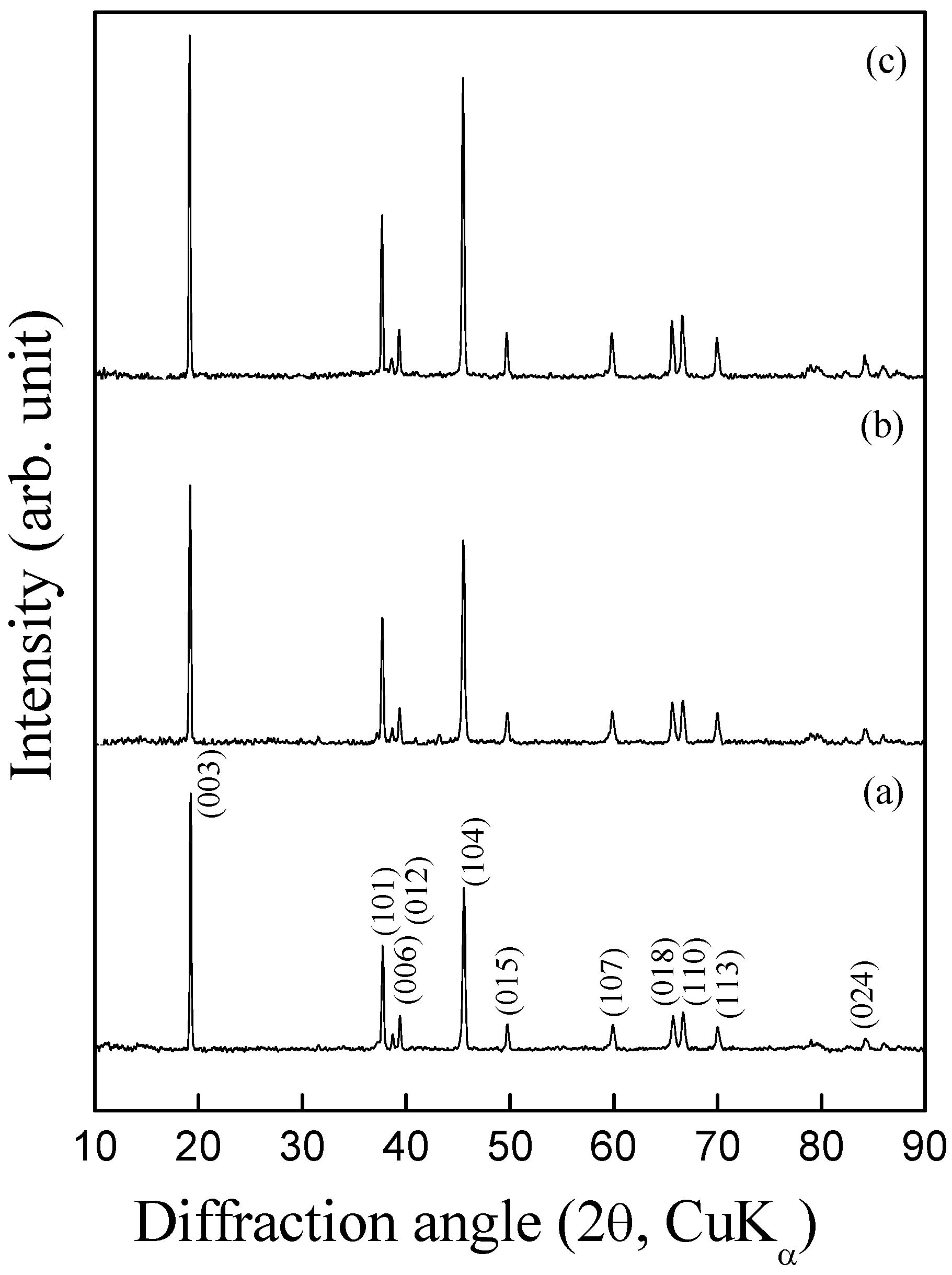
Figure 4 shows powder X-ray diffraction patterns for LiCo
0.95Mg
0.05O
2 and LiCo
0.95Al
0.05O
2 samples along with the XRD pattern for pristine LiCoO
2. All these XRD patterns could be indexed to a layered α-NaFeO
2 structure (space group: R
3m). The samples are phase pure and no impurity peaks corresponding to Li
2CO
3 and metal oxides are present. The refined lattice parameters for LiCo
0.95Mg
0.05O
2 and LiCo
0.95Al
0.05O
2 are presented in
Table 1 which agrees well with those reported in the literature for similar compositions [
23,
24]. Substitution of Co
3+ by larger Mg
2+-ion increases the
a and
c lattice parameters with
c/
a ratio of 4.99 while substitution by smaller Al
3+-ion decreases the
a-lattice parameter and increases the
c-lattice parameter resulting in an increased
c/
a ratio of 5.004.
Figure 4.
Powder X-ray diffraction patterns for (a) pristine LiCoO2, (b) LiCo0.95Mg0.05O2 and (c) LiCo0.95Al0.05O2.
Figure 4.
Powder X-ray diffraction patterns for (a) pristine LiCoO2, (b) LiCo0.95Mg0.05O2 and (c) LiCo0.95Al0.05O2.
The electrochemical performances of all the LiCoO
2 samples, prepared at different temperatures with varying heat durations, have been evaluated using Swagelok-type cells. The voltage-composition curves for galvanostatic charge/discharge cycling of Li/LiCoO
2-700 cells at 1 Li/5 h in different voltage ranges are shown in
Figures 5(a) and (b) as representative examples.
The smooth charge-discharge curves between 3.5 V and 4.2V show the absence of spinel-phase formation during cycling with the plateau due to Co3+/Co4+ redox process at ~3.9 V. A higher capacity of 150 mAhg-1 is obtained when the cells are cycled between 3.5 V and 4.3 V but the irreversible capacity loss, polarisability and capacity fade are higher compared to cells cycled between 3.5 V and 4.2 V. This is probably due to the change in crystal structure associated with the higher lithium extraction during higher voltage windows.
Figure 5.
The voltage-composition curve for a Li/LixCoO2-700 cell cycled galvanostatically at 1 Li/5 h rate between the voltage window (a) 3.5 - 4.2 V and (b) 3.5 - 4.3 V at room temperature (~30 ºC).
Figure 5.
The voltage-composition curve for a Li/LixCoO2-700 cell cycled galvanostatically at 1 Li/5 h rate between the voltage window (a) 3.5 - 4.2 V and (b) 3.5 - 4.3 V at room temperature (~30 ºC).
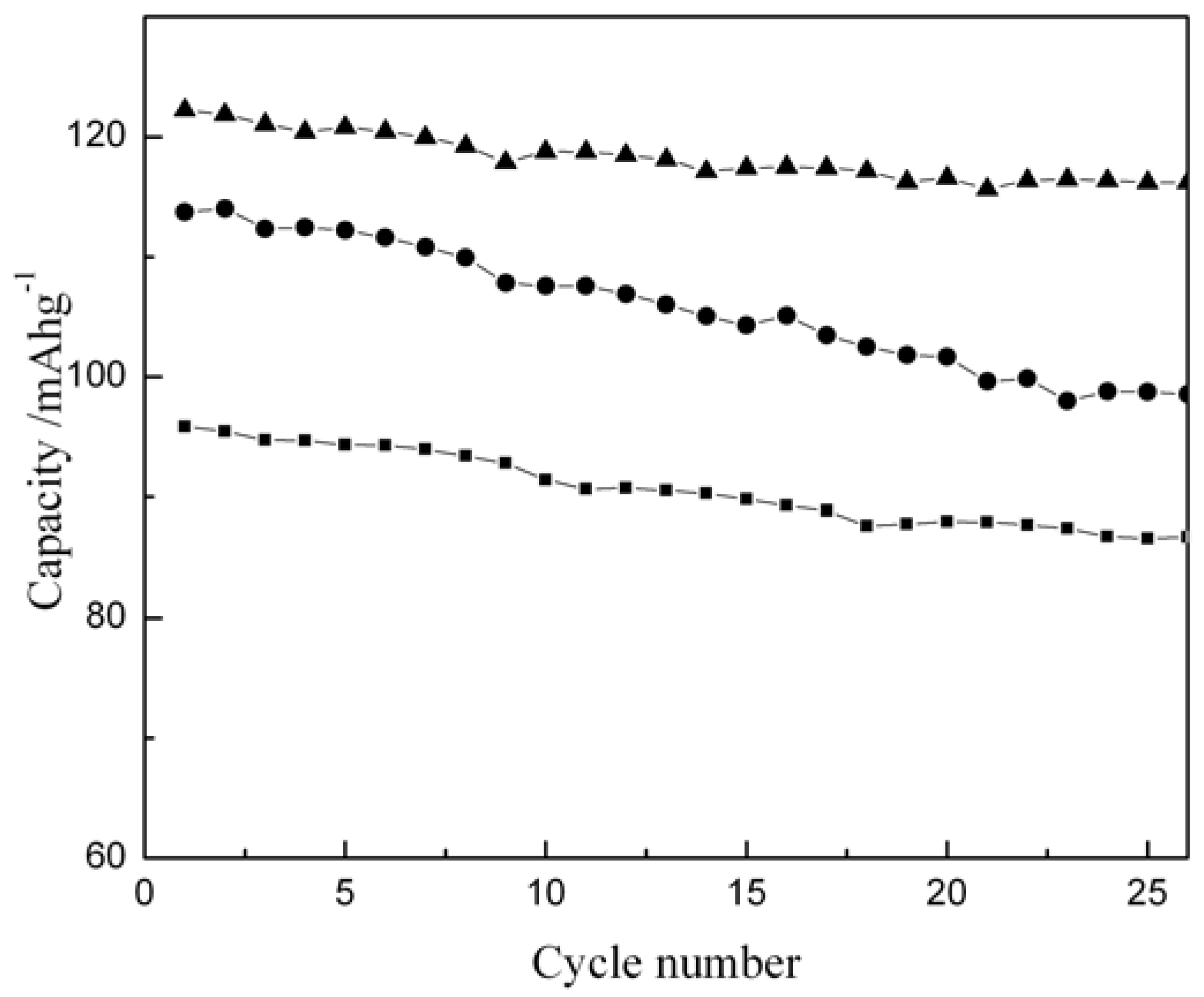
Figure 6 shows the specific capacity values versus cycle number for Li/LiCoO
2 cells with LiCoO
2 samples prepared at different temperatures. The first charge/discharge capacities of the samples prepared at 600 °C, 700 °C and 800 °C are 106/91, 132/122 and 131/114 mAhg
-1, respectively. As obvious from the data, the sample obtained by heating at 600 ºC shows lower capacity and retains only 92% of its initial capacity after 25 cycles, presumably due to the absence of perfect-layered structure as revealed by its X-ray diffraction pattern. By contrast, the sample prepared at 700 °C shows a maximum capacity with an irreversible capacity loss of about 10 mAh/g; the sample retains a capacity value of ~ 116mAhg
-1 after 25 cycles with a capacity loss of only ~ 5%. The initial charge capacity for the sample synthesized at 800 °C is comparable to the sample prepared at 700 °C but its irreversible capacity loss in the first cycle is 17 mAhg
-1 that pushes its specific capacity to ~114 mAhg
-1. This is due to the Li-deficiency in the sample brought about by lithium loss at higher temperature. Accordingly, the sintering temperature clearly affects electrochemical behavior of the samples. In this study, the optimum electrochemical performance is observed for stoichiometric LiCoO
2 prepared at 700 °C.
Figure 6.
Discharge capacity versus cycle number for Li/LiCoO2 cells cycled between 3.5-4.2 V at room temperature ( ~30 ºC ) with LiCoO2 prepared at different temperatures: -●- 800 ºC, -▲- 700 ºC and -■-600 ºC.
Figure 6.
Discharge capacity versus cycle number for Li/LiCoO2 cells cycled between 3.5-4.2 V at room temperature ( ~30 ºC ) with LiCoO2 prepared at different temperatures: -●- 800 ºC, -▲- 700 ºC and -■-600 ºC.
In order to compare the electrochemical performance of HT-LiCoO
2 prepared by nitrate-melt method with conventionally prepared samples, the cells of LiCoO
2 prepared by solid state (LiCoO
2-SS) and nitrate-melt (LiCoO
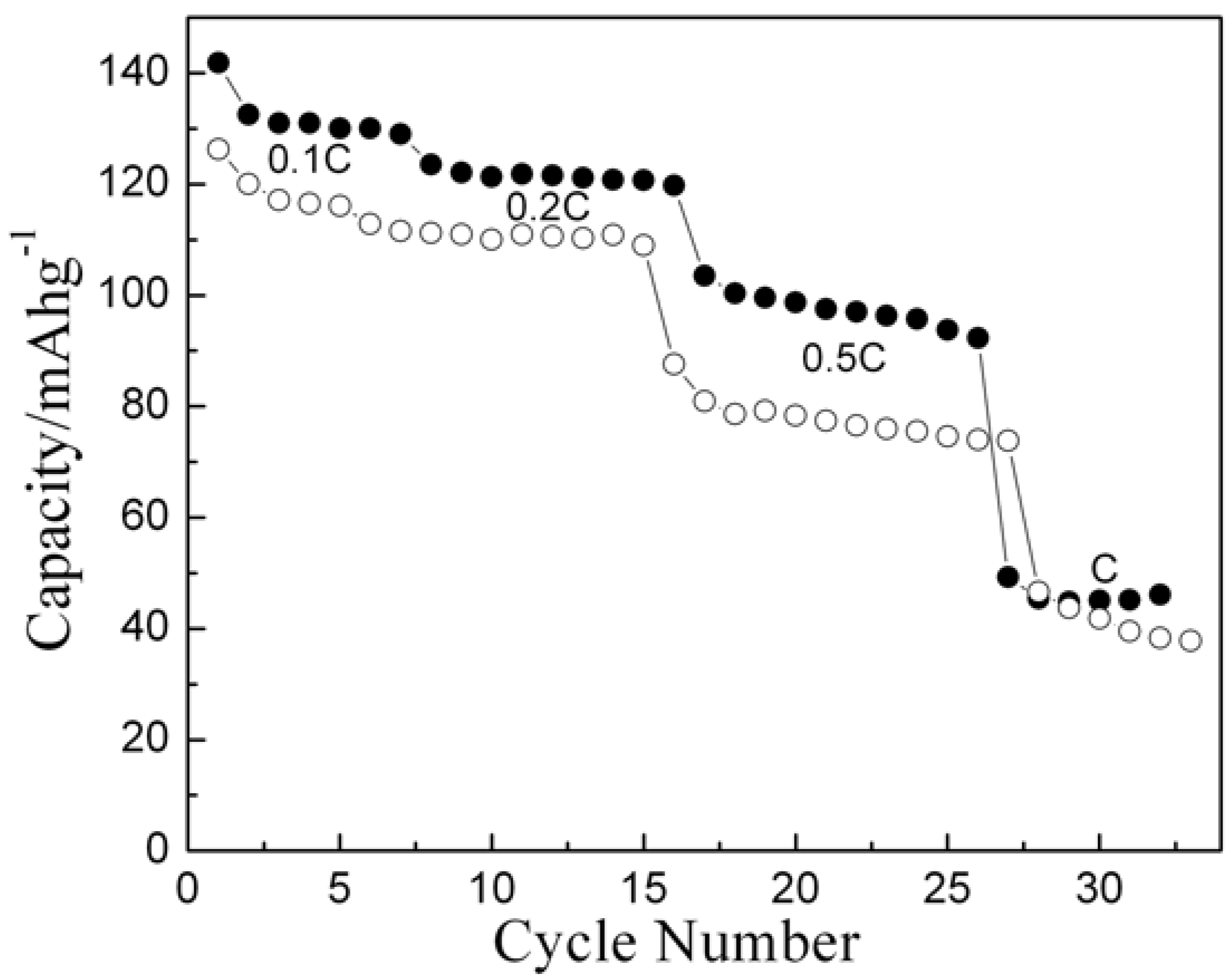
2-700) methods have been subjected to cycling at varying rates. The capacity Vs cycle number plot is shown in
Figure 7. The data reflect that the sample derived by nitrate-melt method exhibits higher capacity and better rate capability as compared to the sample prepared by conventional ceramic method.
Figure 7.
Rate capability plot for Li/LiCoO2 cells for nitrate derived (-●-) and solid-state prepared (-○-) samples.
Figure 7.
Rate capability plot for Li/LiCoO2 cells for nitrate derived (-●-) and solid-state prepared (-○-) samples.
Further, we have also evaluated electrochemical performances of Mg- or Al-doped LiCoO
2 samples prepared by nitrate-melt-decomposition method.
Figures 8(a) and (b) show the voltage versus composition curves for Li/LiCo
0.95Mg
0.05O
2 and Li/LiCo
0.95Al
0.05O
2 cells, respectively. The corresponding plots of capacity versus cycle number are shown as insets to
Figures 8 (a) and (b).
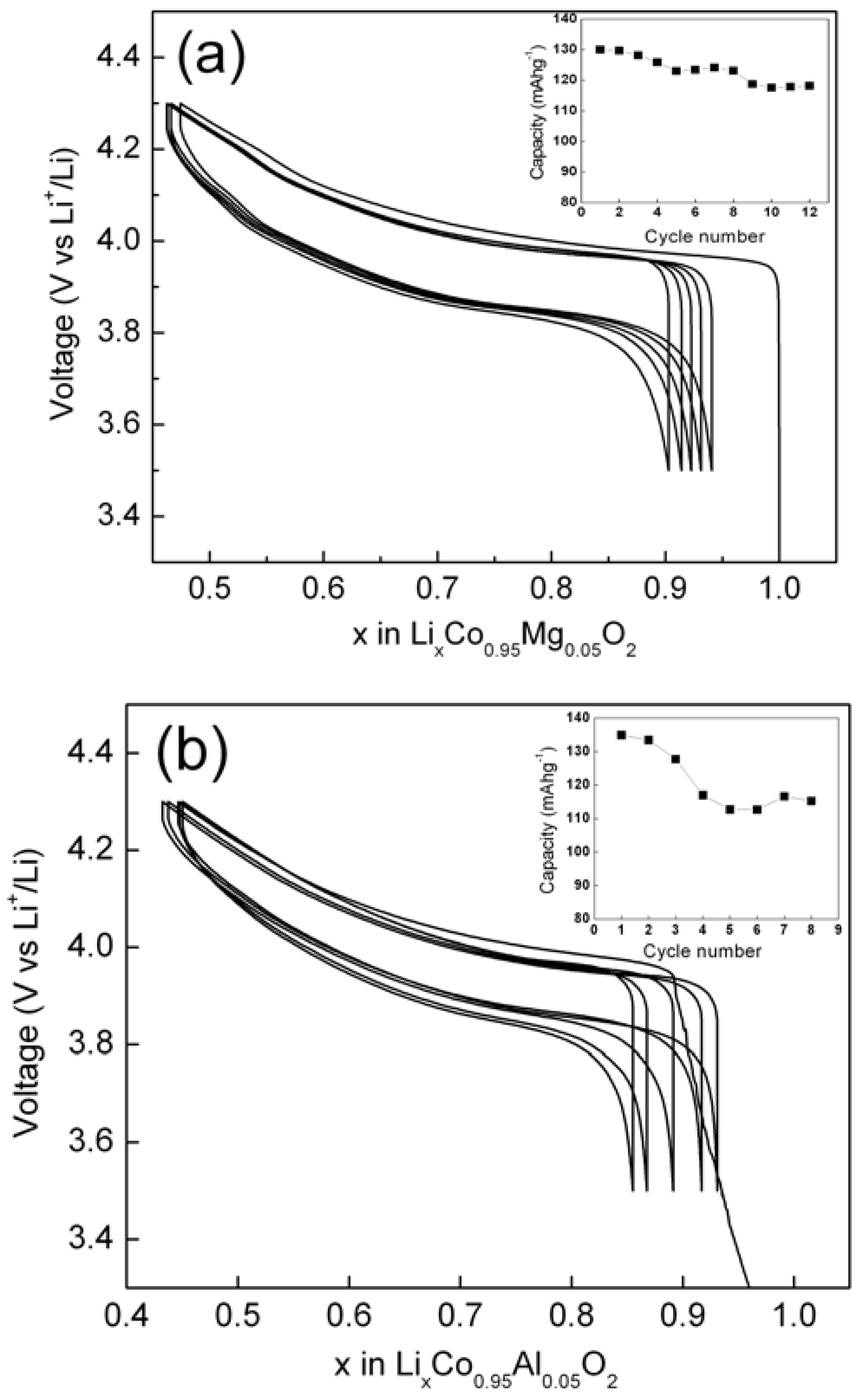
Figure 8.
The voltage versus composition curves for (a) Li/LiCo0.95Mg0.05O2 and (b) Li/LiCo0.95Al0.05O2 cells in the voltage window 3.5-4.3 V. Corresponding capacity versus cycle number data are shown as insets.
Figure 8.
The voltage versus composition curves for (a) Li/LiCo0.95Mg0.05O2 and (b) Li/LiCo0.95Al0.05O2 cells in the voltage window 3.5-4.3 V. Corresponding capacity versus cycle number data are shown as insets.
The Mg-doped samples show good reversibility during charge/discharge cycling with first cycle capacity as high as 146 mAhg-1, a value comparable to un-doped LiCoO2. As expected, Mg doping enhances the structural stability with good capacity retention between 3.5 V and 4.3 V in relation to pristine LiCoO2. Interestingly, Li/LiCo0.95Al0.05O2 cell shows very high initial capacity of ~ 154 mAhg-1 but has poor capacity retention
We have demonstrated that nitrate-melt-decomposition route leads to electrochemically active pristine and doped LiCoO
2. Although, synthesis of electrochemically active LiCoO
2 through nitrate precursors followed by combustion, sol-gel or flame pyrolysis methods have been reported previously [
18,
19,
20], these methods require a fuel or chelating agent in addition with metal precursors. Generally, the product obtained using these methods have residual carbonaceous matter which requires high-temperature heating for long duration. Besides, in many cases, the product formed is amorphous and requires further calcination for crystallization. In this context, the metal-nitrate-melt-decomposition route is attractive and new. The method is simple as well as cost effective for synthesizing battery grade LiCoO
2 in bulk.