Sol–Gel Synthesis of Endodontic Cements: Post-Synthesis Treatment to Improve Setting Performance and Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioactive Endodontic Cement (BEC) Synthesis Procedure
2.2. Ethanol Post-Synthesis Treatment (EPT)
2.3. Material Characterization
2.3.1. X-ray Diffraction (XRD) Analysis
2.3.2. Particle Size Measurements
2.3.3. Fourier-Transform Infrared (FT-IR) Spectroscopy
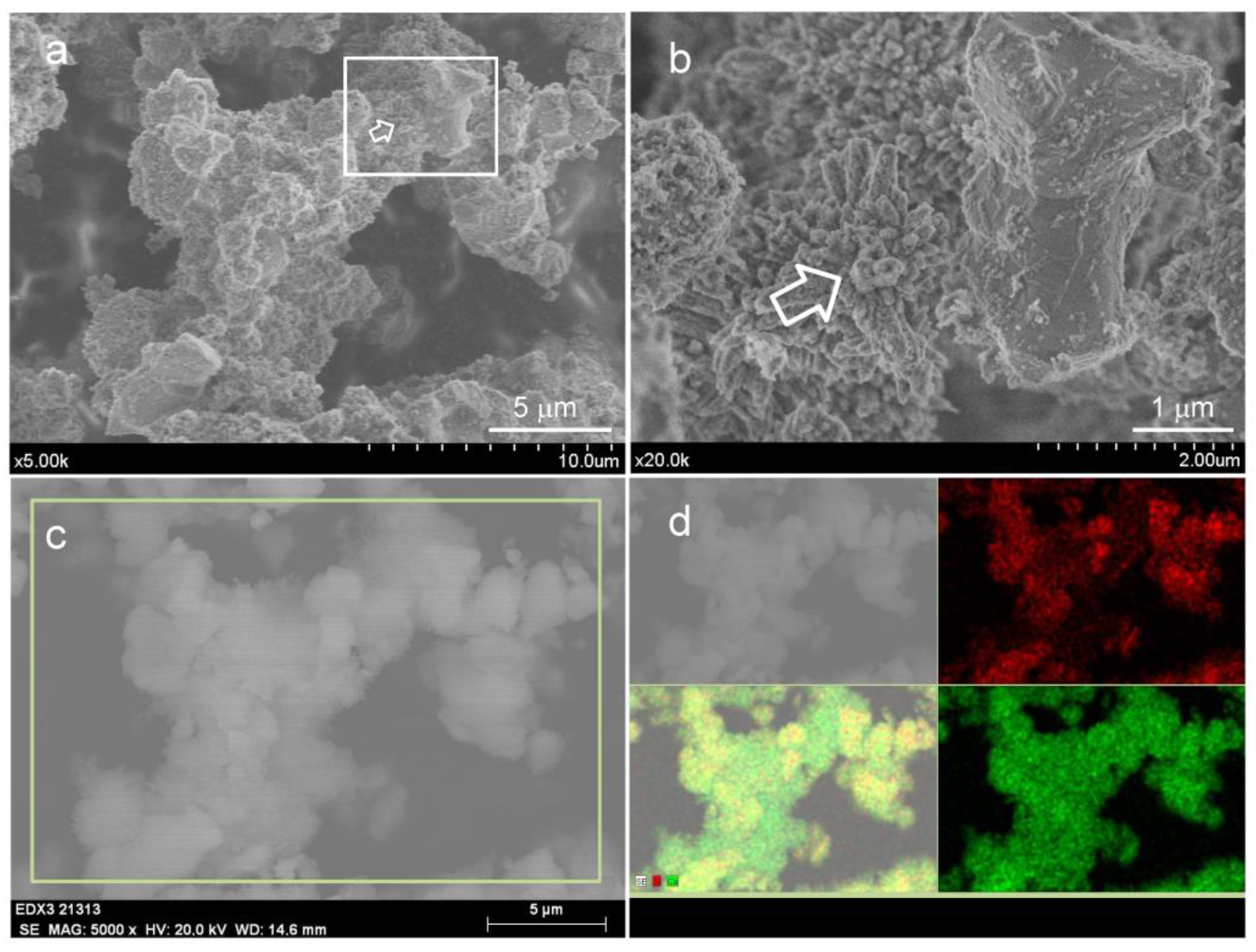
2.3.4. Field-Emission Gun Scanning Electron Microscopy (FEG-SEM)
2.3.5. Characterization of Textural Properties
2.3.6. Hydration Protocol and Setting Time Analysis
2.3.7. Bioactivity Assays
3. Results
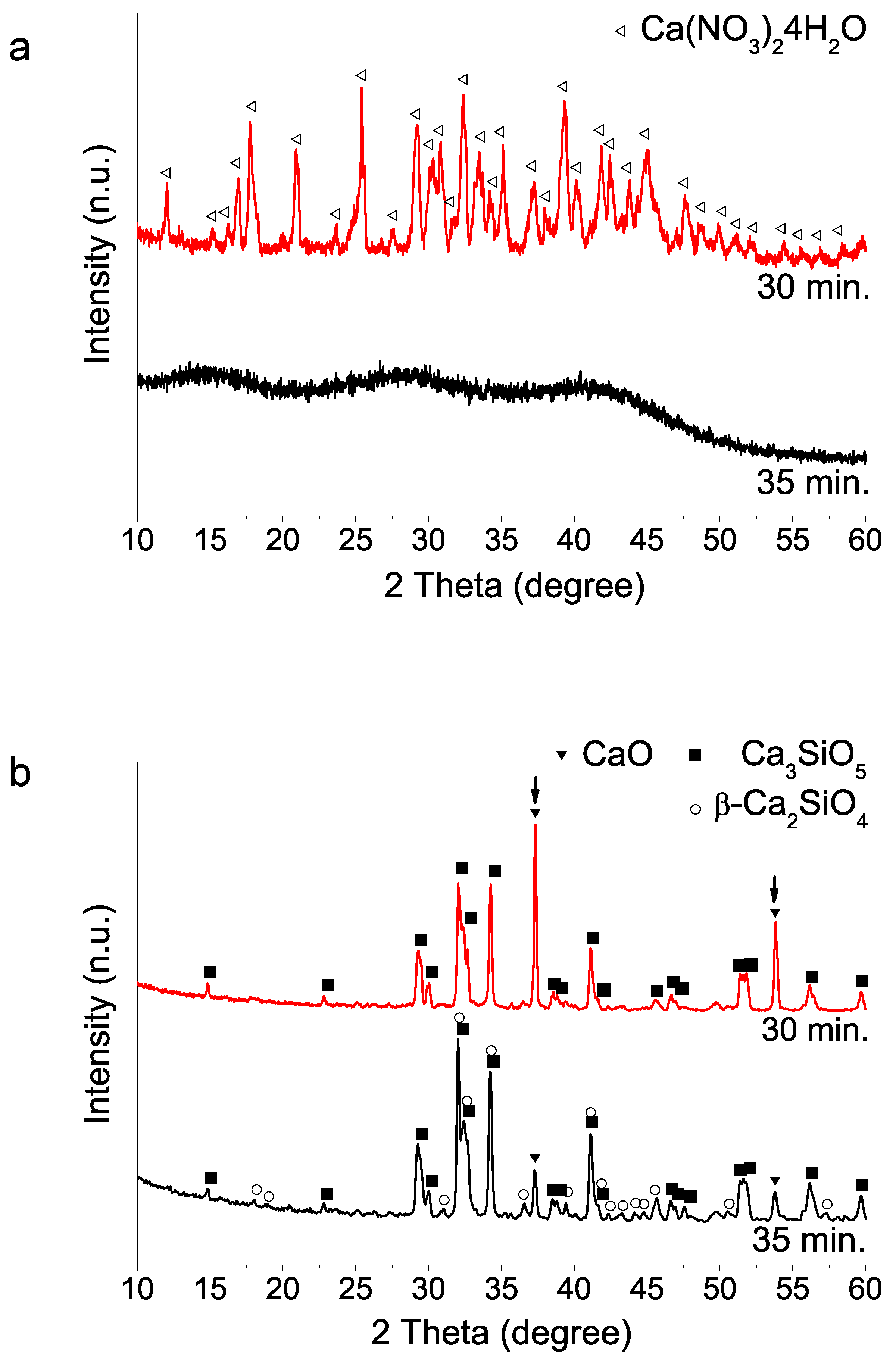
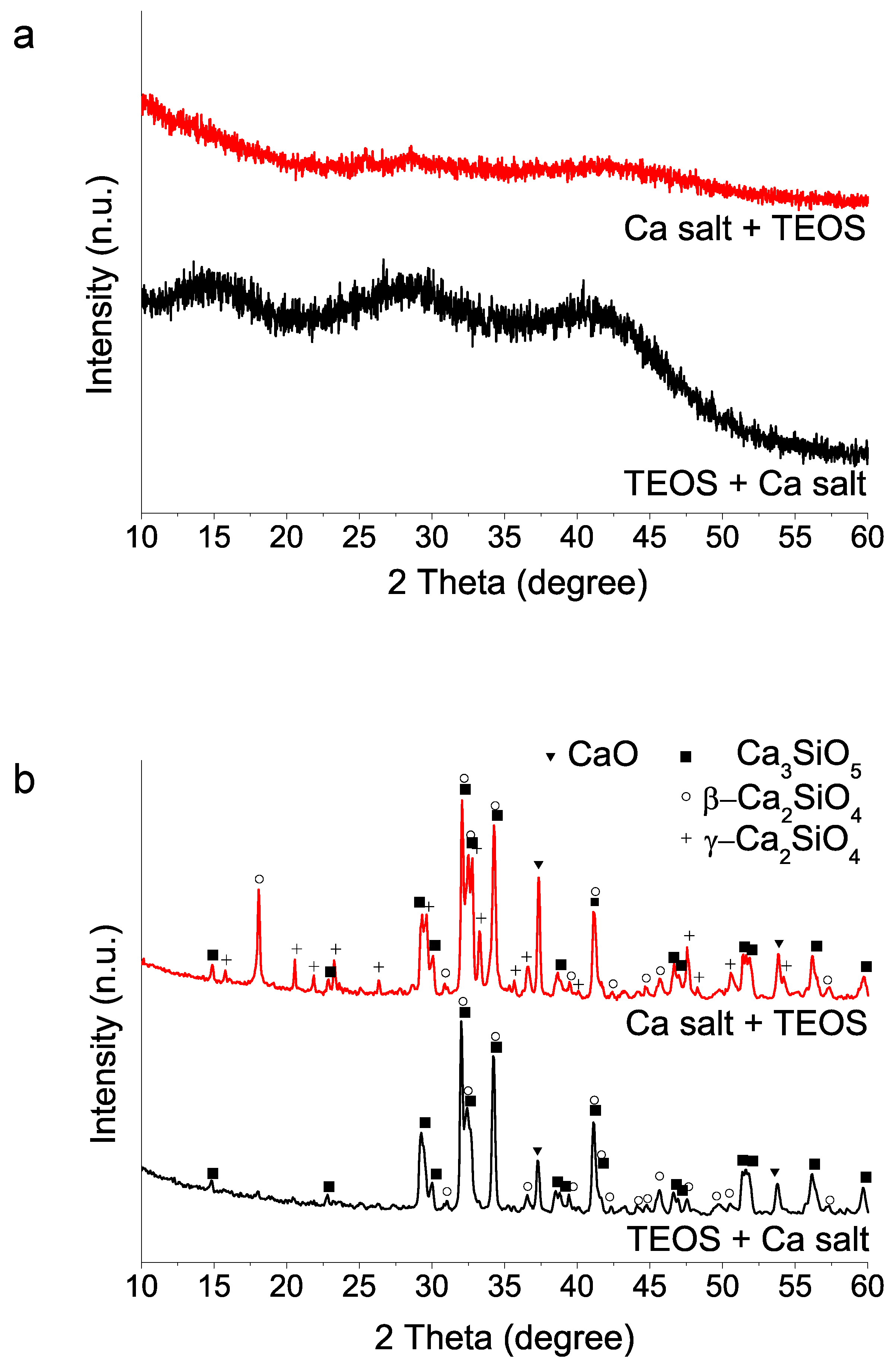
3.1. Sol–Gel Synthesis Variable Influence in the BEC Powder Product Standardization and Optimization
3.2. Effect on the BEC Properties of the Ethanol-Based Post-Synthesis Treatment
3.2.1. Influence of EPT on the Physicochemical Properties of Unhydrated Sol–Gel BEC Material
3.2.2. Influence of EPT on Hydration and the Setting Properties of the Sol–Gel Synthesized BEC Material
3.2.3. Influence of EPT on the Bioactivity of the Sol–Gel Synthesized BEC Material
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Result Lot Numbers | C4955 Sigma Aldrich MKBQ0375V | 42,353 Acros Organics A0413021 |
|---|---|---|
| Chloride (Cl) | ≤0.005% | ≤0.005% |
| Sulfate (SO4) | ≤0.002% | ≤0.002% |
| Magnesium (Mg) | ≤0.01% | 0.0068 |
| Potassium (K) | ≤0.001% | 0.0006% |
| Sodium (Na) | ≤0.001% | 0.0012% |
| Iron (Fe) | ≤0.0001% | ≤0.0005% |
| Aluminum (Al) | ≤0.001% | - |
| Phosphorous (P) | ≤0.0002% | - |
| Strontium (Sr) | - | 0.0017% |
| Barium (Ba) | - | ≤0.005% |
References
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. Higher hydration performance and bioactive response of the new endodontic bioactive cement MTA HP repair compared with ProRoot MTA white and NeoMTA Plus. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Primus, C.M.; Tay, F.R.; Niu, L.-N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomat. 2019, 96, 35–54. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic sealers based on calcium silicates: A systematic review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef]
- ISO 23317:2014; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. ISO: Geneva, Switzerland, 2014.
- Hosoya, N.; Takigawa, T.; Horie, T.; Maeda, H.; Yamamoto, Y.; Momoi, Y.; Yamamoto, K.; Okiji, T. A review of the literature on the efficacy of mineral trioxide aggregate in conservative dentistry. Dent. Mater. J. 2019, 38, 693–700. [Google Scholar] [CrossRef]
- Liu, W.; Chang, J.; Yue, Z. Physicochemical properties and biocompatibility of tricalcium and dicalcium silicate composite cements after hydration. Int. J. Appl. Ceram. Technol. 2011, 8, 560–565. [Google Scholar] [CrossRef]
- Ha, W.N.; Bentz, D.P.; Kahler, B.; Walsh, L.J. D90: The strongest contributor to setting time in mineral trioxide aggregate and Portland cement. J. Endod. 2015, 41, 1146–1150. [Google Scholar] [CrossRef]
- Stephan, D.; Maleki, H.; Knöfel, D.; Eber, B.; Härdtl, R. Influence of Cr, Ni, and Zn on the properties of pure clinker phases Part I. C3S. Cem. Concr. Res. 1999, 29, 545–552. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lin, H.-P.; Chan, J.C.-C.; Wang, W.-C.; Hung, P.-H.; Tsai, Y.-H.; Lee, Y.-L. A novel sol-gel-derived calcium silicate cement with short setting time for application in endodontic repair of perforations. Int. J. Nanomed. 2018, 13, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Ramiro-Gutiérrez, M.L.; Santos-Ruiz, L.; Borrego-González, S.; Becerra, J.; Díaz-Cuenca, A. In vitro stimulation of MC3T3-E1 sells and sustained drug delivery by a hierarchical nanostructured SiO2-CaO-P2O5 scaffold. Microporous Mesoporous Mater. 2016, 229, 31–43. [Google Scholar] [CrossRef]
- Romero-Sánchez, L.B.; Borrego-González, S.; Díaz-Cuenca, A. High surface area biopolymeric-ceramic scaffolds for hard tissue engineering. Biomed. Phys. Eng. Express 2017, 3, 035012. [Google Scholar] [CrossRef]
- Díaz, A.; López, T.; Manjarrez, J.; Basaldella, E.; Martínez-Blanes, J.M.; Odriozola, J.A. Growth of hydroxyapatite in a biocompatible mesoporous ordered silica. Acta Biomat. 2006, 2, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol-Gel materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Borrego-González, S.; Romero-Sánchez, L.B.; Blázquez, J.; Díaz-Cuenca, A. Nanostructured hybrid device mimicking bone extracellular matrix as local and sustained antibiotic delivery system. Microporous Mesoporous Mater. 2018, 256, 165–176. [Google Scholar] [CrossRef]
- Valdés-Sánchez, L.; Borrego-González, S.; Montero-Sánchez, A.; Massalini, S.; de la Cerda, B.; Díaz-Cuenca, A.; Díaz-Corrales, F.J. Mesoporous silica-based nanoparticles as non-viral gene delivery platform for treating retinitis pigmentosa. J. Clin. Med. 2022, 11, 2170. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. A microstructure insight of MTA Repair HP of rapid setting capacity and bioactive response. Materials 2020, 13, 1641. [Google Scholar] [CrossRef]
- Gou, Z.; Chang, J. Synthesis and in vitro bioactivity of dicalcium silicate powders. J. Eur. Ceram. Soc. 2004, 24, 93–99. [Google Scholar] [CrossRef]
- Zhao, W.; Chang, J. Sol-Gel synthesis and in vitro bioactivity of tricalcium silicate powders. Mater. Lett. 2004, 58, 2350–2353. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. Physicochemical parameters—Hydration performance relationship of the new endodontic cement MTA Repair HP. J. Clin. Exp. Dent. 2019, 11, e739–e744. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. MTA HP Repair stimulates in vitro an homogeneous calcium phosphate phase coating deposition. J. Clin. Exp. Dent. 2019, 11, e322–e326. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Ratner, B.D. Differentiation of calcium carbonate polymorphs by surface analysis techniques—An XPS and TOF-SIMS study. Surf. Interface Anal. 2008, 40, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, W.; Ye, J. FTIR study on the polymorphic structure of tricalcium silicate. Cem. Concr. Res. 2017, 99, 129–136. [Google Scholar] [CrossRef]
- Taddei, P.; Modena, E.; Tinti, A.; Siboni, F.; Prati, C.; Gandolfi, M.G. Vibrational investigation of calcium silicate cements for endodontics in simulated body fluids. J. Mol. Struct. 2011, 993, 367–375. [Google Scholar] [CrossRef]
- Chen, C.-C.; Ho, C.-C.; Chen, C.-H.D.; Ding, S.-J. Physicochemical properties of calcium silicate cements for endodontic treatment. J. Endod. 2009, 35, 1288–1291. [Google Scholar] [CrossRef]
- Durgun, E.; Manzano, H.; Pellenq, R.J.M.; Grossman, J.C. Understanding and controlling the reactivity of the calcium silicate phases from first principles. Chem. Mater. 2012, 24, 1262–1267. [Google Scholar] [CrossRef]
- Hong, S.-H.; Young, J.F. Hydration kinetics and phase stability od dicalcium silicate synthesized by the Pechini process. J. Am. Ceram. Soc. 1999, 82, 1681–1686. [Google Scholar] [CrossRef]
- Nettleship, I.; Slavick, K.G.; Kim, Y.J.; Kriven, W.M. Phase transformations in dicalcium silicate: I, fabrication and phase stability of fine-grained β phase. J. Am. Ceram. Soc. 1992, 75, 2400–2406. [Google Scholar] [CrossRef]
- Groves, G.W. Phase transformations in dicalcium silicate. J. Mater. Sci. 1983, 18, 1615–1624. [Google Scholar] [CrossRef]
- Smith, D.K.; Majumdar, A.J.; Ordway, F. Re-Examination of the polymorphism of dicalcium silicate. J. Am. Ceram. Soc. 1961, 44, 405–411. [Google Scholar] [CrossRef]
- Pritts, I.M.; Daugherty, K.E. The effect of stabilizing agents on the hydratation rate of β-C2S. Cem. Concr. Res. 1976, 6, 783–796. [Google Scholar] [CrossRef]
- Belío-Reyes, I.A.; Bucio, L.; Cruz-Chavez, E. Phase composition of ProRoot mineral trioxide aggregate by X-ray powder diffraction. J. Endod. 2009, 35, 875–878. [Google Scholar] [CrossRef]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Dunstetter, F.; De Noirfontaine, M.-N.; Courtial, M. Polymorphism of tricalcium silicate, the major compound of Portland cement clinker: 1. Structural data: Review and unified analysis. Cem. Concr. Res. 2006, 36, 39–53. [Google Scholar] [CrossRef]
- Pustovgar, E.; Sangodkar, R.P.; Andreev, A.S.; Palacios, M.; Chmelka, B.F.; Flatt, R.J.; D’Espinose De Lacaillerie, J.-B. Understanding silicate hydration from quatitative analyses of hydrating tricalcium silicates. Nat. Commun. 2016, 7, 10952. [Google Scholar] [CrossRef]
- Domingos Pires, M.; Cordeiro, J.; Vasconcelos, I.; Alves, M.; Quaresma, S.A.; Ginjeira, A.; Camilleri, J. Effect of different manipulations on the physical, chemical and microstructural characteristics of Biodentine. Dent. Mater. 2021, 37, e399–e406. [Google Scholar] [CrossRef]
- Zajac, M.; Lechevallier, A.; Durdzinski, P.; Bullerjahn, F.; Skibsted, J.; Ben Haha, M. CO2 mineralisation of Portland cement: Towards understanding the mechanisms of enforced carbonation. J. CO2 Util. 2020, 38, 398–415. [Google Scholar] [CrossRef]
- Parvan, M.-G.; Voicu, G.; Badanoiu, A.-I.; Nicoara, A.-I.; Vasile, E. CO2 sequestration in the production of Portland cement mortars with calcium carbonate additions. Nanomaterials 2021, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, P.; Gao, X.; Wang, T.; Qin, L. New sights in early carbonation of calcium silicates: Performance, mechanism and nanostructure. Constr. Build. Mater. 2022, 314, 125622. [Google Scholar] [CrossRef]
- MicroMega. Available online: https://micro-mega.com (accessed on 10 March 2022).
- Liu, X.; Lin, W.; Chen, B.; Zhang, F.; Zhao, P.; Parsons, A.; Rau, C.; Robinson, I. Coherent diffraction study of calcite crystallisation during the hydration of tricalcium silicate. Mater. Des. 2018, 157, 251–257. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Primathena, I.; Nurdin, D.; Hermawan, H.; Cahyanto, A. Synthesis, characterization, and antibacterial evaluation of a cost-effective endodontic sealer based on tricalcium silicate-white Portland cement. Materials 2021, 14, 417. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- Muramatsu, T.; Kashiwagi, S.; Ishizuka, H.; Matsuura, Y.; Furusawa, M.; Kimura, M.; Shibukawa, Y. Alkaline extracellular conditions promote the proliferation and mineralization of a human cementoblast cell line. Int. Endod. J. 2019, 52, 639–645. [Google Scholar] [CrossRef]
- Do Nascimento, C.; Issa, J.P.M.; Iyomasa, M.M.; Regalo, S.C.H.; Siéssere, S.; Pitol, D.L.; Wolga, N.d.O.; Pedrazzi, V. Bone repair using mineral trioxide aggregate combined to a material carrier, associated or not with calcium hydroxide in bone defects. Micron 2008, 39, 868–874. [Google Scholar] [CrossRef]
- Seux, D.; Couble, M.L.; Hartmann, D.J.; Gauthier, J.P.; Maglore, H. Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Archs. Oral Biol. 1991, 36, 117–128. [Google Scholar] [CrossRef]
- Faraco, I.M., Jr.; Holland, R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent. Traumatol. 2001, 17, 163–166. [Google Scholar] [CrossRef]
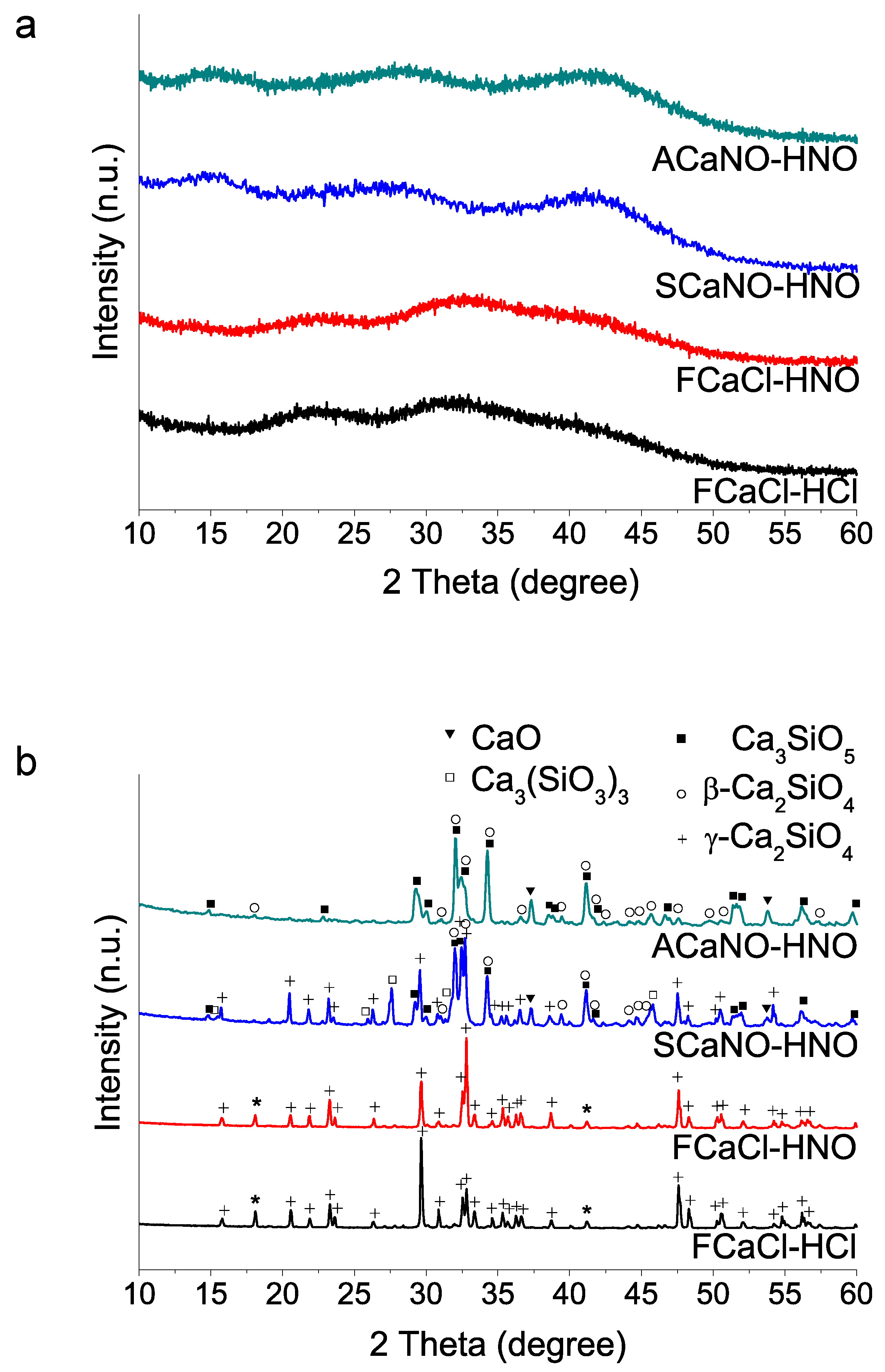




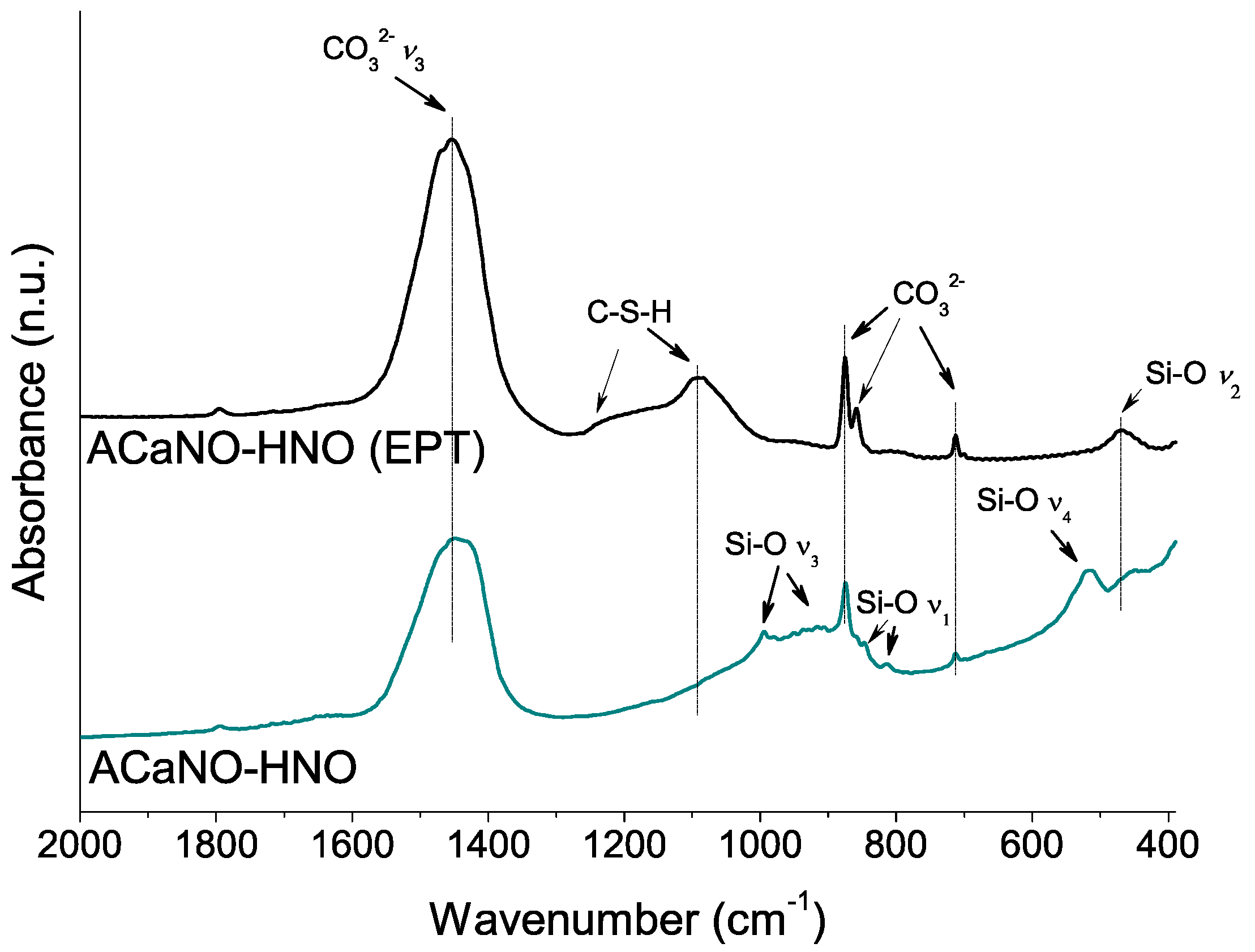
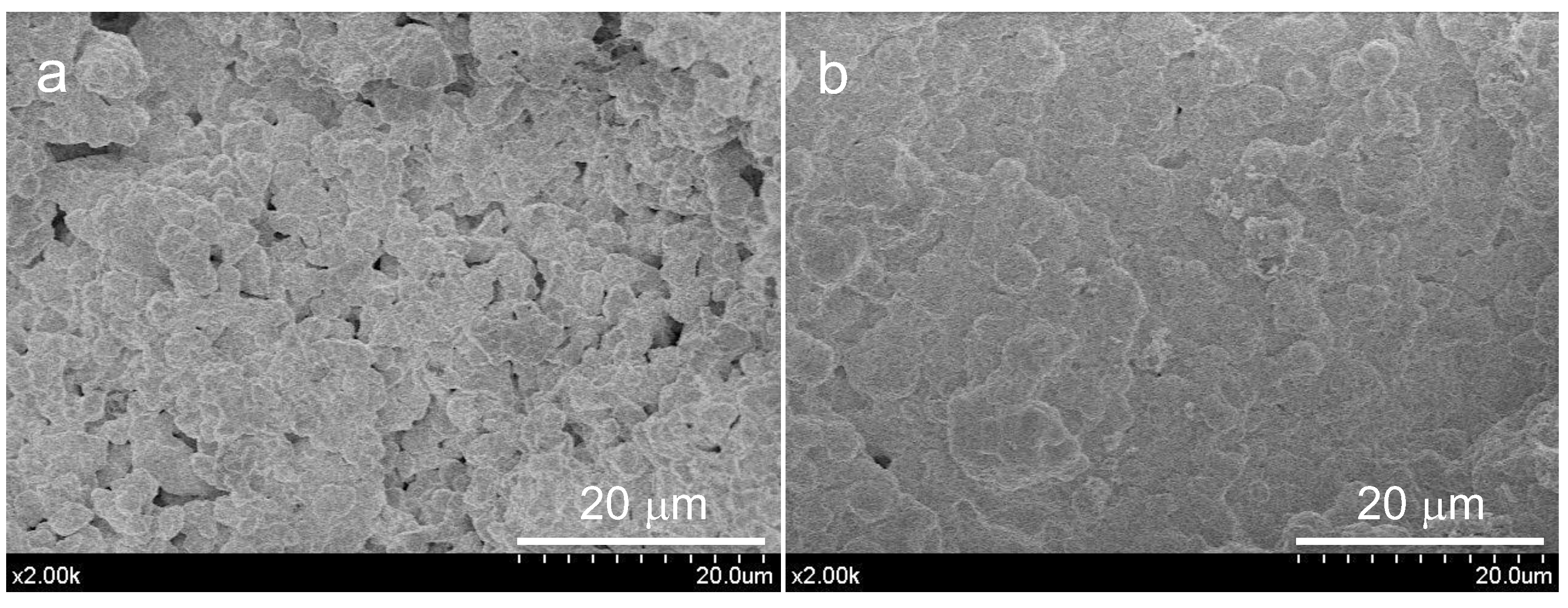

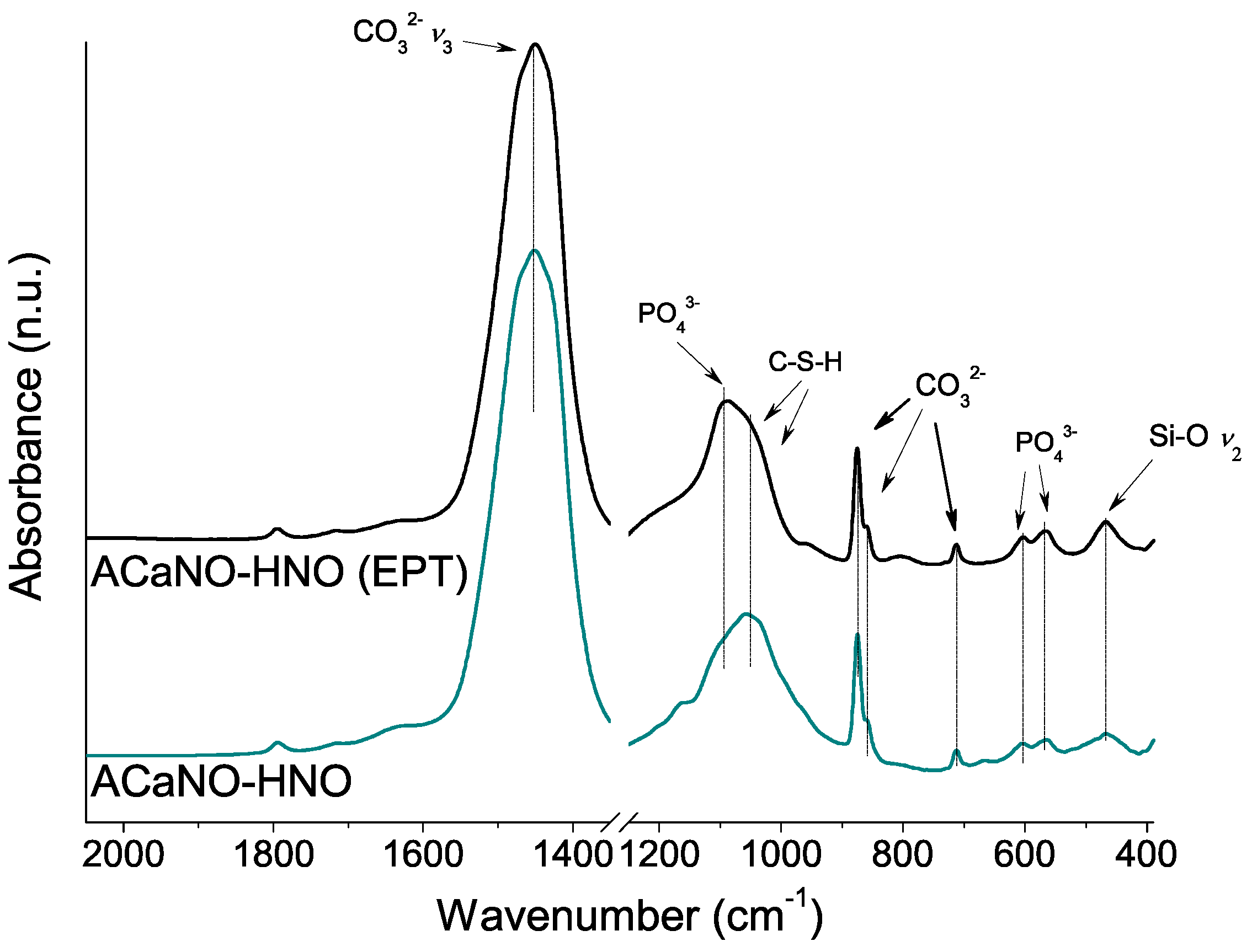

| Sample Name | Ca Salt Reactive | Acid-Type Reactive |
|---|---|---|
| ACaNO-HNO | Ca(NO3)2·4H2O Acros Organics | HNO3 Carlo Erba |
| SCaNO-HNO | Ca(NO3)2·4H2O Sigma Aldrich | HNO3 Carlo Erba |
| FCaCl-HNO | CaCl2·2H2O Alfa Aesar | HNO3 Carlo Erba |
| FCaCl-HCl | CaCl2·2H2O Alfa Aesar | HCl Fluka |
| Sample | SBET (m2 g−1) | Vt (cm3 g−1) |
|---|---|---|
| ACaNO-HNO | 2.6 ± 0.3 | 0.006 ± 0.000 |
| ACaNO-HNO (EPT) | 6.9 ± 0.2 | 0.021 ± 0.001 |
| Sample | Initial Setting (min) | Final Setting (min) |
|---|---|---|
| ACaNO-HNO | 26 ± 1 | 95 ± 7 |
| ACaNO-HNO (EPT) | 6 ± 0 | 53 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Díaz-Cuenca, A. Sol–Gel Synthesis of Endodontic Cements: Post-Synthesis Treatment to Improve Setting Performance and Bioactivity. Materials 2022, 15, 6051. https://doi.org/10.3390/ma15176051
Song X, Díaz-Cuenca A. Sol–Gel Synthesis of Endodontic Cements: Post-Synthesis Treatment to Improve Setting Performance and Bioactivity. Materials. 2022; 15(17):6051. https://doi.org/10.3390/ma15176051
Chicago/Turabian StyleSong, Xiaozhe, and Aránzazu Díaz-Cuenca. 2022. "Sol–Gel Synthesis of Endodontic Cements: Post-Synthesis Treatment to Improve Setting Performance and Bioactivity" Materials 15, no. 17: 6051. https://doi.org/10.3390/ma15176051





