Effect of Atmospheric Cold Plasma Treatment on the Adhesion and Tribological Properties of Polyamide 66 and Poly(Tetrafluoroethylene)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
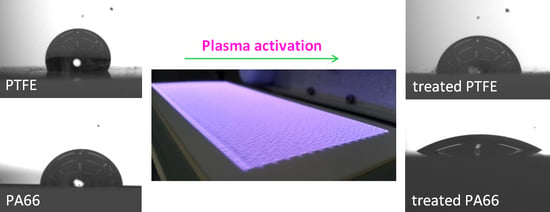
2.2. Plasma Treatment
2.3. Adhesive Testing
2.4. Surface Characterization
3. Results and Discussion
3.1. Contact Angle Measurements
3.2. Surface Analysis
3.3. Surface Topography
3.4. Adhesive Tests
3.5. Tribology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baldan, A. Adhesively-bonded joints in metallic alloys, polymers and composite materials: Mechanical and environmental durability performance. J. Mater. Sci. 2004, 39, 4729–4797. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Mandolfino, C.; Lertora, E.; Gambaro, C.; Bruno, M. Improving adhesion performance of polyethylene surfaces by cold plasma treatment. Meccanica 2014, 49, 2299–2306. [Google Scholar] [CrossRef]
- Horakova, M.; Spatenka, P.; Hladik, J.; Hornik, J.; Steidl, J.; Polachova, A. Investigation of adhesion between metal and plasma-modified polyethylene. Plasma Process. Polym. 2011, 8, 983–988. [Google Scholar] [CrossRef]
- Abdul Samad, M.; Satyanarayana, N.; Sinha, S.K. Tribology of UHMWPE film on air-plasma treated tool steel and the effect of PFPE overcoat. Surf. Coat. Technol. 2010, 204, 1330–1338. [Google Scholar] [CrossRef]
- Van Vrekhem, S.; Cools, P.; Declercq, H.; Van Tongel, A.; Vercruysse, C.; Cornelissen, M.; De Geyter, N.; Morent, R. Application of atmospheric pressure plasma on polyethylene for increased prosthesis adhesion. Thin Solid Films 2015, 596, 256–263. [Google Scholar] [CrossRef]
- Blackman, B.R.K.; Guild, F.J. Forced air plasma treatment for enhanced adhesion of polypropylene and polyethylene. J. Adhes. Sci. Technol. 2013, 27, 2714–2726. [Google Scholar] [CrossRef]
- Favia, P.; Oehr, C.; Wertheimer, M.R. Plasma Processes and Polymers; d’Agostino, R., Pietro Favia, C.O., Wertheimer, M.R., Eds.; Wiley-VCH: Weinheim, Germany, 2005; Volume 14, ISBN 3527404872. [Google Scholar]
- Kusano, Y. Atmospheric Pressure Plasma Processing for Polymer Adhesion: A Review. J. Adhes. 2014, 90, 755–777. [Google Scholar] [CrossRef]
- Thomas, M.; Mittal, K.L. Atmospheric Pressure Plasma Treatment of Polymers: Relevance to Adhesion; Michael Thomas, K.L.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 9781118596210. [Google Scholar]
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; West, A.; Bredin, J.; Wagenaars, E. Mechanisms behind surface modification of polypropylene film using an atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 2016, 25, 065018. [Google Scholar] [CrossRef] [Green Version]
- Bormashenko, E.; Whyman, G.; Multanen, V.; Shulzinger, E.; Chaniel, G. Physical Mechanisms of Interaction of Cold Plasma with Polymer Surfaces. J. Colloid Interface Sci. 2015, 448, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lambare, C.; Tessier, P.-Y.; Poncin-Epaillard, F.; Debarnot, D. Plasma functionalization and etching for enhancing metal adhesion onto polymeric substrates. RSC Adv. 2015, 5, 62348–62357. [Google Scholar] [CrossRef]
- Saranko, A.; Szakal, Z.; Kalacska, G.; Samyn, P.; Sukumaran, J.; Klebert, S.; Karoly, Z. Adhesion and sliding tribological properties of polyolefins treated by diffuse coplanar surface barrier discharges. Express Polym. Lett. 2018, 12, 972–985. [Google Scholar] [CrossRef]
- Šourková, H.; Primc, G.; Špatenka, P.; Šourková, H.; Primc, G.; Špatenka, P. Surface Functionalization of Polyethylene Granules by Treatment with Low-Pressure Air Plasma. Materials (Basel) 2018, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Bismarck, A.; Brostow, W.; Chiu, R.; Hagg Lobland, H.E.; Ho, K.K.C. Effects of surface plasma treatment on tribology of thermoplastic polymers. Polym. Eng. Sci. 2008, 48, 1971–1976. [Google Scholar] [CrossRef]
- Mandolfino, C.; Lertora, E.; Gambaro, C. Influence of cold plasma treatment parameters on the mechanical properties of polyamide homogeneous bonded joints. Surf. Coat. Technol. 2017, 313, 222–229. [Google Scholar] [CrossRef]
- Tóth, A.; Kereszturi, K.; Mohai, M.; Bertóti, I. Plasma based ion implantation of engineering polymers. Surf. Coat. Technol. 2010, 204, 2898–2908. [Google Scholar] [CrossRef]
- López-García, J.; Cupessala, F.; Humpolíček, P.; Lehocký, M.; López-García, J.; Cupessala, F.; Humpolíček, P.; Lehocký, M. Physical and Morphological Changes of Poly(tetrafluoroethylene) after Using Non-Thermal Plasma-Treatments. Materials (Basel) 2018, 11, 2013. [Google Scholar] [CrossRef]
- Novák, I.; Popelka, A.; Valentín, M.; Chodák, I.; Špírková, M.; Tóth, A.; Kleinová, A.; Sedliačik, J.; Lehocký, M.; Marônek, M. Surface Behavior of Polyamide 6 Modified by Barrier Plasma in Oxygen and Nitrogen. Int. J. Polym. Anal. Charact. 2014, 19, 31–38. [Google Scholar] [CrossRef]
- Šimor, M.; Ráhel’, J.; Vojtek, P.; Černák, M.; Brablec, A. Atmospheric-pressure diffuse coplanar surface discharge for surface treatments. Appl. Phys. Lett. 2002, 81, 2716–2718. [Google Scholar] [CrossRef]
- Černák, M.; Černáková, L.; Hudec, I.; Kováčik, D.; Zahoranová, A. Diffuse Coplanar Surface Barrier Discharge and its applications for in-line processing of low-added-value materials. Eur. Phys. J. Appl. Phys. 2009, 47, 22806. [Google Scholar] [CrossRef] [Green Version]
- Károly, Z.; Kalácska, G.; Zsidai, L.; Mohai, M.; Klébert, S. Improvement of Adhesion Properties of Polyamide 6 and Polyoxymethylene-Copolymer by Atmospheric Cold Plasma Treatment. Polymers (Basel) 2018, 10, 1380. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Mohai, M. XPS MultiQuant: Multimodel XPS quantification software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Evans, S.; Pritchard, R.G.; Thomas, J.M. Relative differential subshell photoionisation cross-sections (MgKα) from lithium to uranium. J. Electron Spectrosc. Relat. Phenom. 1978, 14, 341–358. [Google Scholar] [CrossRef]
- Reilman, R.F.; Msezane, A.; Manson, S.T. Relative intensities in photoelectron spectroscopy of atoms and molecules. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 389–394. [Google Scholar] [CrossRef]
- Dixon, D.; Meenan, B.J. Atmospheric Dielectric Barrier Discharge Treatments of Polyethylene, Polypropylene, Polystyrene and Poly(ethylene terephthalate) for Enhanced Adhesion. J. Adhes. Sci. Technol. 2012, 26, 2325–2337. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.A.; Brown, N.M.D. The surface oxidation of selected polymers using an atmospheric pressure air dielectric barrier discharge. Part I. Appl. Surf. Sci. 2004, 221, 203–214. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. XPS Database of Polymers in High Resolution, 2nd ed.; [CD-ROM]; SurfaceSpectra: Manchester, UK, 2000. [Google Scholar]
- Yeh, J.T.; Lai, Y.C.; Suen, M.C.; Chen, C.C. An improvement on the adhesion-strength of laminated ultra-high-molecular-weight polyethylene fabrics: Surface-etching/modification using highly effective helium/oxygen/nitrogen plasma treatment. Polym. Adv. Technol. 2011, 22, 1971–1981. [Google Scholar] [CrossRef]
- Inagaki, N. Selective Surface Modification of Polymeric Materials by Atmospheric-Pressure Plasmas: Selective Substitution Reactions on Polymer Surfaces by Different Plasmas. In Atmospheric Pressure Plasma Treatment of Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 83–130. [Google Scholar]







| Property | PA66 | PTFE |
|---|---|---|
| Density (g·cm−3) | 1.15 | 2.16 |
| Tensile strength (MPa) | 85 | 22 |
| Elasticity modulus MPa) | 1850 | 400–550 |
| Shore D hardness | 78–88 | 55 |
| Thermal conductivity (W·m−1·K−1) | 0.25–0.35 | 0.25–0.30 |
| Melting temperature (°C) | 260 | - |
| Sample | θw (°) | θCH2l2 (°) | γpol (mJ/m2) | γdisp (mJ/m2) | γtot (mJ/m2) |
|---|---|---|---|---|---|
| PA6.6 pristine | 63 ± 2.3 | 42 ± 2.5 | 11.2 | 38.7 | 50.0 ± 2.4 |
| PA6.6 treated, 30 s | 33 ± 2.6 | 27 ± 2.7 | 24.0 | 45.5 | 69.5 ± 4.9 |
| PA6.6 treated, 60 s | 29 ± 3.6 | 27 ± 1.2 | 25.7 | 45.7 | 71.4 ± 4.8 |
| PA6.6 treated, 180 s | 30 ± 2.6 | 24 ± 2.5 | 25.1 | 46.7 | 71.8 ± 5.4 |
| PTFE pristine | 108 ± 1.5 | 73 ± 3.2 | 0.2 | 21.2 | 21.5 ± 0.5 |
| PTFE treated, 30 s | 101 ± 3.4 | 73 ± 4.6 | 1.2 | 21.1 | 22.4 ± 0.7 |
| PTFE treated, 60 s | 105 ± 2.5 | 62 ± 3.9 | 0.1 | 27.6 | 27.7 ± 0.7 |
| PTFE treated, 180 s | 75 ± 1.0 | 56 ± 1.0 | 7.5 | 30.9 | 38.4 ± 0.2 |
| PTFE treated, 300 s | 68 ± 3.7 | 61 ± 1.6 | 12.4 | 28.2 | 40.6 ± 0.5 |
| PTFE treated, 600 s | 65 ± 1.5 | 60 ± 2.4 | 13.8 | 28.5 | 42.3 ± 0.4 |
| Chemical Composition, % | Atomic Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Sample | C | O | N | F | O/C | N/C | F/C |
| PA66 pristine | 76.1 | 12.0 | 11.9 | - | 0.16 | 0.15 | - |
| PA66 treated | 58.5 | 27.8 | 13.7 | - | 0.47 | 0.23 | - |
| PTFE pristine | 33.5 | - | - | 66.5 | 0 | 0 | 1.98 |
| PTFE treated | 49.5 | 3.4 | 2.1 | 45.0 | 0.07 | 0.04 | 0.91 |
| C Components | Composition (Atomic %) | Binding Energy (eV) | Chemical States | |
|---|---|---|---|---|
| Pristine | Plasma Treated | |||
| PA66 | ||||
| C1 | 38.0 | 23.8 | 285.0 ± 0.1 | C–C, C–H |
| C2 | 12.7 | 7.9 | 285.3 ± 0.1 | CH–C=O |
| C3 | 12.7 | 7.9 | 286.0 ± 0.1 | *C–N–C=O |
| C4 | 12.7 | 7.9 | 288.0 ± 0.1 | C–N–*C=O |
| C5 | 7.7 | 289.0 ± 0.2 | O=*C–O(H) | |
| C6 | 3.3 | 287.0 ± 0.2 | *C–O–C=O, epoxy | |
| PTFE | ||||
| C1 | 0 | 21.0 | 286.4 ± 0.1 | –*CH2–CF2 C–O–C, C–OH, C–N |
| C2 | 0 | 6.1 | 287.9 ± 0.1 | –CH2–*CHF– C=O, O–C–O, N–C–O |
| C3 | 0 | 3.1 | 289.8 ± 0.1 | *CF(CF3) –CF2– –CF2–CH2– |
| CF | 33.5 | 19.3 | 292.5 ± 0.1 | –CF2–CF2– |
| Test Materials | PTFE | PA66 | ||||||
|---|---|---|---|---|---|---|---|---|
| 3D Parameters | Sa | Sz | Sku | Ssk | Sa | Sz | Sku | Ssk |
| Untreated | 0.56 | 5.4 | 3.99 | −0.78 | 0.91 | 14.7 | 10.9 | 1.89 |
| Treated 24 h | 0.5 | 4.29 | 3.56 | −0.6 | 0.44 | 7.06 | 6.27 | 0.03 |
| Treated 800 h | 0.76 | 9.12 | 4.47 | −0.7 | 0.36 | 4.84 | 4.12 | −0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Károly, Z.; Kalácska, G.; Sukumaran, J.; Fauconnier, D.; Kalácska, Á.; Mohai, M.; Klébert, S. Effect of Atmospheric Cold Plasma Treatment on the Adhesion and Tribological Properties of Polyamide 66 and Poly(Tetrafluoroethylene). Materials 2019, 12, 658. https://doi.org/10.3390/ma12040658
Károly Z, Kalácska G, Sukumaran J, Fauconnier D, Kalácska Á, Mohai M, Klébert S. Effect of Atmospheric Cold Plasma Treatment on the Adhesion and Tribological Properties of Polyamide 66 and Poly(Tetrafluoroethylene). Materials. 2019; 12(4):658. https://doi.org/10.3390/ma12040658
Chicago/Turabian StyleKároly, Zoltán, Gábor Kalácska, Jacob Sukumaran, Dieter Fauconnier, Ádám Kalácska, Miklós Mohai, and Szilvia Klébert. 2019. "Effect of Atmospheric Cold Plasma Treatment on the Adhesion and Tribological Properties of Polyamide 66 and Poly(Tetrafluoroethylene)" Materials 12, no. 4: 658. https://doi.org/10.3390/ma12040658