Physicochemical Study on the Strength Development Characteristics of Cold Weather Concrete Using a Nitrite–Nitrate Based Accelerator
Abstract
:1. Introduction
2. Experimental Overview
2.1. Materials and Methods
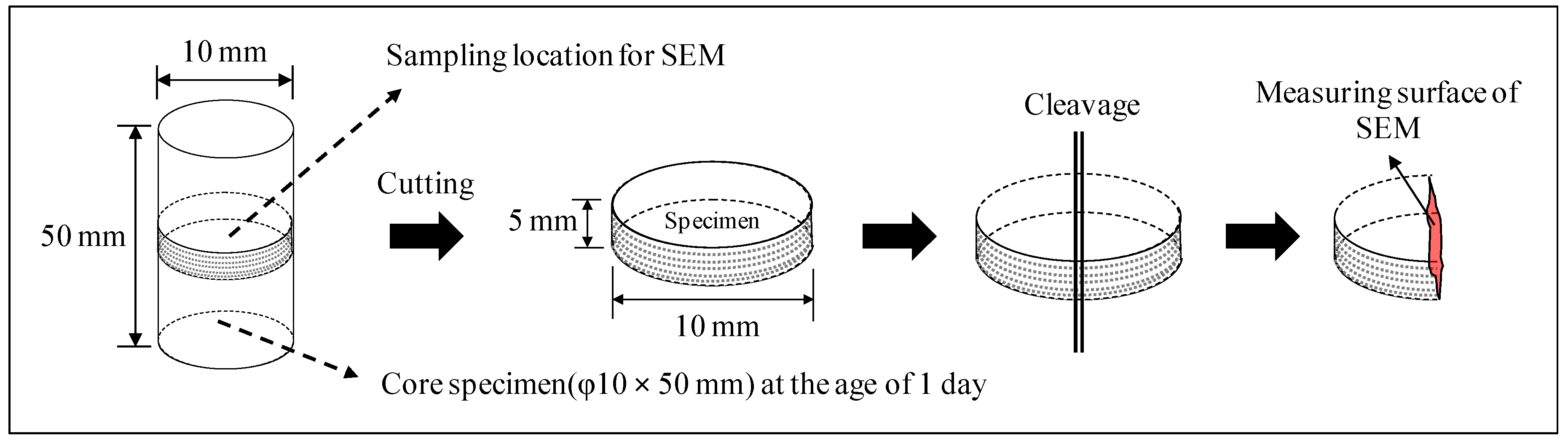
2.2. Experimental Method
3. Results and Discussion
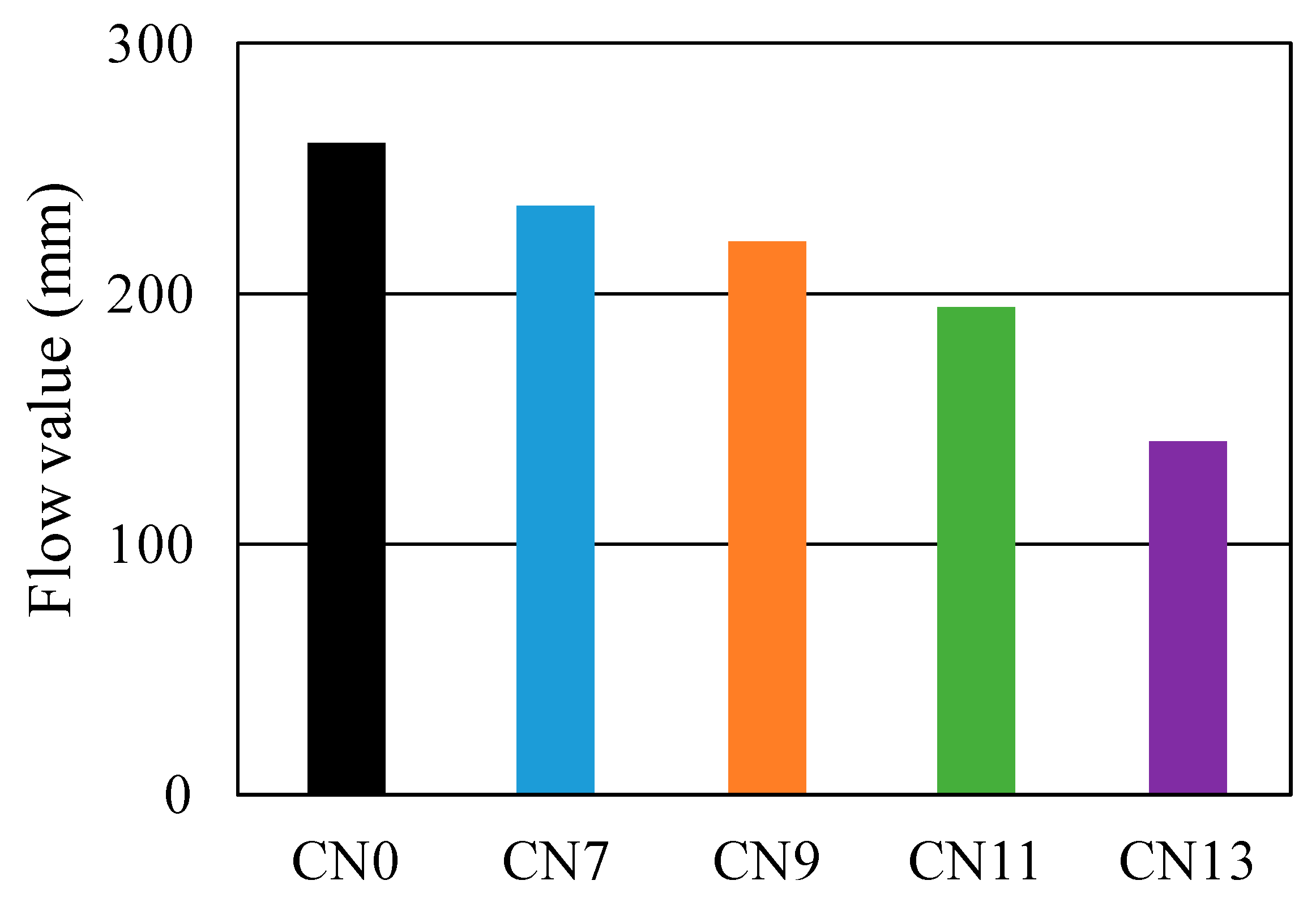
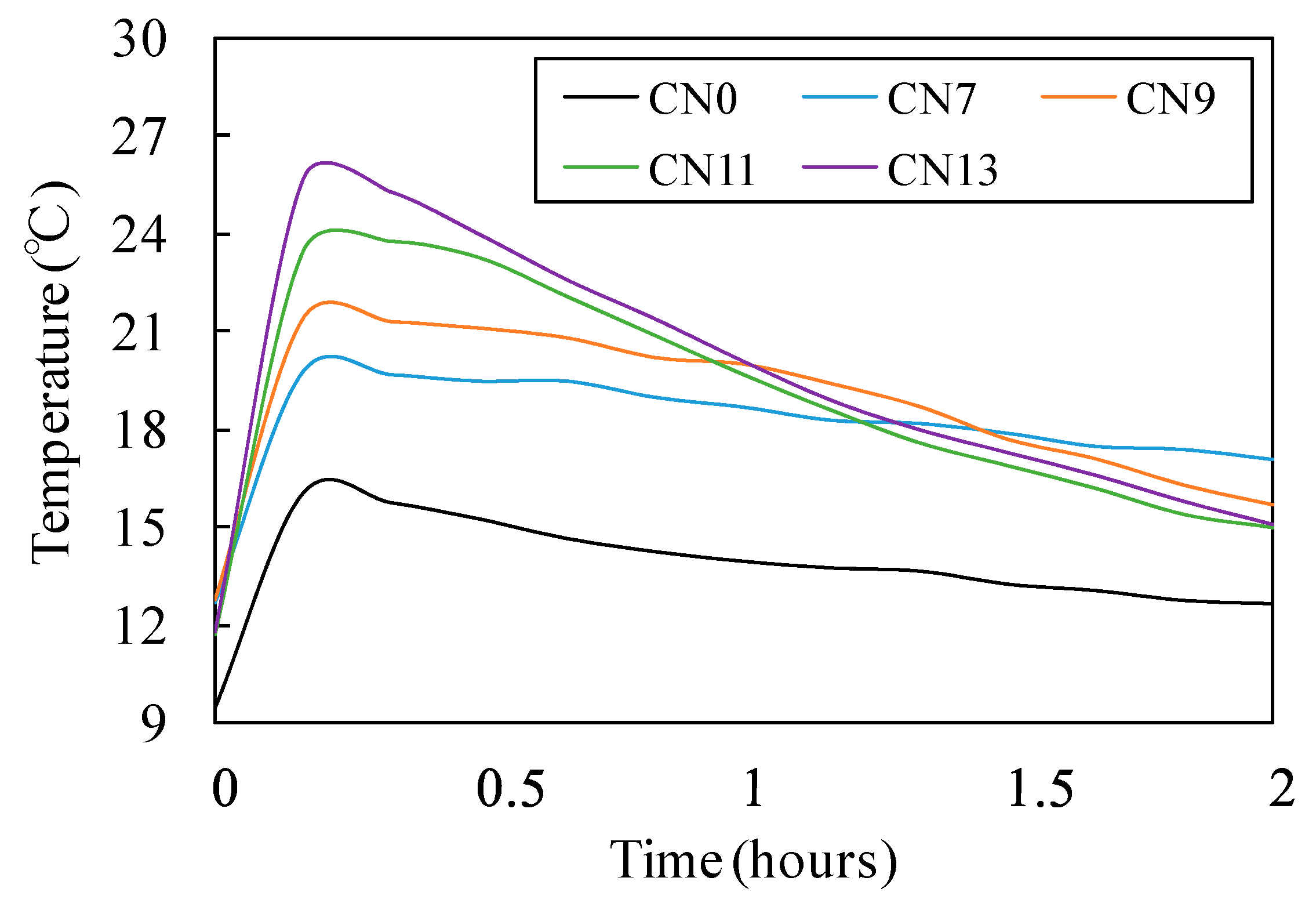
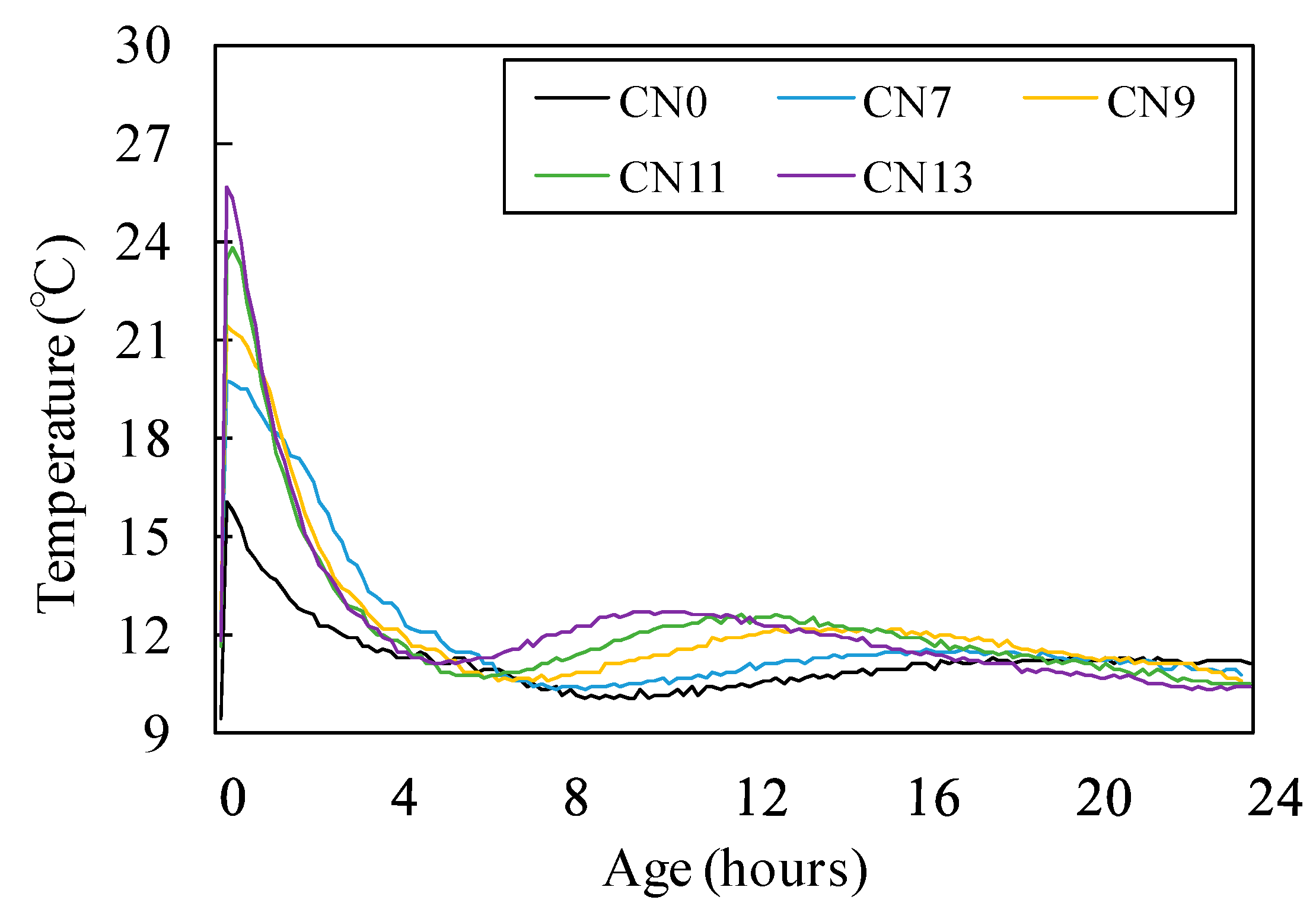
3.1. Fresh State Characteristics
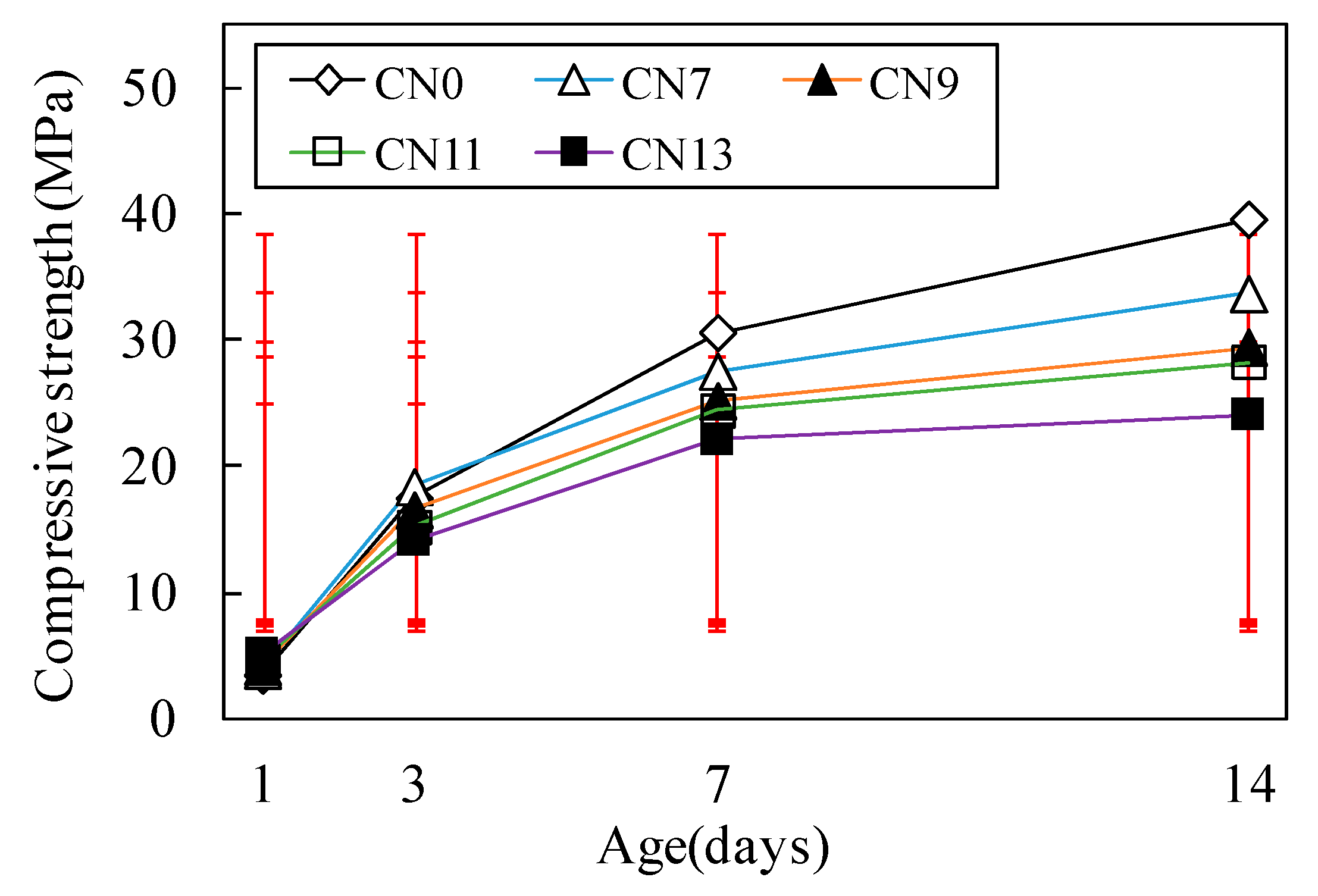
3.2. Compressive Strength Characteristics
3.3. Correlation Between Hydration Products and Strength of the Ca(No2)2·Ca(No3)2 Concrete by Micro-Analysis
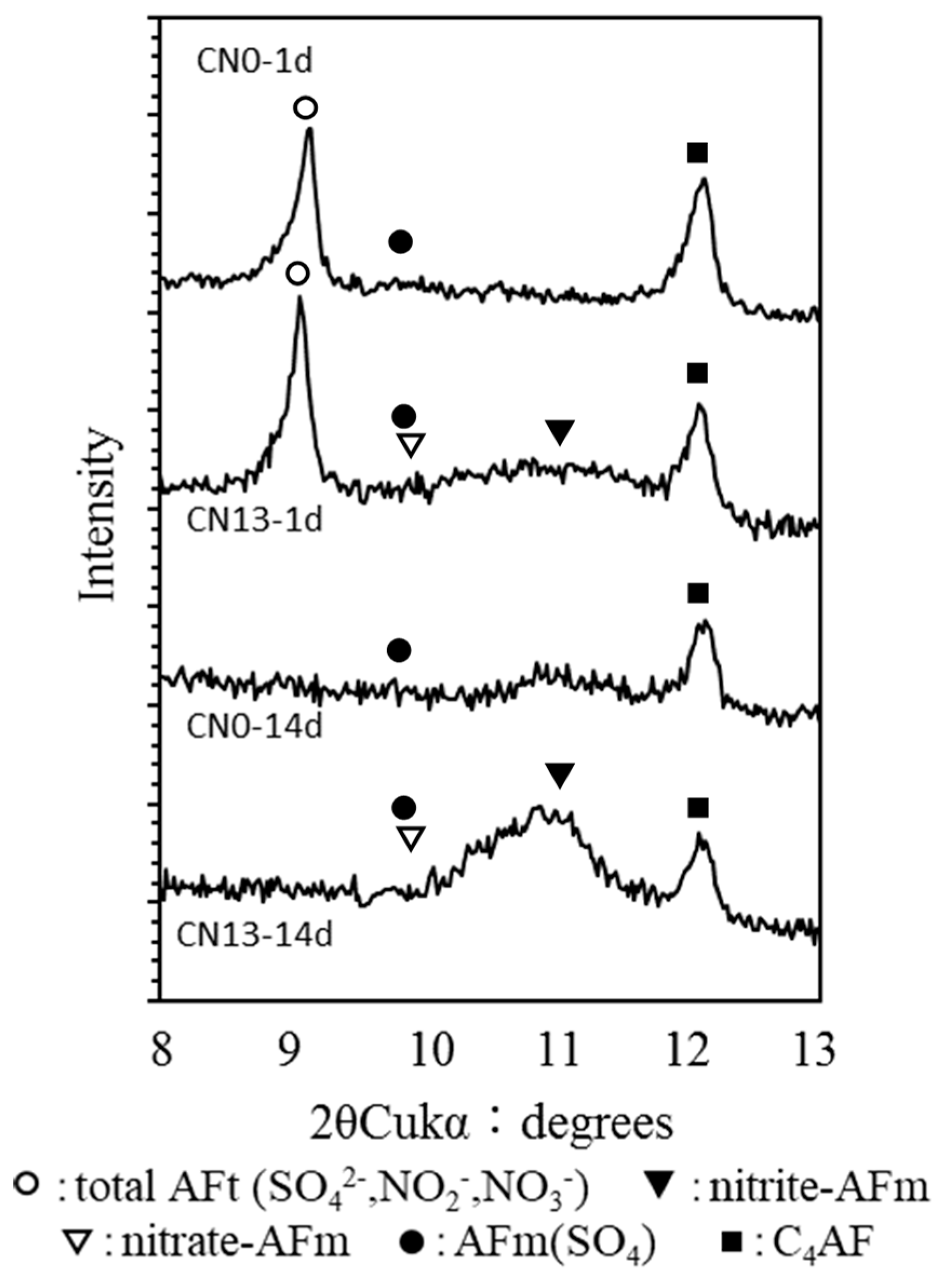
3.3.1. X-Ray Diffraction (XRD)
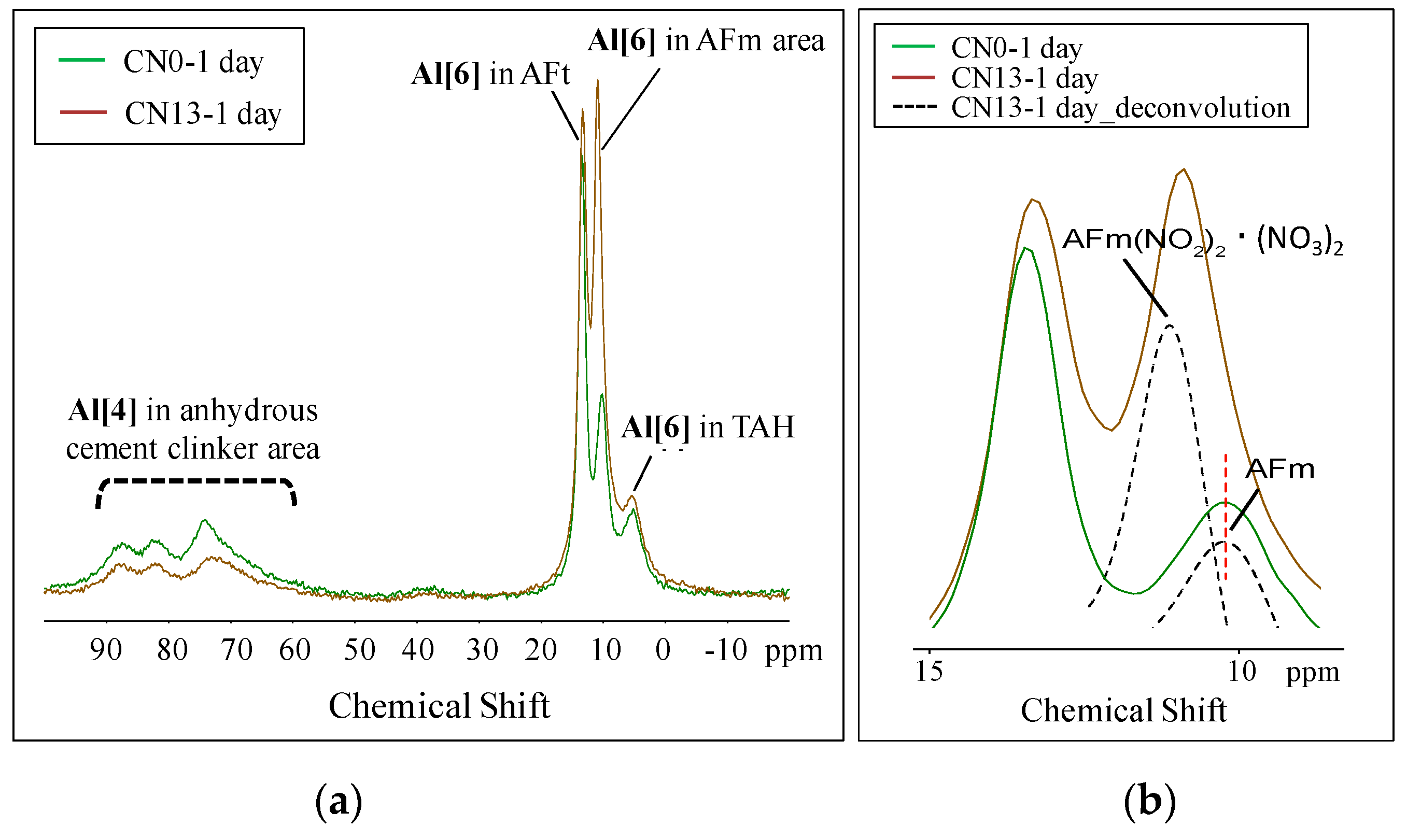
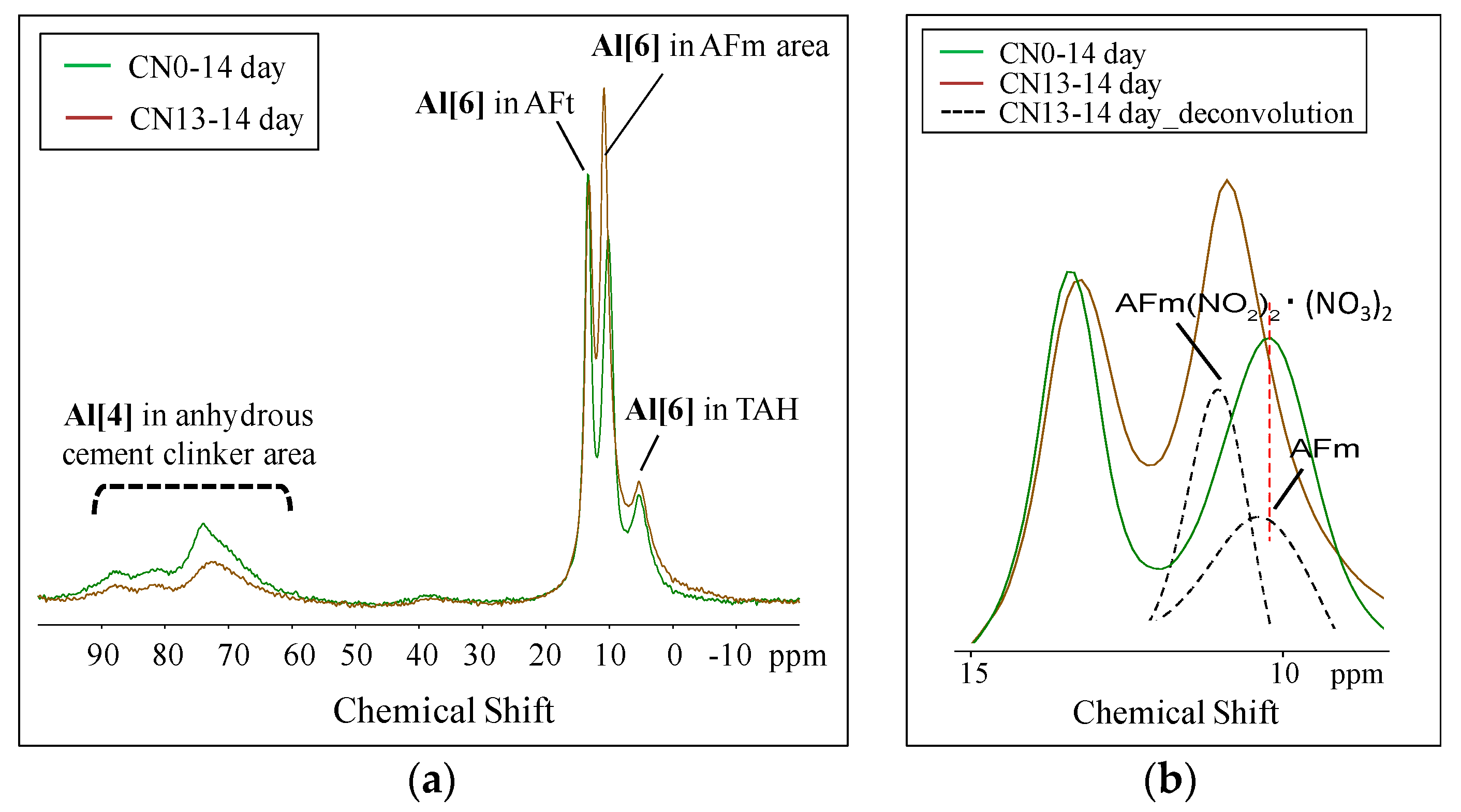
3.3.2. Nuclear Magnetic Resonance (NMR)
3.3.3. Scanning Electron Microscope (SEM)
4. Conclusions
- (1)
- With regards to fluidity, an increase in CN raised the rate of reaction between the nitrite (NO2−) and nitrate ions (NO3−), which are a part of the CN, and Al2O3 of C3A in the cement, which in turn caused the heat of hydration to increase. This consequently raised the internal temperature of the mortar and reduced its fluidity;
- (2)
- Concerning strength development over time, an increase in CN raised the rate and amount at which nitrite-AFt and nitrate-AFt were formed, and this in turn increased the amount of nitrite-AFm and nitrate-AFm. This increased strength at the early age; however, due to the production of large amounts of hydrates with vulnerable crystal structures, we conclude that the strength would be reduced at the later ages;
- (3)
- The XRD and NMR results showed that the cases where CN was added resulted in a larger peak height and area of ettringite, that is an Al hydrate, than the case without CN, due to the influence of nitrite (NO2−) and nitrate ions (NO3−) at the early age. Nitrite-AFm and nitrate-AFm peaks were also observed. Further, at the late ages, the needle-type nitrite-AFt and nitrate-Aft, which were produced in large amounts at the early age, reacted with Ca(OH)2 in the cement over time, resulting in the transformation of their crystal structure into those of nitrite-AFm and nitrate-AFm;
- (4)
- The NMR results showed that an increase in Al hydrates such as AFm, nitrite-AFm, nitrate-AFm, and TAH, resulting from the addition of the CN, caused a relative decrease in the production of other hydrates such as C-S-H gel and Ca(OH)2, thereby lowering strength at mid and late ages.
- (5)
- The SEM examination results showed that adding CN to concrete as an accelerator resulted in the production of a large amount of needle-type nitrite and nitrate hydrates. This is due to the reactions between the nitrite (NO2−) and nitrate ions (NO3−) from the CN and C3A in the cement, in addition to the production of sulfate (SO42−) ettringite from the reaction of normal Portland cement. We also observed the presence of nitrite-AFm and nitrate-AFm produced from the reactions between nitrite-AFt and nitrate-AFt and Ca(OH)2 in the cement.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Akama, T.; Inoue, M.; Sudoh, U.; Mikami, S. Fresh properties and early strength development of concrete using calcium nitrite and water-reducing agents. Proc. Jap. Concr. Inst. 2012, 34, 155–159. [Google Scholar]
- Iwasawa, M.; Inoue, M.; Choi, H.; Sudoh, Y.; Ayuta, K. Characteristics of concrete using nitrite-based accelerator and chemical admixtures in low-temperature environments. In Proceedings of the 7th International Conference of Asian Concrete Federation, Hanoi, Vietnam, 30 October–20162 November 2016; pp. 1–8. [Google Scholar]
- Practical Guideline for Investigation. Recommendation for Practice of Cold Weather Concreting; Architectural Institute of Japan: Tokyo, Japan, 2010; p. 57. (In Japanese) [Google Scholar]
- Grochoski, C. Cold Weather Concreting with Hydronic Heaters. J. Am. Concr. Inst. ACI 2000, 22, 51–55. [Google Scholar]
- ACI Committee 306. Cold-Weather Concreting, ACI 306R-88; American Concrete Institute (ACI): Farmington Hills, MI, USA, 1997. [Google Scholar]
- Choi, H. Control of curing temperature of cold-weather concrete through effective utilization of energy-saving heat-curing systems. In Proceedings of the 2017 World Congress on Advances in Structural Engineering and Mechanics, Seoul, Korea, 28 August–1 September 2017; pp. 1–8. [Google Scholar]
- Kojima, T. Concrete Admixture Handbook; The Society of Materials Science: Tokyo, Japan, 2004; pp. 172–175. (In Japanese) [Google Scholar]
- Construction Promotion Council. Operation manual of Anti-Freezing Agent; Ministry of Land, Infrastructure, Transport and Tourism: Tokyo, Japan, 2005. (In Japanese)
- Ramachanran, V.S. Concrete Admixture Handbook; Noyes Publications: Park Ridge, NJ, USA, 1995; pp. 741–799. [Google Scholar]
- Hama, Y.; Kamada, E. Effects on Protection Fresh Concrete against Frost Damage by Non-chloride and Non-alkali Type Antifreezing Admixtures. Concr. Res. Technol. 1996, 7, 113–122. (In Japanese) [Google Scholar] [CrossRef]
- Japanese Industrial Standards. Physical Testing Methods for Cement, JIS R 5201; Japanese Standards Association: Tokyo, Japan, 2015; pp. 20–21. [Google Scholar]
- Paulo, J.M. CONCRETE, Microstructure, Properties, and Materials, 2nd ed.; Mc Graw Hill: New York, NY, USA, 1995; pp. 181–227. [Google Scholar]
- Balonis, M. Influence of calcium nitrate and nitrite on the constitution of AFm and AFt cement hydrates. Adv. Cem. Res. 2011, 23, 129–143. [Google Scholar] [CrossRef]
- Dumm, J.Q.; Brown, P.W. Phase assemblages in the system Ca(OH)2-Al2O3-Ca(NO3)2-H2O. Adv. Cem. Res. 1996, 8, 143–153. [Google Scholar] [CrossRef]
- Sakai, E.; Ueda, Y.; Aikawa, Y.; Nito, N. Influence of calcium nitrite on the hydration of high volume slag cement. J. Cem. Sci. Concr. Technol. 2017, 71, 62–67. [Google Scholar] [CrossRef]
- Kondo, R. Influence of inorganic salts on the hydration of tricalcium silicate. J. appl. Chem. Biotechnol. 1977, 27, 191–197. [Google Scholar] [CrossRef]
- Chudek, J.A.; Hunter, G.; Jones, M.R.; Scrimgeour, S.N.; Hewlett, P.C.; Kudryavtsev, A.B. Aluminium-27 solid state NMR spectroscopic studies of chloride binding in Portland cement and blends. J. Materi. Sci. 2000, 35, 4275–4288. [Google Scholar] [CrossRef]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterization of calcium aluminate phases in cements by 27Al MAS NMR spectroscopy. Inorg. Chem. 1993, 32, 1013–1027. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. A new Aluminium-hydrate species in hydrated Portland cements characterized by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2006, 36, 3–17. [Google Scholar] [CrossRef]
- L’Hopital, E.; Lothenbach, B.; Le Saout, G.; Kulik, D.; Scrivener, K. Incorporation of aluminium in calcium-silicate-hydrates. Cem. Concr. Res. 2015, 75, 91–103. [Google Scholar] [CrossRef]
- Richardson, I.G.; Brough, A.R.; Brydson, R.; Groves, G.W.; Dobson, C.M. Location of Aluminum in Substituted Calcium Silicate Hydrate (C-S-H) Gels as Determined by Si-29 and Al-27 Nmr and Eels. J. Am. Ceram. Soc. 1993, 76, 2285–2288. [Google Scholar] [CrossRef]
- Rodriguez, E.D.; Bernal, S.A.; Provis, J.L.; Paya, J.; Monzo, J.M.; Borrachero, M.V. Structure of Portland Cement Pastes Blended with Sonicated Silica Fume. J. Mater. Civil. Eng. 2012, 24, 1295–1304. [Google Scholar] [CrossRef]
- Richardson, I.G.; Girão, A.V.; Taylor, R.; Jia, S. Hydration of water- and alkali-activated white Portland cement pastes and blends with low-calcium pulverized fuel ash. Cem. Concr. Res. 2016, 83, 1–18. [Google Scholar] [CrossRef] [Green Version]






| Materials (Code) | Properties |
|---|---|
| Cement (C) | Normal Portland cement, Density: 3.16 g/cm3 |
| Fine aggregate (S) | No. 5 silica sand, Absolute dry density: 2.61 g/cm3, Water absorption: 0.26%, Fineness modulus: 2.16 |
| Anti-freezing agent (CN) | nitrite–nitrate based accelerator = Calcium nitrite (Ca(NO2)2); Calcium nitrate (Ca(NO3)2) |
| Code | Component | Component Ratio | pH | Specific Gravity |
|---|---|---|---|---|
| CN | Ca(NO2)2 | 23.02% | 9.3 | 1.43 |
| Ca(NO3)2 | 22.81% |
| Type | W/C (%) | S/C | Unit Content (kg/m3) | Anti-Freezing Agent (C × %) | ||
|---|---|---|---|---|---|---|
| W | C | S | CN | |||
| CN0 | 50 | 2.0 | 315 | 631 | 1262 | 0 |
| CN7 | 7 | |||||
| CN9 | 9 | |||||
| CN11 | 11 | |||||
| CN13 | 13 | |||||
| Temperature Condition | Experimental Period | Subject and Method of Evaluation | |
|---|---|---|---|
| Physical Properties | Chemical Properties | ||
| 10 °C | Casting—14 days | -Flow test -Compressive strength -Temperature history | -XRD -NMR -SEM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Inoue, M.; Choi, H.; Kim, J.; Sudoh, Y.; Kwon, S.; Lee, B.; Yoneyama, A. Physicochemical Study on the Strength Development Characteristics of Cold Weather Concrete Using a Nitrite–Nitrate Based Accelerator. Materials 2019, 12, 2706. https://doi.org/10.3390/ma12172706
Choi H, Inoue M, Choi H, Kim J, Sudoh Y, Kwon S, Lee B, Yoneyama A. Physicochemical Study on the Strength Development Characteristics of Cold Weather Concrete Using a Nitrite–Nitrate Based Accelerator. Materials. 2019; 12(17):2706. https://doi.org/10.3390/ma12172706
Chicago/Turabian StyleChoi, Heesup, Masumi Inoue, Hyeonggil Choi, Jihoon Kim, Yuhji Sudoh, Sukmin Kwon, Bokyeong Lee, and Akira Yoneyama. 2019. "Physicochemical Study on the Strength Development Characteristics of Cold Weather Concrete Using a Nitrite–Nitrate Based Accelerator" Materials 12, no. 17: 2706. https://doi.org/10.3390/ma12172706





