On The Microstructures and Hardness of The Nb-24Ti-18Si-5Al-5Cr-5Ge and Nb-24Ti-18Si-5Al-5Cr-5Ge-5Hf (at.%) Silicide Based Alloys
Abstract
:1. Introduction
2. Experimental
3. Results
3.1. Nb-24Ti-18Si-5Al-5Cr-5Ge (Alloy ZF6)
3.2. Nb-24Ti-18Si-5Ge -5Cr-5Al-5Hf (Alloy ZF9)
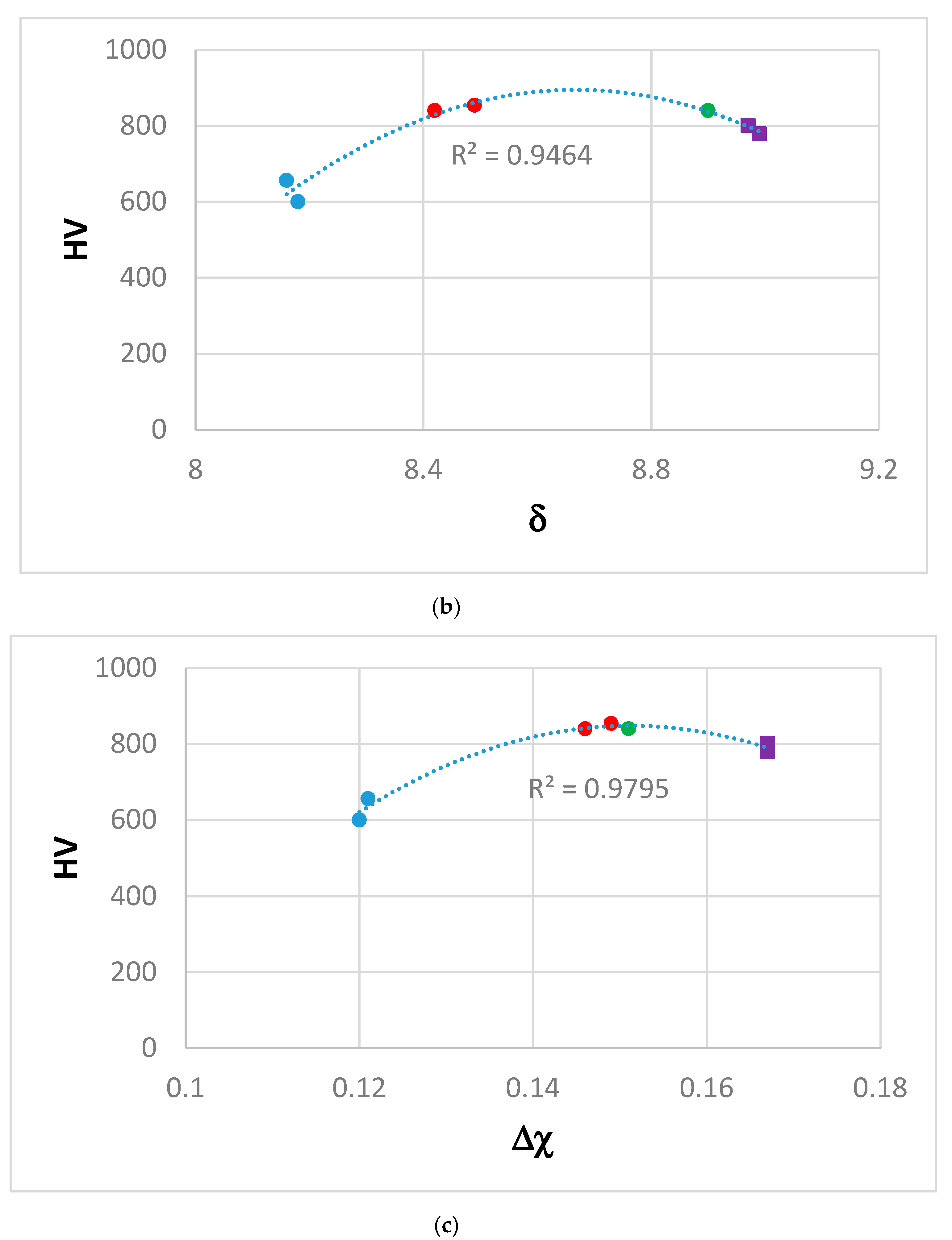
4. Discussion
4.1. Macrosegregation
4.2. Microstructures
4.2.1. Cast Alloys
4.2.2. Heat Treated Alloys
4.2.3. Lattice Parameter of The Nbss
4.3. Comparison with High Entropy Alloys
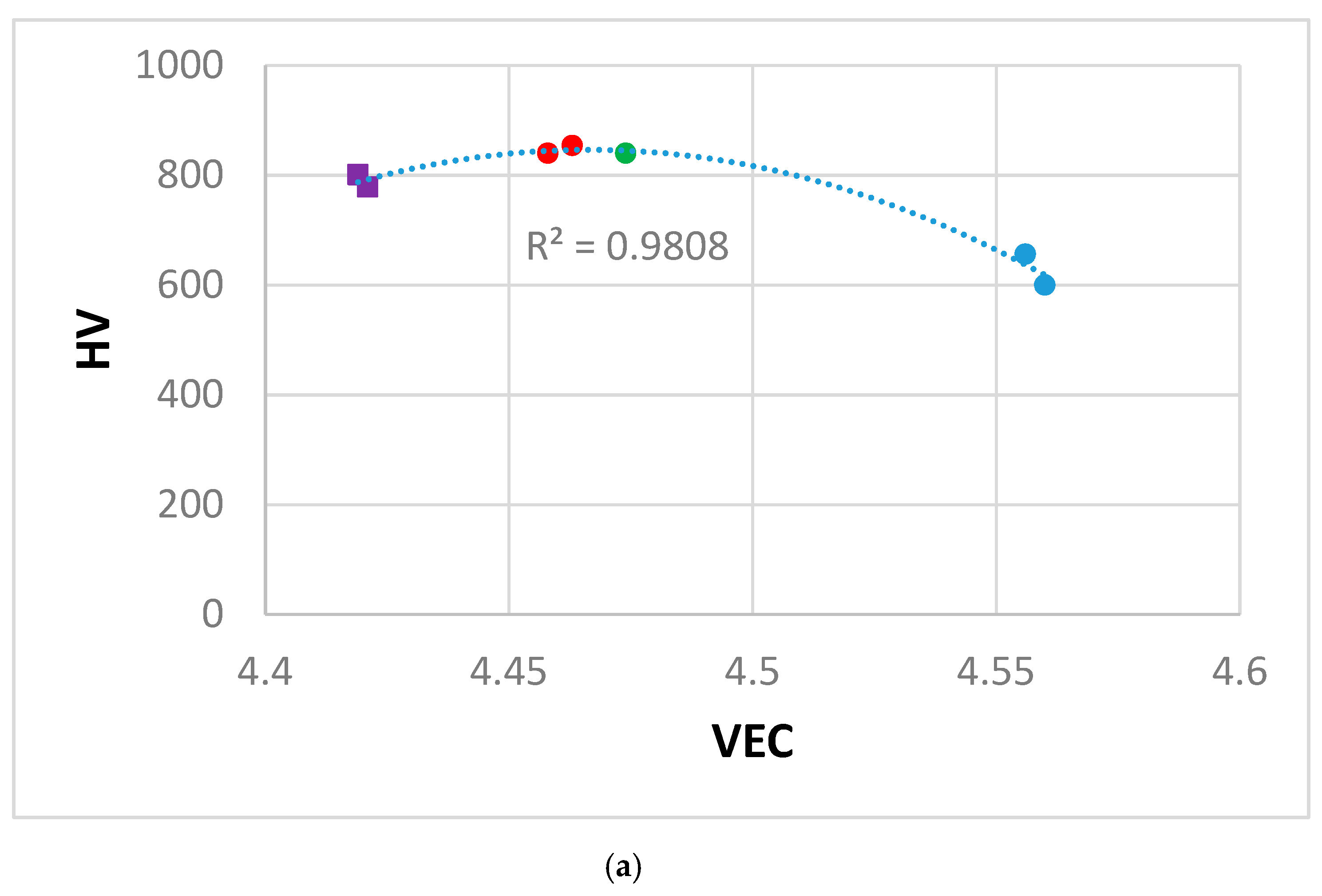
4.4. Hardness
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Alloy Code | Nominal Composition (at.%) | Reference |
|---|---|---|
| KZ3 | Nb-24Ti-18Si | [12] |
| KZ4 | Nb-24Ti-18Si-5Cr | [12] |
| KZ7 | Nb-24Ti-18Si-5Al | [12] |
| KZ5 | Nb-24Ti-18Si-5Al-5Cr | [12] |
| ZF1 | Nb-18Si-5Ge | [9] |
| ZF2 | Nb-18Si-10Ge | [9] |
| ZF3 | Nb-24Ti-18Si-5Ge | [18] |
| ZF7 | Nb-18Si-5Cr-5Ge | [15] |
| ZF4 | Nb-24Ti-18Si-5Cr-5Ge | [15] |
| ZF8 | Nb-18Si-5Al-5Ge | [13] |
| ZF5 | Nb-24Ti-18Si-5Al-5Ge | [13] |
References
- Zhao, J.C.; Westbrook, J.H. Ultrahigh-temperature materials for jet engines. MRS Bull. 2003, 28, 622–627. [Google Scholar] [CrossRef]
- Balsone, S.J.; Bewlay, B.P.; Jackson, M.R.; Subramanian, P.R.; Zhao, J.C.; Chatterjee, A.; Heffernan, T.M. Materials beyond superalloys-exploiting high temperature composites. In Proceedings of the International Symposium on Structural Intermetallics, Jackson Hole, WY, USA, 23–27 September 2001; pp. 99–108. [Google Scholar]
- Tsakiropoulos, P. On Nb silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Tsakiropoulos, P. Alloying and hardness of eutectics with Nbss and Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.C. Niobium silicide based composites resistant to high temperature oxidation. U.S. Patent 6,913,655 B2, 5 July 2005. [Google Scholar]
- Begley, R.T. Columbium alloy development at Westinghouse. In Evolution of Refractory Metals and Alloys; Dalder, E.N.C., Grobenstein, T., Olsen, C.S., Eds.; TMS: Warrendale, PA, USA, 1994; pp. 29–48. [Google Scholar]
- Chan, K.S. Alloying effects on fracture mechanisms in Nb-based intermetallic in-situ composites. Mater. Sci. Eng. 2002, A329, 513–522. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Tsakiropoulos, P. Study of the effect of Ge addition on the microstructure of Nb-18Si in situ composites. Intermetallics 2010, 18, 1072–1078. [Google Scholar]
- Chan, K.S.; Davison, D.L. Improving the fracture toughness of constituent phases and Nb-based in-situ composites by a computational alloy design approach. Metall. Mater. Trans. 2003, 34A, 1833–1849. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J. Ti5Si3 and Ti5Si3-based alloys: Alloying behaviour, microstructure and mechanical property evaluation. Acta Metall. 1998, 46, 3535–3546. [Google Scholar]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Al and Cr additions in the microstructure of Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Li, Z.F.; Tsakiropoulos, P. The microstructure of Nb-18Si-5Ge-5Al and Nb-24Ti-18Si-5Ge-5Al in situ composites. J. Alloy. Compd. 2013, 550, 553–560. [Google Scholar] [CrossRef]
- Cretegny, L.; Bewlay, B.P.; Ritter, A.M.; Jackson, M.R. The effects of substitutional additions on tensile behaviour of Nb-siliicde based composites. Mater. Res. Soc. Symp. Proc. 2005, 842, S2.10.1–S2.10.6. [Google Scholar]
- Li, Z.F.; Tsakiropoulos, P. Study of the effects of Cr and Ti additions in the microstructure of Nb-18Si-5Ge based in situ composites. Intermetallics 2012, 26, 18–25. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the macrosegregation of silicon in niobium silicide based alloys. Intermetallics 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Li, X.J.; Chen, H.F.; Sha, J.B.; Zhang, H. The effects of melting technologies on the microstructure and properties of Nb-16Si-22Ti-2Al-2Hf-17Cr alloy. Mater. Sci. Eng. 2010, A527, 6140–6152. [Google Scholar] [CrossRef]
- Li, Z.F.; Tsakiropoulos, P. Study of the effect of Ti and Ge in the microstructure of Nb-24Ti-18Si-5Ge in situ composite. Intermetallics 2011, 19, 1291–1297. [Google Scholar] [CrossRef]
- Beckermann, C. Modelling of macrosegregation: Application and future needs. Int. Mater. Rev. 2002, 47, 243–261. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Gokhale, A.B.; Abbaschian, R. The Nb-Si (Niobium-Silicon) System. J. Phase Equilib. 1993, 14, 502–509. [Google Scholar] [CrossRef]
- Okamoto, H. Desk Handbook: Phase Diagrams for Binary Alloys, 2nd ed.; ASM International: Metals Park, Ohio, OH, USA, 2000. [Google Scholar]
- Bewlay, B.P.; Jackson, M.R.; Gigliotti, M.F.X. Niobium silicide high temperature in situ composites. In Intermetallic compounds: Principles and practice, vol. 3; Fleisher, R.L., Westbrook, J.H., Eds.; John Wiley: New York, NY, USA, 2001; pp. 541–560, chapter 26. [Google Scholar]
- Hu, Y.L.; Zhang, L.; Shuman, D.; Huey, B.D.; Aindow, M. Atomic site occupancies and mechanical response of the eutectic C14 and A15 phases in a quinternary Nb-Mo-Cr-Al-Si alloy. Scripta Mater. 2009, 60, 309–312. [Google Scholar] [CrossRef]
- Goldschmidt, H.J.; Brand, J.A. The constitution of chromium-niobium-silicon system. J. Less Common Met. 1961, 3, 34–43. [Google Scholar] [CrossRef]
- Hunt, C.R.; Raman, A. Alloy chemistry of SIGMA-BETA-U-related phases. PT. 1. Extension of μ and occurrence of μ prime-phases in the ternary systems Nb-Ta-X-Al (X equals Fe, Co, Ni, Cu, Cr, Mo). Z. Metallkd. 1968, 59, 701–707. [Google Scholar]
- Geng, J.; Tsakiropoulos, P.; Shao, G. The effects of Ti and Mo additions on the microstructure of Nb-silicide based in situ composites. Intermetallics 2006, 14, 227–235. [Google Scholar] [CrossRef]
- Kotula, P.G.; Carter, C.B.; Chen, K.C.; Thoma, D.J.; Chu, F.; Mitchell, T.E. Defects and site occupancies Nb-Cr-Ti C15 Laves phase alloys. Scripta Mater. 1998, 39, 619–623. [Google Scholar] [CrossRef]
- Fujita, M.; Kaneno, Y.; Takasugi, T. Phase field and room-temperature mechanical properties of C15 Laves phase in Nb-Hf-Cr and Nb-Ta-Cr alloy systems. J. Alloy. Compd. 2006, 424, 283–288. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G. A study of the effects of Hf and Sn additions on the microstructure of Nbss/Nb5Si3 based in situ composites. Intermetallics 2007, 15, 69–76. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Al, Cr and Ti additions in the microstructure of Nb-18Si-5Hf base alloys. Intermetallics 2010, 18, 242–253. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T. Smithells Metals Reference Book; Oxford-Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Kim, J.H.; Tabaru, T.; Sakamoto, M.; Hanada, S. Mechanical properties and fracture behaviour of an Nbss /Nb5Si3 in-situ composite modified by Mo and Hf alloying. Mater. Sci. Eng. A 2004, 372, 137–144. [Google Scholar] [CrossRef]
- Prokoshkin, D.A.; Vasileva, E.V. Alloys of Niobium; Israel Programme for Scientific Translations: Jerusalem, Israel, 1965; p. 127. [Google Scholar]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2016, 122, 1–64. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys; Part II. J. Alloy. Compd. 2018, 748, 569–576. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the Nb silicide based alloys: Part I—The bcc Nb solid solution. J. Alloy. Compd. 2017, 708, 961–971. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure and properties of Aluminium-containing refractory high-entropy alloys. JOM 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Erratum to: Phase stability of low density, multi-principal component alloys containing Al, Mg and Li. JOM 2014, 66, 2009–2020. [Google Scholar] [CrossRef]
- Liu, W.H.; Wu, Y.; He, J.Y.; Zhang, Y.; Liu, C.T. The phase competition and stability of high-entropy alloys. JOM 2014, 66, 1973–1983. [Google Scholar] [CrossRef]
- Wang, Z.J.; Guo, S.; Liu, C.T. Phase selection in high-entropy alloys: From non-equilibrium to equilibrium. JOM 2014, 66, 1966–1972. [Google Scholar] [CrossRef]
- Koppenaal, T.J.; Kuhlmann-Wilsdorf, D. The effect of pre-stressing on the strength of neutron-irradiated copper single crystals. Appl. Phys. Lett. 1964, 4, 59. [Google Scholar] [CrossRef]
- Zacharis, E.; Utton, C.; Tsakiropoulos, P. A study of the effects of Hf and Sn on the microstructure, hardness and oxidation of Nb-18Si silicide based alloys without Ti addition. Materials 2018, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Christman, T.; Needleman, A.; Nutt, S.; Suresh, S. On microstructural evolution and micromechanical modelling of deformation of a whisker reinforced metal matrix composite. Mater. Sci. Eng. 1989, 107A, 49. [Google Scholar] [CrossRef]
- Drucker, D.C. Engineering and Continuum Aspects of High Strength Materials in High Strength Materials; John Wiley: New York, NY, USA, 1965; pp. 795–833. [Google Scholar]
- Nutt, S.R.; Needleman, A. Void nucleation at fibre ends in Al-SiC composites. Scripta Metallurgica 1987, 21, 705–710. [Google Scholar] [CrossRef]
- Nelson, J.; Ghadyani, M.; Utton, C.; Tsakiropoulos, P. A study of the effect of Al, Cr, Hf and Ti additions on the microstructure and oxidation of Nb-24Ti-18Si silicide based alloys. Materials 2018, 11, 1579. [Google Scholar] [CrossRef]

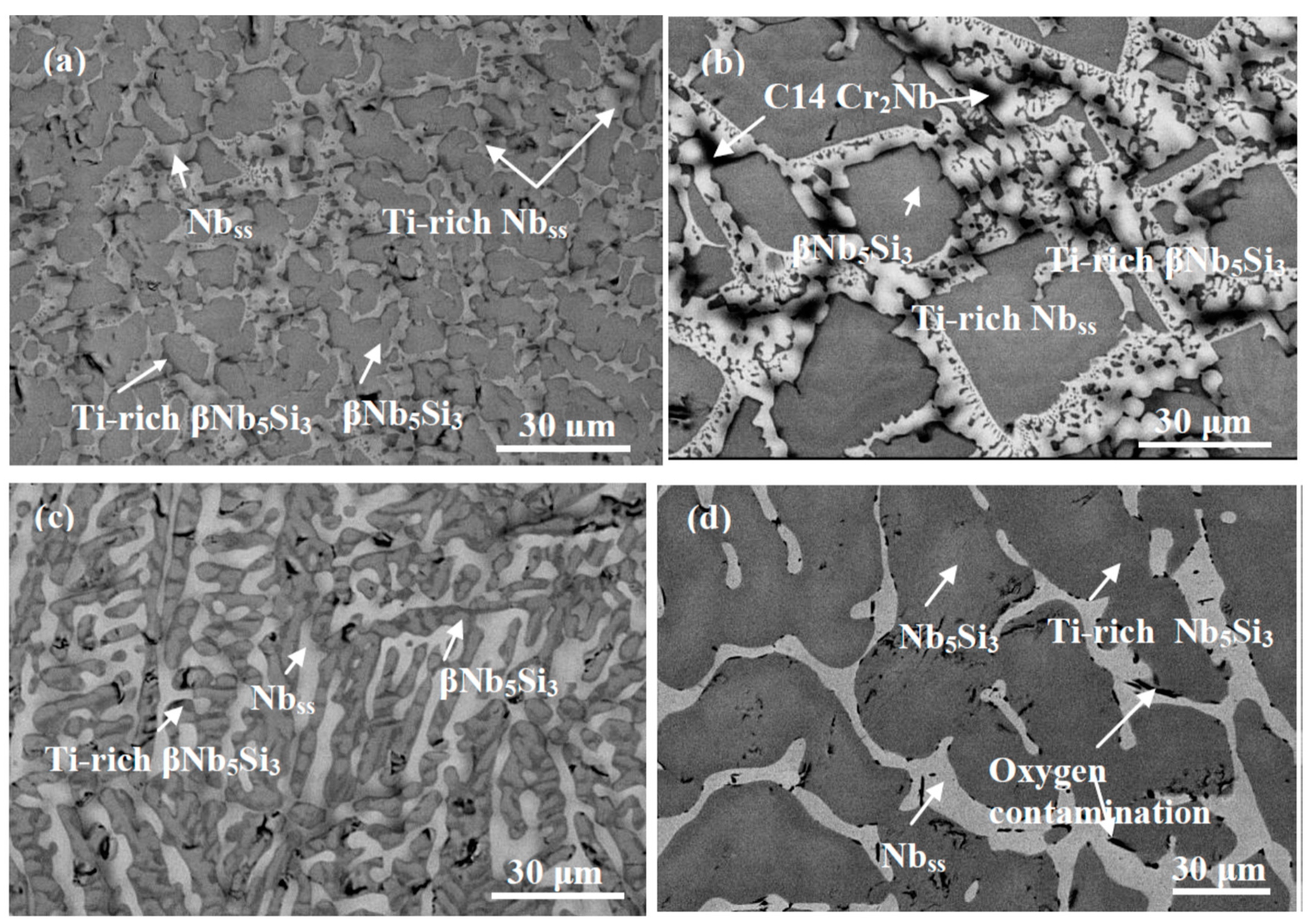


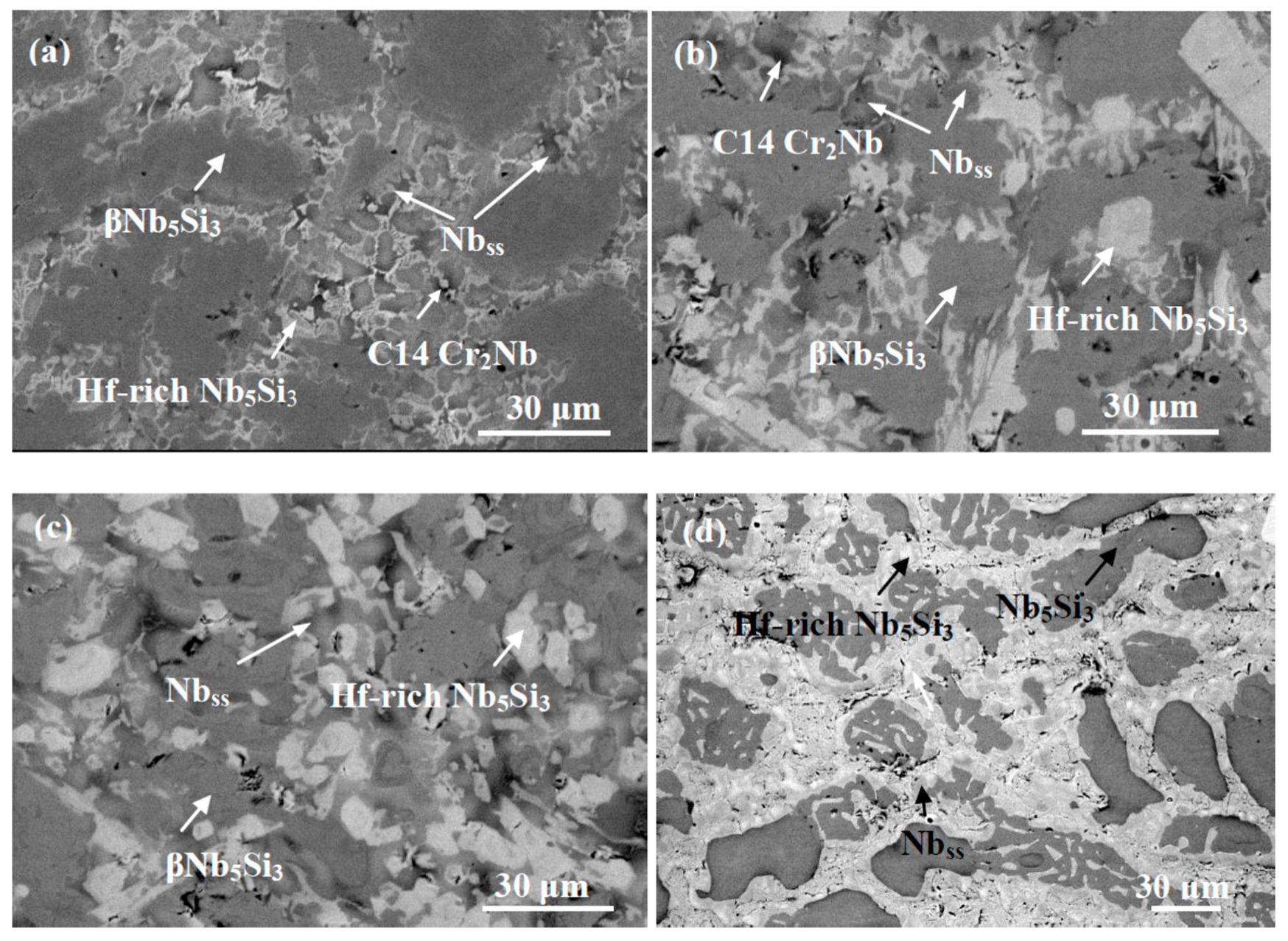
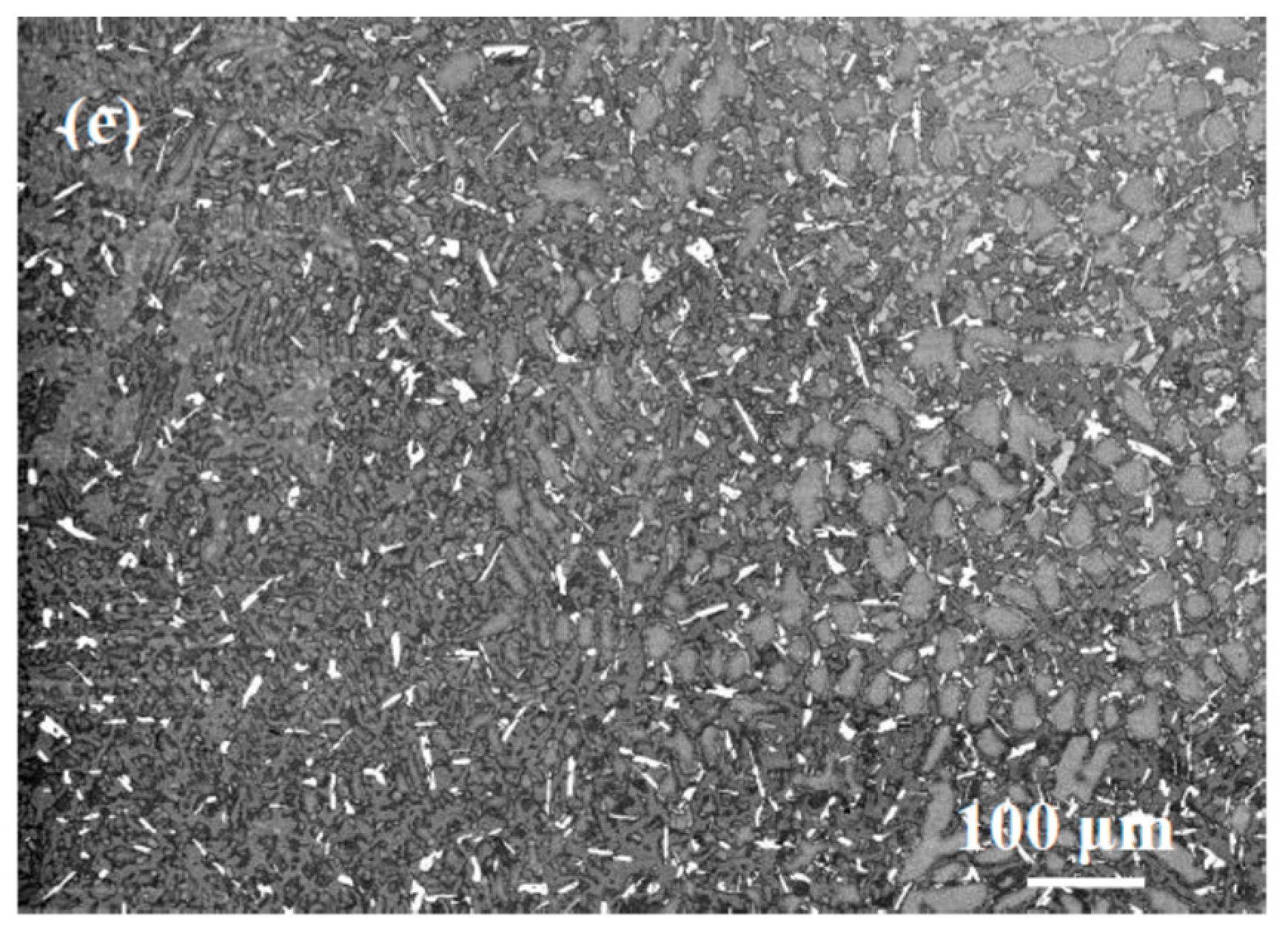


| Alloy | Density (g/cm3) | % Area | ||
|---|---|---|---|---|
| Top | Bulk | Bottom | ||
| ZF6-AC | 6.54 ± 0.01 | 22.4 ± 1.8 | 17.1 ± 3.7 | 29.2 ± 2.8 |
| ZF6-HT | - | - | 17.3 ± 3.5 | - |
| ZF9-AC | 6.96 ± 0.02 | - | - | - |
| ZF9-HT | - | - | - | - |
| Area/Phase | Nb | Ti | Si | Ge | Cr | Al |
|---|---|---|---|---|---|---|
| ZF6-AC | - | - | - | - | - | - |
| Top* | 41.6 ± 0.4 | 26.2 ± 0.1 | 16.1 ± 0.1 | 4.9 ± 0.1 | 5.4 ± 0.2 | 5.8 ± 0.6 |
| 41.4–42.0 | 26.1–26.2 | 16.0–16.1 | 4.8–5.0 | 5.2–5.5 | 5.3–6.2 | |
| Bulk* | 41.9 ± 1.0 | 24.9 ± 0.8 | 18.0 ± 1.9 | 5.1 ± 0.3 | 4.5 ± 1.0 | 5.6 ± 1.1 |
| 40.9–43.3 | 23.9–26.0 | 16.1–20.1 | 4.9–5.6 | 3.5–5.6 | 4.1–6.6 | |
| Bottom* | 41.1 ± 0.4 | 26.1 ± 0.4 | 16.3 ± 0.4 | 5.4 ± 0.1 | 5.2 ± 0.2 | 6.1 ± 0.5 |
| 40.7–41.6 | 25.5–26.3 | 15.8–16.6 | 5.3–5.5 | 5.0–5.4 | 5.9–6.7 | |
| Nbss | 52.3 ± 1.6 | 27.9 ± 0.7 | 2.1 ± 0.2 | 1.7 ± 0.3 | 9.2 ± 1.1 | 6.8 ± 0.4 |
| 50.2–53.6 | 27.0–28.8 | 2.0–2.4 | 1.4–2.2 | 8.0–10.6 | 6.6–7.3 | |
| Ti-rich Nbss | 45.4 ± 3.1 | 31.3 ± 1.4 | 1.8 ± 0.4 | 1.6 ± 0.3 | 12.9 ± 1.2 | 7.0 ± 0.3 |
| 42.0–48.7 | 29.9–32.7 | 1.3–2.1 | 1.3–1.9 | 11.5–14.4 | 6.7–7.7 | |
| βNb5Si3 | 42.4 ± 1.1 | 22.1 ± 0.9 | 23.2 ± 1.3 | 7.3 ± 0.4 | 1.4 ± 0.2 | 3.6 ± 1.0 |
| 41.3–43.9 | 20.9–23.0 | 22.1–24.8 | 6.9–7.9 | 1.1–1.5 | 2.3–4.8 | |
| Ti-rich βNb5Si3 | 34.1 ± 0.4 | 29.5 ± 0.3 | 20.9 ± 1.4 | 8.4 ± 0.4 | 2.4 ± 0.4 | 4.7 ± 0.7 |
| 33.8–34.5 | 29.1–29.7 | 19.3–27.1 | 8.1–8.9 | 2.1–2.8 | 4.1–5.4 | |
| C14-Cr2Nb+ | 18.4 | 24.8 | 6.2 | 1.2 | 46.3 | 3.1 |
| Eutectic | 45.5 ± 1.2 | 28.4 ± 0.6 | 8.1 ± 1.5 | 3.7 ± 0.5 | 8.0 ± 1.1 | 6.3 ± 0.4 |
| 44.2–46.9 | 27.7–29.0 | 5.8–9.0 | 3.2–4.3 | 6.6–9.3 | 6.0–6.8 | |
| ZF6-HT* (1400 °C/100 h) | 43.1 ± 1.0 | 24.3 ± 1.2 | 17.4 ± 0.8 | 5.8 ± 0.2 | 4.2 ± 0.3 | 5.2 ± 0.8 |
| 41.9–44.6 | 23.0–25.9 | 16.4–18.7 | 5.5–6.2 | 4.0–4.7 | 4.1–6.1 | |
| Nbss | 52.6 ± 0.5 | 26.9 ± 0.3 | 0.9 ± 0.5 | 1.0 ± 0.2 | 12.3 ± 0.2 | 6.3 ± 0.4 |
| 52.1–53.0 | 26.4–27.2 | 0–1.2 | 0.8–1.2 | 11.9–12.4 | 5.7–6.7 | |
| Nb5Si3 | 42.2 ± 0.9 | 21.7 ± 0.9 | 23.1 ± 1.2 | 7.4 ± 0.5 | 1.8 ± 0.4 | 3.8 ± 0.6 |
| 41.4–43.4 | 20.5–22.6 | 21.7–24.4 | 7.0–8.1 | 1.5–2.3 | 3.1–4.3 | |
| Ti-rich Nb5Si3 | 37.6 ± 1.1 | 26.1 ± 1.1 | 21.0 ± 0.3 | 7.8 ± 0.2 | 2.7 ± 0.1 | 4.8 ± 0.3 |
| 35.9–39.1 | 25.0–27.9 | 20.8–21.6 | 7.6–8.0 | 2.5–2.8 | 4.6–5.2 |
| Area/Phase | Nb | Ti | Si | Ge | Cr | Al | Hf |
|---|---|---|---|---|---|---|---|
| ZF9-AC | - | - | - | - | - | - | - |
| Top* | 35.6 ± 0.1 | 27.3 ± 0.2 | 16.0 ± 0.6 | 5.3 ± 0.2 | 5.9 ± 0.3 | 4.4 ± 0.3 | 5.5 ± 0.1 |
| 35.7–35.9 | 27.0–27.5 | 15.3–16.5 | 5.0–5.4 | 5.5–6.1 | 4.1–4.7 | 5.5–5.6 | |
| Bulk* | 36.7 ± 0.4 | 24.8 ± 0.6 | 17.6 ± 0.8 | 5.1 ± 0.2 | 4.6 ± 0.2 | 5.8 ± 0.4 | 5.4 ± 0.2 |
| 36.3–37.3 | 24.1–25.5 | 16.4–18.4 | 4.9–5.4 | 4.3–4.9 | 5.2–6.1 | 5.2–5.6 | |
| Bottom* | 35.3 ± 0.1 | 27.1 ± 0.2 | 15.5 ± 0.1 | 5.5 ± 0.5 | 6.2 ± 0.3 | 5.0 ± 0.2 | 5.4 ± 0.2 |
| 35.2–35.4 | 27.0–27.3 | 15.4–15.6 | 5.1–6.0 | 5.9–6.4 | 4.9–5.2 | 5.2–5.5 | |
| Nbss | 40.4 ± 3.4 | 31.5 ± 2.3 | 1.6 ± 0.2 | 1.0 ± 0.2 | 15.0 ± 1.2 | 7.8 ± 0.3 | 2.7 ± 0.2 |
| 35.5–44.4 | 29.1–35.1 | 1.5–1.8 | 0.7–1.1 | 13.4–16.1 | 7.5–8.4 | 2.5–3.0 | |
| βNb5Si3 | 37.2 ± 0.2 | 21.0 ± 0.3 | 24.2 ± 0.5 | 6.6 ± 0.1 | 1.2 ± 0.1 | 5.0 ± 0.4 | 4.8 ± 0.1 |
| 36.8–37.3 | 20.6–21.2 | 23.8–25.1 | 6.5–6.8 | 1.2–1.3 | 4.4–5.5 | 4.7–4.9 | |
| Hf-rich Nb5Si3 | 30.9 ± 0.7 | 24.0 ± 0.3 | 24.5 ± 0.6 | 7.0 ± 0.3 | 0.8 ± 0.2 | 3.8 ± 0.6 | 9.0 ± 0.2 |
| 29.9–31.8 | 23.6–24.2 | 23.7–25.1 | 6.6–7.4 | 0.5–0.9 | 3.3–4.8 | 8.8–9.2 | |
| Cr2Nb+ | 20.5 | 19.7 | 5.6 | 1.3 | 43.7 | 4 | 5.2 |
| ZF9-HT* | 38.0 ± 2.0 | 24.2 ± 1.5 | 17.1 ± 1.3 | 5.2 ± 0.2 | 4.8 ± 0.6 | 5.5 ± 0.8 | 5.2 ± 0.2 |
| 36.4–40.2 | 22.8–25.7 | 15.8–18.4 | 5.0–5.4 | 4.1–5.3 | 4.9–6.4 | 5.0–5.4 | |
| Nbss | 56.6 ± 3.3 | 25.3 ± 1.2 | 0.7 ± 0.5 | 0.6 ± 0.6 | 11.8 ± 0.8 | 3.8 ± 1.2 | 1.2 ± 0.1 |
| 56.8–59.8 | 24.3–26.7 | 0.0–1.4 | 0.0–1.2 | 10.6–12.6 | 2.4–5.4 | 1.1–1.3 | |
| Nb5Si3 | 37.0 ± 0.6 | 21.2 ± 0.4 | 24.8 ± 0.5 | 6.7 ± 0.3 | 2.3 ± 0.3 | 3.2 ± 0.4 | 4.8 ± 0.2 |
| 36.4–37.9 | 20.6–21.7 | 24.2–25.2 | 6.3–7.2 | 2.0–2.7 | 2.7–3.4 | 4.5–4.9 | |
| Hf-rich Nb5Si3 | 30.2 ± 1.2 | 24.3 ± 0.9 | 25.4 ± 0.6 | 7.0 ± 0.2 | 1.0 ± 0.3 | 3.3 ± 0.5 | 8.8 ± 0.1 |
| 29.0–31.3 | 23.6–25.3 | 25.0–26.1 | 6.8–7.2 | 0.7–1.3 | 2.7–3.6 | 8.8–8.9 |
| Alloy | ZF6-AC | ZF6-HT | ZF9-AC | ZF9-HT |
|---|---|---|---|---|
| Parameter | 3.252 | 3.260 | 3.256 | 3.264 |
| Alloy | Hardness | Microhardness | |
|---|---|---|---|
| Nbss | Nb5Si3 | ||
| ZF6-AC | 840 ± 34 | —+ | 1644 ± 89 |
| ZF6-HT | 854 ± 31 | 691 ± 37 | 1576 ± 60 |
| ZF9-AC | 801 ± 37 | —+ | 1495 ± 88 |
| ZF9-HT | 779 ± 11 | —+ | 1391 ± 21 |
| Elements in Synergy | Alloy Code* & Reference | CmaxSi–CminSi | Macrosegregation |
|---|---|---|---|
| Ti + Cr + Ge | ZF4 [15] | 5.3 |  |
| Ti + Cr + Al + Ge | ZF6 | 4.3 | |
| Ti + Ge | ZF3 [18] | 3.2 | |
| Ti + Cr + Al + Ge + Hf | ZF9 | 3.1 | |
| Ti + Al + Ge | ZF5 [13] | 2.9 | |
| Al + Ge | ZF8 [13] | 2.5 | |
| Ti + Al | KZ7 [12] | 2.3 | |
| Ge | ZF1 [9] | 2.0 | |
| Ti + Cr | KZ4 [12] | 1.9 | |
| Ti | KZ3 [12] | 1.6 | |
| Cr + Ge | ZF7 [15] | 1.5 | |
| Ti + Cr + Al | KZ5 [12] | 1.3 |
| Alloying Additions | Alloy Code* & Reference | Cmaxi–Cmini (i = Ti, Cr, Al) | ||
|---|---|---|---|---|
| Ti | Cr | Al | ||
| Ti + Ge + Cr + Al + Hf | ZF9 | 3.4 | 2.1 | 2 |
| Ti + Ge + Cr | ZF4 [15] | 3.3 | 2.2 | - |
| Ti + Ge + Cr + Al | ZF6 | 2.4 | 2.1 | 2.6 |
| Ti + Al | KZ7 [12] | 2.3 | - | - |
| Ti + Cr + Al | KZ5 [12] | 1.4 | - | - |
| Ti + Ge | ZF3 [18] | 1.4 | - | - |
| Ti + Cr | KZ4 [12] | 1.4 | 1.8 | - |
| Ti | KZ3 [12] | 3.1 | - | - |
| Alloy Code | Nominal Composition (at.%) | Si + Ge +Al in The Eutectic (at.%) |  |
| ZF8-AC [13] | Nb-18Si-5Ge-5Al | 22.6 | |
| ZF5-AC [13] | Nb-24Ti-18Si-5Ge-5Al | 18.8 | |
| ZF6-AC | Nb-24Ti-18Si-5Ge-5Cr-5Al | 18.1 |
| Alloy Code & Reference | Nominal Composition (at.%) | Si + Ge in The Eutectic (at.%) |  |
| ZF1-AC [9] | Nb-18Si-5Ge | 17.7 | |
| ZF2-AC [9] | Nb-18Si-10Ge | 17.6 | |
| ZF8-AC [13] | Nb-18Si-5Ge-5Al | 16.3 | |
| ZF7-AC [15] | Nb-18Si-5Ge-5Cr | 15.2 | |
| ZF4-AC [15] | Nb-24Ti-18Si-5Ge-5Cr | 14.8 | |
| ZF5-AC [13] | Nb-24Ti-18Si-5Ge-5Al | 12.6 | |
| ZF6-AC | Nb-24Ti-18Si-5Ge-5Cr-5Al | 11.8 |
| Condition | Alloy | Nbss | Parameter | |||||
|---|---|---|---|---|---|---|---|---|
| - | - | - | ΔHchem (Kj·mol−1) | ΔSmix (J·mol−1·K) | VEC | δ | Δχ | Ω |
| AC | ZF6 | Nbss Ti-rich Nbss | −39.4 | 12.31 | 4.458 | 8.42 | 0.146 | 0.672 |
| −12.87 | 10.38 | 4.639 | 5.12 | 0.078 | 1.855 | |||
| −13 | 10.9 | 4.642 | 5.52 | 0.077 | 1.888 | |||
| HT | ZF6 | Nbss | −40.4 | 12.16 | 4.463 | 8.49 | 0.149 | 0.650 |
| −9.7 | 10.07 | 4.709 | 5.02 | 0.063 | 2.411 | |||
| AC | ZF9 | Nbss | −40.09 | 13.65 | 4.419 | 8.97 | 0.167 | 0.730 |
| −12.29 | 11.83 | 4.626 | 5.95 | 0.084 | 2.146 | |||
| HT | ZF9 | Nbss | −40.73 | 13.52 | 4.421 | 8.99 | 0.167 | 0.714 |
| −6.87 | 9.68 | 4.764 | 4.96 | 0.063 | 3.367 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tsakiropoulos, P. On The Microstructures and Hardness of The Nb-24Ti-18Si-5Al-5Cr-5Ge and Nb-24Ti-18Si-5Al-5Cr-5Ge-5Hf (at.%) Silicide Based Alloys. Materials 2019, 12, 2655. https://doi.org/10.3390/ma12172655
Li Z, Tsakiropoulos P. On The Microstructures and Hardness of The Nb-24Ti-18Si-5Al-5Cr-5Ge and Nb-24Ti-18Si-5Al-5Cr-5Ge-5Hf (at.%) Silicide Based Alloys. Materials. 2019; 12(17):2655. https://doi.org/10.3390/ma12172655
Chicago/Turabian StyleLi, Zifu, and Panos Tsakiropoulos. 2019. "On The Microstructures and Hardness of The Nb-24Ti-18Si-5Al-5Cr-5Ge and Nb-24Ti-18Si-5Al-5Cr-5Ge-5Hf (at.%) Silicide Based Alloys" Materials 12, no. 17: 2655. https://doi.org/10.3390/ma12172655





