Mo-La2O3 Multilayer Metallization Systems for High Temperature Surface Acoustic Wave Sensor Devices
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Composition of the La Interlayers
3.2. Mo-(LaO-Mo) Multilayers
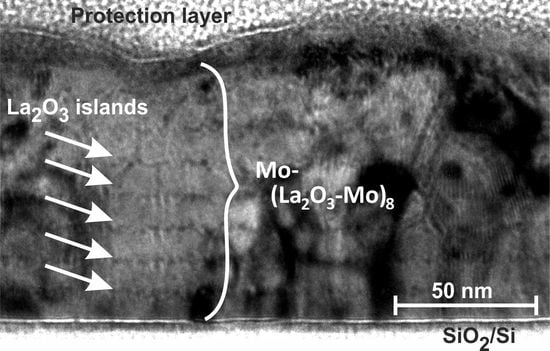
3.2.1. Microstructure
3.2.2. Phase Formation
3.2.3. Roughness
3.2.4. Electrical Resistivity
4. Conclusions
- All Mo-(LaO-Mo) multilayers deposited at room temperature (RT) are polycrystalline and free of significant cracks and they exhibit a similar morphology in the as-deposited state.
- In all multilayers the in-plane grain size reduces with an increasing number of bilayers and, thus decreasing thickness of the Mo layers, both in as-deposited state and after thermal treatment.
- There is no clear trend in the RMS roughness with the number n of (LaO-Mo) bilayers. However, the roughness upon annealing at 800 C for 120 h is lowest for the Mo-(LaO-Mo) system.
- The influence of the increase in RMS roughness during the annealing on the electrical resistivity is overcompensated by defect annihilation.
- Annealing of the multilayer systems at 800 C for 120 h leads to a reduction in the resistivity due to grain coarsening which results in reduced electron scattering. The annealed Mo layers show the lowest resistivity values of ≈8.2 cm. For the Mo-(LaO-Mo) multilayer films with and (LaO-Mo) bilayers a slight increase in resistivity is observed after annealing at 800 C for 24 h compared to the as-deposited state.
- The results show that the multilayer systems retain a clearly visible periodic structure of Mo and LaO along the growth direction even after annealing at 800 C for 120 h.
- In case of 1 nm thickness of the La deposition, a continuous and closed LaO layer was formed on top of Mo. Thus the LaO in Mo-(LaO-Mo) provided a layer of complete chemical and physical discontinuity to the Mo layers. However, it was shown that extremely thin La interlayers (<1 nm) were present as a discontinuous layer of LaO particles.
- The results indicate that Mo-(LaO-Mo) multilayer films can be appropriate material systems for IDT electrodes for applications in the high-temperature range above 600 C.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | as deposited |
| AFM | atomic force microscopy |
| bcc | body centered cubic |
| MFPE | mean free path of electrons |
| FIB | focussed ion beam |
| IDT | interdigital transducer |
| ODS | oxide particle strengthened |
| RMS | root mean square roughness |
| RT | room temperature |
| SAW | surface acoustic wave |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| vdP | van der Pauw |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Hosaka, N.; Yuhara, A.; Watanabe, H.; Yamada, J.; Kajiyama, M. A Study of Al-Alloy Electrodes for High Power SAW Filters. Jpn. J. Appl. Phys. 1988, 27, 175. [Google Scholar] [CrossRef] [Green Version]
- Ota, Y.; Yuhara, A. Study on Power Durability of Ti-Containing Al Surface Acoustic Wave Electrodes, Using Interdigitated Interdigital Transducer Filters. Jpn. J. Appl. Phys. 1995, 34, 2693–2697. [Google Scholar] [CrossRef]
- Nishihara, T.; Uchishiba, H.; Ikata, O.; Satoh, Y. Improved Power Durability of Surface Acoustic Wave Filters for an Antenna Duplexers. Jpn. J. Appl. Phys. 1995, 34, 2688–2692. [Google Scholar] [CrossRef]
- Menzel, S.; Pekarčikova, M.; Hofmann, M.; Gemming, T.; Wetzig, K. Material transport in Al-metallizations of power-loaded SAW structures. Appl. Surf. Sci. 2005, 252, 215–217. [Google Scholar] [CrossRef]
- Pekarcikova, M.; Hofmann, M.; Menzel, S.; Schmidt, H.; Gemming, T.; Wetzig, K. Investigation of high power effects on Ti/Al and Ta-Si-N/Cu/Ta-Si-N electrodes for SAW devices. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 911–917. [Google Scholar] [CrossRef]
- Hornsteiner, J.; Born, E.; Fischerauer, G.; Riha, E. Surface acoustic wave sensors for high-temperature applications. In Proceedings of the 1998 IEEE International Frequency Control Symposium (Cat. No. 98CH36165), Pasadena, CA, USA, 29 May 1998; pp. 615–620. [Google Scholar] [CrossRef]
- Aubert, T.; Bardong, J.; Elmazria, O.; Bruckner, G.; Assouar, B. Iridium interdigital transducers for high-temperature surface acoustic wave applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 194–197. [Google Scholar] [CrossRef]
- Behanan, R.; Moulzolf, S.C.; Call, M.; Bernhardt, G.; Frankel, D.; Lad, R.J.; da Cunha, M.P. Thin films and techniques for SAW sensor operation above 1000 °C. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 1013–1016. [Google Scholar] [CrossRef]
- Richter, D.; Sakharov, S.; Forsén, E.; Mayer, E.; Reindl, L.; Fritze, H. Thin Film Electrodes for High Temperature Surface Acoustic Wave Devices. Procedia Eng. 2011, 25, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, M.P.; Moonlight, T.; Lad, R.; Frankel, D.; Bernhard, G. High temperature sensing technology for applications up to 1000 °C. IEEE Sens. J. 2008, 752–755. [Google Scholar] [CrossRef]
- Thiele, J.A.; Da Cunha, M.P. Platinum and palladium high-temperature transducers on langasite. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 545–549. [Google Scholar] [CrossRef]
- Hamidon, M.; Skarda, V.; White, N.; Krispel, F.; Krempl, P.; Binhack, M.; Buff, W. Fabrication of high temperature surface acoustic wave devices for sensor applications. Sens. Actuators A Phys. 2005, 123–124, 403–407. [Google Scholar] [CrossRef]
- Freund, L.B.; Suresh, S. Thin Film Materials Stress, Defect Formation and Surface Evolution; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Moulzolf, S.C.; Frankel, D.J.; da Cunha, M.P.; Lad, R.J. High temperature stability of electrically conductive Pt–Rh/ZrO2 and Pt–Rh/HfO2 nanocomposite thin film electrodes. Microsyst. Technol. 2014, 20. [Google Scholar] [CrossRef]
- Frankel, D.J.; Moulzolf, S.C.; da Cunha, M.P.; Lad, R.J. Influence of composition and multilayer architecture on electrical conductivity of high temperature Pt-alloy films. Surf. Coat. Technol. 2015, 284, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Rane, G.K.; Menzel, S.; Gemming, T.; Eckert, J. Microstructure, electrical resistivity and stresses in sputter deposited W and Mo films and the influence of the interface on bilayer properties. Thin Solid Films 2014, 571, 1–8. [Google Scholar] [CrossRef]
- Rane, G.K.; Menzel, S.; Seifert, M.; Gemming, T.; Eckert, J. Tungsten/molybdenum thin films for application as interdigital transducers on high temperature stable piezoelectric substrates La3Ga5SiO14 and Ca3TaGa3Si2O14. Mater. Sci. Eng. B 2015, 202, 31–38. [Google Scholar] [CrossRef]
- Seifert, M.; Rane, G.K.; Menzel, S.B.; Gemming, T. TEM studies on the changes of the composition in LGS and CTGS substrates covered with a RuAl metallization and on the phase formation within the RuAl film after heat treatment at 600 and 800 °C. J. Alloy Compd. 2016, 664, 510–517. [Google Scholar] [CrossRef]
- Spindler, M.; Menzel, S.B.; Eckert, J.; Eggs, C. Influence of Al on resistance and power durability of Cu-based SAW metallizations. IOP Conf. Ser. Mater. Sci. Eng. 2010, 8, 012013. [Google Scholar] [CrossRef]
- Lattner, E.; Seifert, M.; Gemming, T.; Heicke, S.; Menzel, S.B. Coevaporation and structuring of titanium–aluminum alloy thin films. J. Vac. Sci. Technol. A 2017, 35, 061603. [Google Scholar] [CrossRef]
- Seifert, M.; Rane, G.K.; Oswald, S.; Menzel, S.B.; Gemming, T. The Influence of the Composition of Ru100−xAlx (x = 50, 55, 60, 67) Thin Films on Their Thermal Stability. Materials 2017, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Rane, G.K.; Menzel, S.B.; Oswald, S.; Gemming, T. Improving the oxidation resistance of RuAl thin films with Al2O3 or SiO2 cover layers. J. Alloy Compd. 2019, 776, 819–825. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry & Physics, 89th ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Maier, K.; Mehrer, H.; Rein, G. Self-Diffusion in Molybdenum. Z. Metallk. 1979, 70, 271–276. [Google Scholar]
- Schumacher, D.; Seeger, A.; Härlin, O. Vacancies, divacancies, and self-diffusion in platinum. Phys. Status Solidi (b) 1968, 25, 359–371. [Google Scholar] [CrossRef]
- Fu, X.; Iacute, V.; Encarnacion, G.; Matsushita, Y.; Kitanaka, Y.; Noguchi, Y.; Miyayama, M.; Shimamura, K.; Ohashi, N. Influence of growth conditions on the optical, electrical resistivity and piezoelectric properties of Ca3TaGa3Si2O14 single crystals. J. Ceram. Soc. Jpn. 2016, 124, 523–527. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, D.; Cheng, X.; Xu, D.; Lv, M.; Pan, L.; Duan, X.; Sun, H.; Shi, X.; Lv, Y.; et al. The growth and properties of Ca3TaGa3Si2O14 single crystals. J. Cryst. Growth 2003, 253, 378–382. [Google Scholar] [CrossRef]
- Samanta, S.K.; Yoo, W.J.; Samudra, G.; Tok, E.S.; Bera, L.K.; Balasubramanian, N. Tungsten nanocrystals embedded in high-k materials for memory application. Appl. Phys. Lett. 2005, 87, 113110. [Google Scholar] [CrossRef]
- Shields, J.A. Application of Molybdenum Metal and Its Alloys; Int. Molybdenum Assoc. (IMOA): London, UK, 2013. [Google Scholar]
- Armstrong, R.W. The influence of polycrystal grain size on several mechanical properties of materials. Metall. Mater. Trans. B 1970, 1, 1169–1176. [Google Scholar] [CrossRef]
- Yoo, M.K.; Hiraoka, Y.; Choi, J. Recrystallization of molybdenum wire doped with lanthanum oxide. Int. J. Refract. Met. Hard Mater. 1995, 13, 221–227. [Google Scholar] [CrossRef]
- Shtansky, D. Multicomponent Nanostructured Thin Films. Deposition, Characterization, Testing and Application. Nanostructured Thin Films and Nanodispersion Strengthened Coatings; Voevodin, A.A., Shtansky, D.V., Levashov, E.A., Moore, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 155–165. [Google Scholar]
- Endo, M.; Kimura, K.; Udagawa, T.; Tanabe, S.; Seto, H. The effects of doping molybdenum wire with rare-earth elements. High Temp.-High Press. 1990, 22, 129–137. [Google Scholar]
- Bianco, R.; Buckman, R.J. Mechanical Properties of Oxide Dispersion Strengthened (ODS) Molybdenum Alloys; Bettis Atomic Power Lab.: West Mifflin, PA, USA, 1998. [Google Scholar]
- Volmer, M.; Weber, A. Keimbildung in übersättigten Gebilden. Z. Phys. Chem. 1926, 119, 277–301. [Google Scholar] [CrossRef]
- Rhonira, L.; Aziz, M.; Yeop Majlis, B. Control of physical and microstructural properties in molybdenum by direct current magnetron sputtering deposition producing bilayer thin film. Thin Solid Films 2018, 665, 17–28. [Google Scholar] [CrossRef]
- Priyadarshi, A. Mo/La2O3 Nano Multilayers and Their Thermal Stability and Electrical Properties. Master’s Thesis, IFW Dresden and Indian Institute of Technology Roorkee, Dresden, Germany, 2018. [Google Scholar]
- Oswald, S.; Lattner, E.; Seifert, M.; Menzel, S. AES and XPS depth profiling of annealed AlN/TiAl/AlN films for high temperature applications in SAW metallization. Surf. Interface Anal. 2018. [Google Scholar] [CrossRef]
- Mayadas, A.F.; Shatzkes, M. Electrical-Resistivity Model for Polycrystalline Films: The Case of Arbitrary Reflection at External Surfaces. Phys. Rev. B 1970, 1, 1382–1389. [Google Scholar] [CrossRef]
- Fuchs, K. The conductivity of thin metallic films according to the electron theory of metals. Math. Proc. Camb. Philos. Soc. 1938, 34, 100–108. [Google Scholar] [CrossRef]
- Sondheimer, E. The mean free path of electrons in metals. Adv. Phys. 1952, 1, 1–42. [Google Scholar] [CrossRef]
- Fawcett, E.; Griffiths, D. The fermi surface areas of chromium, molybdenum and tungsten. J. Phys. Chem. Solids 1962, 23, 1631–1635. [Google Scholar] [CrossRef]
- Desai, P.D.; Chu, T.K.; James, H.M.; Ho, C.Y. Electrical Resistivity of Selected Elements. J. Phys. Chem. Ref. Data 1984, 13, 1069–1096. [Google Scholar] [CrossRef]
- Aubert, T.; Elmazria, O. Stability of langasite regarding SAW applications above 800 °C in air atmosphere. In Proceedings of the 2012 IEEE International Ultrasonics Symposium, Dresden, Germany, 7–10 October 2012; pp. 2098–2101. [Google Scholar] [CrossRef]








| Parameter | Unit | Quantity | |
|---|---|---|---|
| Mo | La | ||
| DC power | W | 500 | 15 |
| Ar gas flow | sccm | 30 | |
| base pressure | mbar | ||
| pressure during deposition | mbar | ||
| substrate temperature | C | ≈25 (RT, not controlled) | |
| deposition time (nominal thickness) | s | 104 (49.5 nm) | 35 (1 nm) |
| 69 (33.0 nm) | 17 (0.5 nm) | ||
| 42 (19.8 nm) | 9 (0.25 nm) | ||
| 23 (11.0 nm) | 4 (0.125 nm) | ||
| total film thickness | nm | 100 | |
| time delay after La was deposited | min | - | 60 (1 nm) |
| 45 (0.5 nm) | |||
| 30 (0.25 nm) | |||
| 25 (0.125 nm) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzel, S.B.; Seifert, M.; Priyadarshi, A.; Rane, G.K.; Park, E.; Oswald, S.; Gemming, T. Mo-La2O3 Multilayer Metallization Systems for High Temperature Surface Acoustic Wave Sensor Devices. Materials 2019, 12, 2651. https://doi.org/10.3390/ma12172651
Menzel SB, Seifert M, Priyadarshi A, Rane GK, Park E, Oswald S, Gemming T. Mo-La2O3 Multilayer Metallization Systems for High Temperature Surface Acoustic Wave Sensor Devices. Materials. 2019; 12(17):2651. https://doi.org/10.3390/ma12172651
Chicago/Turabian StyleMenzel, Siegfried B., Marietta Seifert, Abhinav Priyadarshi, Gayatri K. Rane, Eunmi Park, Steffen Oswald, and Thomas Gemming. 2019. "Mo-La2O3 Multilayer Metallization Systems for High Temperature Surface Acoustic Wave Sensor Devices" Materials 12, no. 17: 2651. https://doi.org/10.3390/ma12172651