Enhanced Multiferroic Properties of YFeO3 by Doping with Bi3+
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Structural Characterization
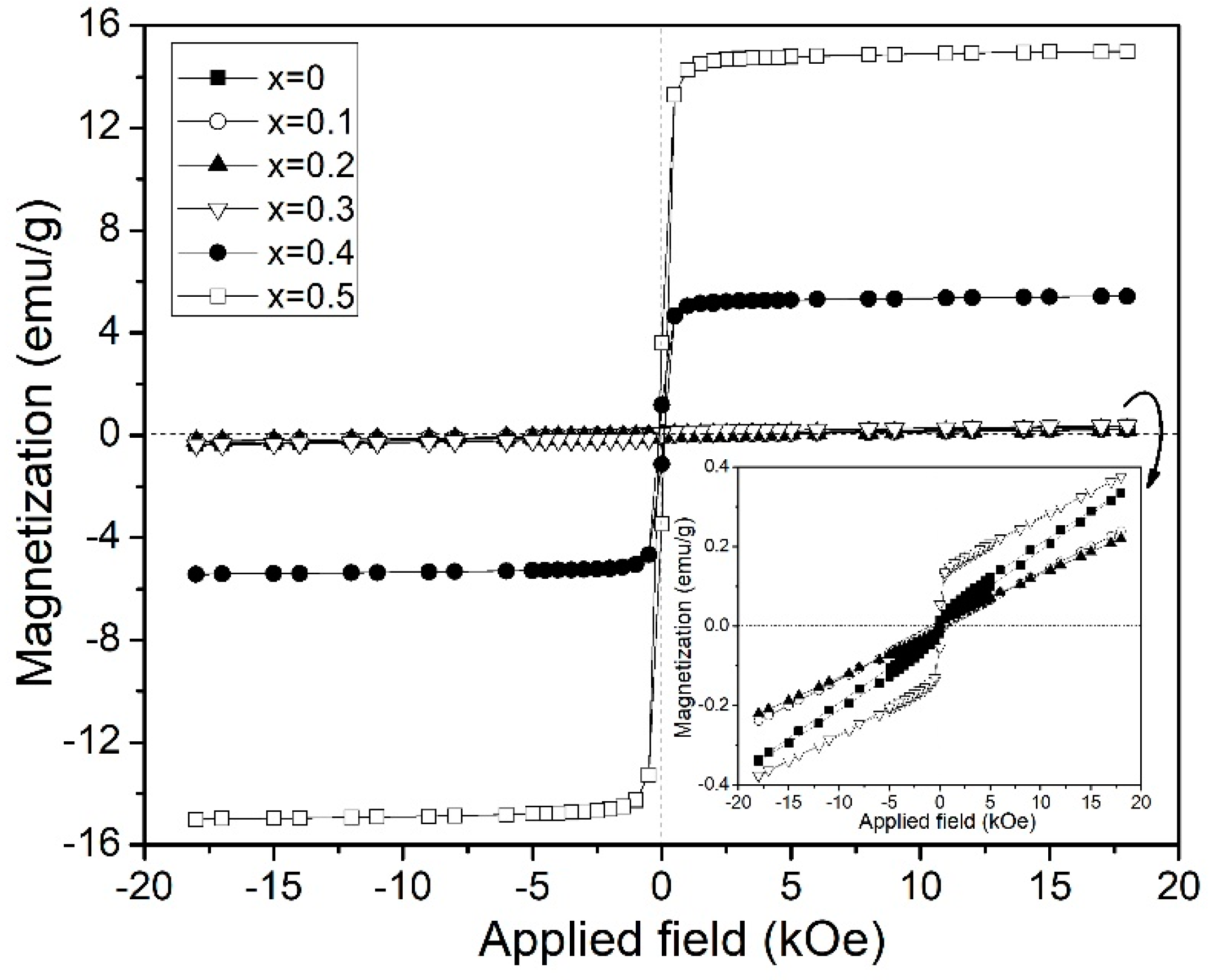
3.2. Magnetic Behavior
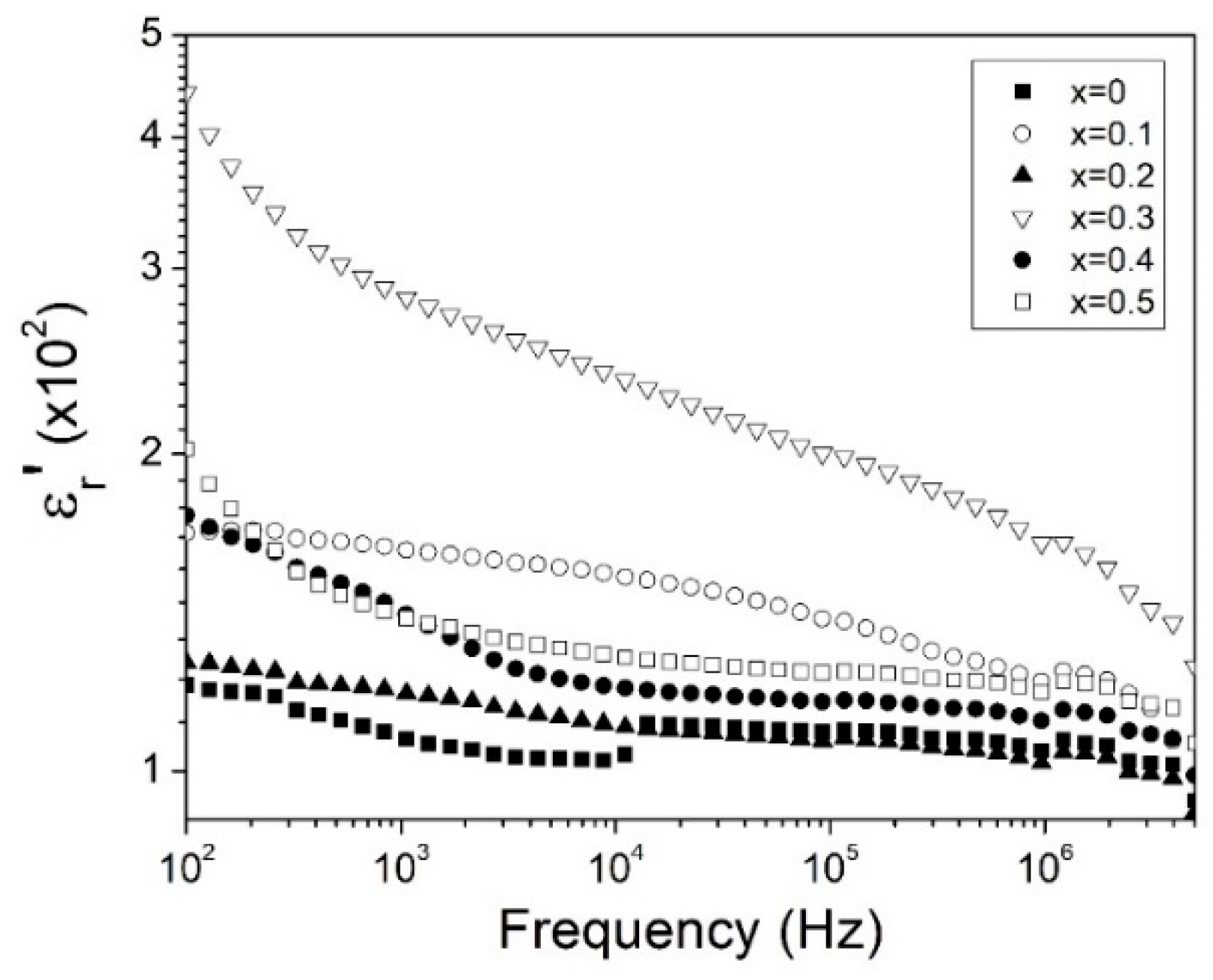
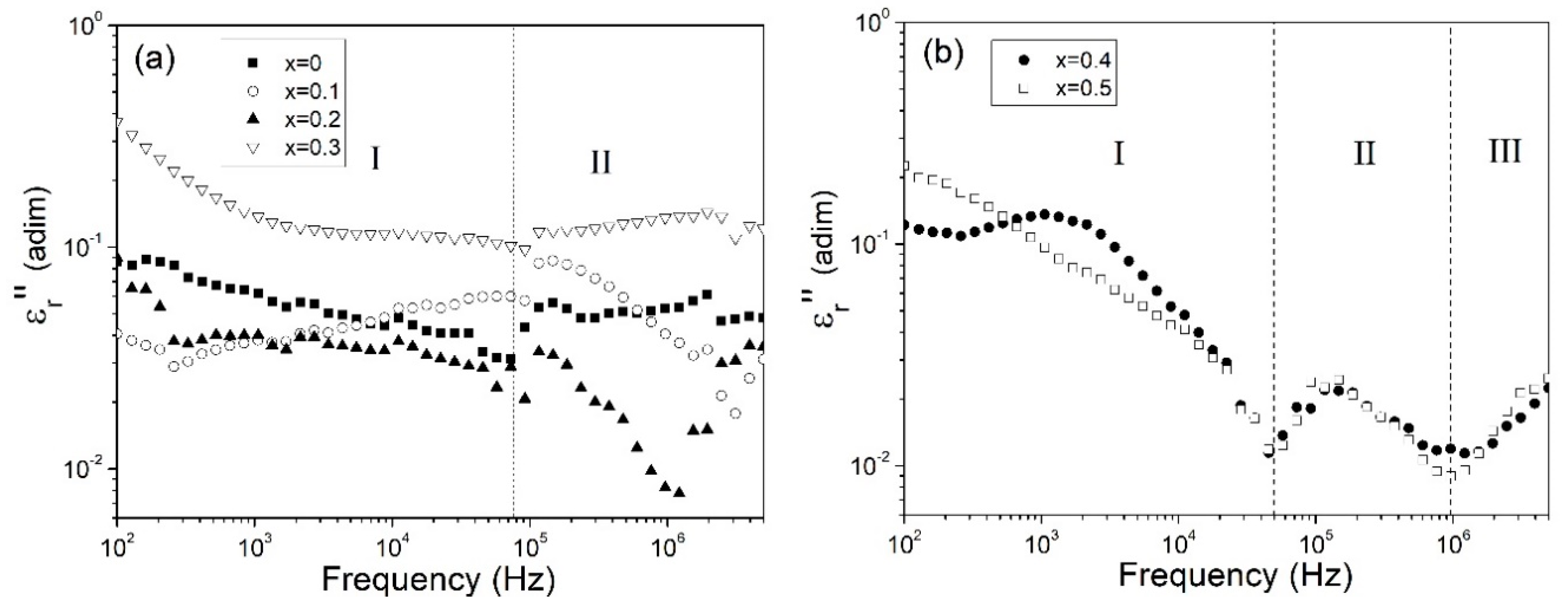
3.3. Dielectric Behavior
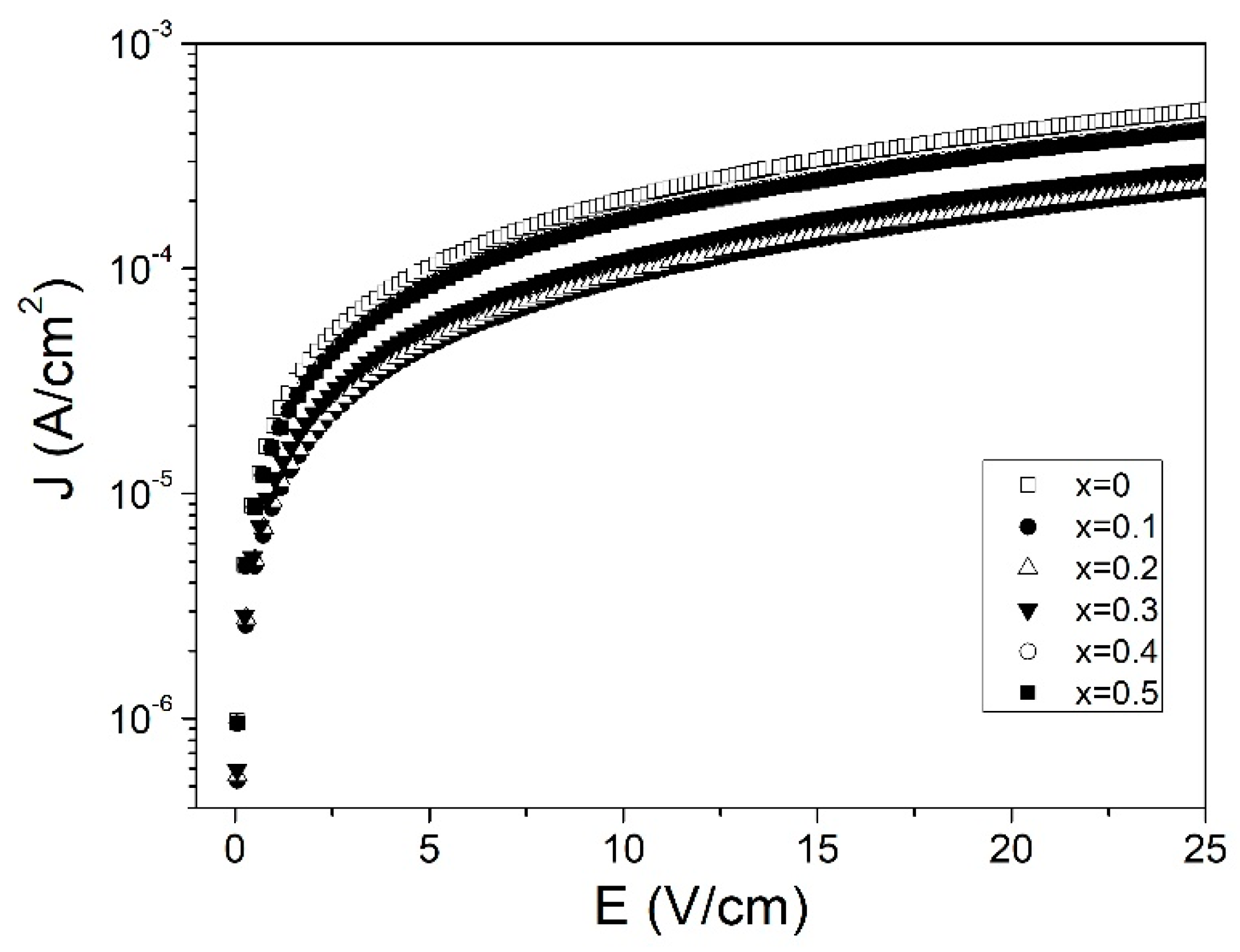
3.4. Conductivity and Leakage Current
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spaldin, N.A. Multiferroics: Past, present, and future. MRS Bull. 2017, 42, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Zhang, Z.; Wang, X.; Ouyang, J.; Feng, Z.; Su, Z.; Chen, Y.; Harris, V.G. Room temperature magnetoelectric effect of YFeO3 –Y3Fe5O12 ferrite composites. J. Alloy. Compd. 2016, 656, 465–469. [Google Scholar] [CrossRef]
- Deka, B.; Ravi, S.; Perumal, A.; Pamu, D. Effect of Mn doping on magnetic and dielectric properties of YFeO3. Ceram. Int. 2017, 43, 1323–1334. [Google Scholar] [CrossRef]
- Stoeffler, D.; Chaker, Z. First principles study of the electronic structure and magnetic properties of YFeO3 oxide. J. Magn. Magn. Mater. 2017, 442, 255–264. [Google Scholar] [CrossRef]
- Zhang, M.; Yasui, S.; Katayama, T.; Rao, B.N.; Wen, H.; Pan, X.; Tang, M.; Ai, F.; Itoh, M. Epitaxial Growth of Orthorhombic GaFeO3 Thin Films on SrTiO3 (111) Substrates by Simple Sol-Gel Method. Materials 2019, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Pedro-García, F.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.; Barba-Pingarrón, A.; Bolarín-Miró, A. Mechanically assisted synthesis of multiferroic BiFeO3: Effect of synthesis parameters. J. Alloy. Compd. 2017, 711, 77–84. [Google Scholar] [CrossRef]
- Hasan, M.; Hakim, M.A.; Basith, M.A.; Hossain, M.S.; Ahmmad, B.; Zubair, M.A.; Hussain, A.; Islam, M.F. Size dependent magnetic and electrical properties of Ba-doped nanocrystalline BiFeO3. AIP Adv. 2016, 6, 35314. [Google Scholar] [CrossRef]
- Kao, M.C.; Chen, H.Z.; Young, S.L.; Chen, K.H.; Chiang, J.L.; Shi, J. Bin Structural, electrical, magnetic and resistive switching properties of the Multiferroic/Ferroelectric bilayer thin films. Materials 2017, 10, 1327. [Google Scholar] [CrossRef]
- Pedro-García, F.; Bolarín-Miró, A.M.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.A.; Valdez-Nava, Z.; Torres-Villaseñor, G. Stabilization of α-BiFeO3 structure by Sr2+ and its effect on multiferroic properties. Ceram. Int. 2018, 44, 8087–8093. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Song, S.-H.; Ravi, M.; Liu, R.-C.; Ji, S.-S. Effect of calcination temperature on structural, magnetic and optical properties of multiferroic YFeO3 nanopowders synthesized by a low temperature solid-state reaction. Ceram. Int. 2017, 43, 10270–10276. [Google Scholar] [CrossRef]
- Madolappa, S.; Ponraj, B.; Bhimireddi, R.; Varma, K.B.R. Enhanced magnetic and dielectric properties of Ti-doped YFeO3 ceramics. J. Am. Ceram. Soc. 2017, 100, 2641–2650. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Song, S.; Tan, M. Structure-Controllable Synthesis of Multiferroic YFeO3 Nanopowders and Their Optical and Magnetic Properties. Materials 2017, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Jiang, G. Effects of Nd, Er Doping on the Structure and Magnetic Properties of YFeO3. J. Supercond. Nov. Magn. 2017, 31, 2511–2517. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Q.; Liu, Y. Magnetic Properties and Spontaneous Polarization of La-, Mn- and N-Doped Tetragonal BiFeO3: A First-Principles Study. Materials 2018, 11, 985. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Zhang, C.; Zhang, T.; Yuan, L.; Ge, L. The multiferroic perovskite YFeO3. Appl. Phys. Lett. 2013, 102, 062903. [Google Scholar] [CrossRef]
- Chowdhury, U.; Goswami, S.; Bhattacharya, D.; Ghosh, J.; Basu, S.; Neogi, S. Room temperature multiferroicity in orthorhombic LuFeO3. Appl. Phys. Lett. 2014, 105, 052911. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, Z.; Yan, H.; Li, H.; Li, L. Dielectric relaxation, electric modulus and ac conductivity of Mn-doped YFeO3. Ceram. Int. 2016, 42, 19461–19465. [Google Scholar] [CrossRef]
- Das, I.; Chanda, S.; Dutta, A.; Banerjee, S.; Sinha, T. Dielectric relaxation of Y1−xRxFeO3 (R=Dy, Er, x=0, 0.5). J. Alloy. Compd. 2013, 571, 56–62. [Google Scholar] [CrossRef]
- Jacob, K.T.; Rajitha, G.; Dasgupta, N. Electrical conductivity of Ca-doped YFeO3. Indian J. Eng. Mater. Sci. 2012, 19, 47–53. [Google Scholar]
- Yuan, X.; Sun, Y.; Xu, M. Effect of Gd substitution on the structure and magnetic properties of YFeO3 ceramics. J. Solid State Chem. 2012, 196, 362–366. [Google Scholar] [CrossRef]
- Bolarín-Miró, A.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.; Valenzuela, R.; Ammar, S. Structure and magnetic properties of GdxY1−xFeO3 obtained by mechanosynthesis. J. Alloy. Compd. 2014, 586, S90–S94. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, G.; Yang, W.; Duan, L.; Peng, W.; Chen, J.; Wang, X. Study of Y1-xErxFeO3 (0<x<1) powder synthesized by sol–gel method and their magnetic properties. J. Magn. Magn. Mater. 2016, 417, 87–91. [Google Scholar]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R.; Schultz, A.S.; Richardson, J.W. Combined texture and structure analysis of deformed limestone from time-of-flight neutron diffraction spectra. J. Appl. Phys. 1997, 81, 594–600. [Google Scholar] [CrossRef]
- Van Hook, H.J. Phase Relations in the System Fe2O3-Fe3O4-YFeO3 in Air. J. Am. Ceram. Soc. 1961, 44, 208–214. [Google Scholar] [CrossRef]
- Van Hook, H.J. Phase Relations in the Ternary System Fe2O3-FeO-YFeO3. J. Am. Ceram. Soc. 1962, 45, 162–165. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Moure, C.; Pena, O. Recent advances in perovskites: Processing and properties. Prog. Solid State Chem. 2015, 43, 123–148. [Google Scholar] [CrossRef]
- Nagashio, K.; Kuribayashi, K. Metastable Phase Formation from an Undercooled Rare-Earth Orthoferrite Melt. J. Am. Ceram. Soc. 2002, 85, 2550–2556. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Hyde, B.G. Some structures topologically related to cubic perovskite (E21), ReO3 (D09) and Cu3Au (L12). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 3802–3813. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Rosales-González, O.; Jesús, F.S.-D.; Cortés-Escobedo, C.; Bolarín-Miró, A. Crystal structure and multiferroic behavior of perovskite YFeO3. Ceram. Int. 2018, 44, 15298–15303. [Google Scholar] [CrossRef]
- Baños-López, E.; Cortés-Escobedo, C.; Jesús, F.S.-D.; Barba-Pingarrón, A.; Bolarín-Miró, A. Crystal structure and magnetic properties of cerium-doped YIG: Effect of doping concentration and annealing temperature. J. Alloy. Compd. 2018, 730, 127–134. [Google Scholar] [CrossRef]
- Anupama, A.; Kumar, R.; Choudhary, H.K.; Sahoo, B. Synthesis of coral-shaped yttrium-aluminium-iron garnets by solution-combustion method. Ceram. Int. 2018, 44, 3024–3031. [Google Scholar] [CrossRef]
- Das, R.; Mandal, K. Magnetic, ferroelectric and magnetoelectric properties of Ba-doped BiFeO3. J. Magn. Magn. Mater. 2012, 324, 1913–1918. [Google Scholar] [CrossRef]
- Treves, D.; Eibschütz, M.; Coppens, P. Dependence of superexchange interaction on Fe3+-O2−-Fe3+ linkage angle. Phys. Lett. 1965, 18, 216–217. [Google Scholar] [CrossRef]
- Mallmann, E.J.J.; Sombra, A.S.B.; Goes, J.C.; Fechine, P.B.A. Yttrium Iron Garnet: Properties and Applications Review. Solid State Phenom. 2013, 202, 65–96. [Google Scholar] [CrossRef]
- Baños, E.; Sánchez-De Jesús, F.; Cortés, C.A.; Bolarin-Miró, A.M. Enhancement in Curie Temperature of Yttrium Iron Garnet by Doping with Neodymium. Materials 2018, 11, 1652. [Google Scholar] [Green Version]
- Elissalde, C.; Ravez, J. Ferroelectric ceramics: defects and dielectric relaxations. J. Mater. Chem. 2001, 11, 1957–1967. [Google Scholar] [CrossRef]
- Betancourt-Cantera, L.; Bolarín-Miró, A.M.; Cortés-Escobedo, C.; Hernández-Cruz, L.; Sánchez-De Jesús, F. Structural transitions and multiferroic properties of high Ni-doped BiFeO3. J. Magn. Magn. Mater. 2018, 456, 381–389. [Google Scholar] [CrossRef]
- Jabbarzare, S.; Abdellahi, M.; Ghayour, H.; Chami, A.; Hejazian, S. Mechanochemically assisted synthesis of yttrium ferrite ceramic and its visible light photocatalytic and magnetic properties. J. Alloy. Compd. 2016, 688, 1125–1130. [Google Scholar] [CrossRef]
- Hunpratub, S.; Thongbai, P.; Yamwong, T.; Yimnirun, R.; Maensiri, S. Dielectric relaxations and dielectric response in multiferroic BiFeO3 ceramics. Appl. Phys. Lett. 2009, 94, 062904. [Google Scholar] [CrossRef]
- Raut, S.; Babu, P.D.; Sharma, R.K.; Pattanayak, R.; Panigrahi, S. Grain boundary-dominated electrical conduction and anomalous optical-phonon behaviour near the Neel temperature in YFeO3 ceramics. J. Appl. Phys. 2018, 123, 174101. [Google Scholar] [CrossRef]
- Ahmad, T.; Lone, I.H.; Ansari, S.; Ahmed, J.; Ahamad, T.; AlShehri, S.M. Multifunctional properties and applications of yttrium ferrite nanoparticles prepared by citrate precursor route. Mater. Des. 2017, 126, 331–338. [Google Scholar] [CrossRef]
- Zahi, S.; Daud, A.; Hashim, M. A comparative study of nickel–zinc ferrites by sol–gel route and solid-state reaction. Mater. Chem. Phys. 2007, 106, 452–456. [Google Scholar] [CrossRef]
- Aakansha; Deka, B.; Ravi, S.; Pamu, D. Impedance spectroscopy and ac conductivity mechanism in Sm doped Yttrium Iron Garnet. Ceram. Int. 2017, 43, 10468–10477. [Google Scholar] [CrossRef]
- Hussain, S.; Hasanain, S.; Jaffari, G.H.; Shah, S.I. Thickness dependent magnetic and ferroelectric properties of LaNiO3 buffered BiFeO3 thin films. Curr. Appl. Phys. 2015, 15, 194–200. [Google Scholar] [CrossRef]
- Reddy, V.R.; Kothari, D.; Upadhyay, S.K.; Gupta, A.; Chauhan, N.; Awasthi, A. Reduced leakage current of multiferroic BiFeO3 ceramics with microwave synthesis. Ceram. Int. 2014, 40, 4247–4250. [Google Scholar] [CrossRef]
- Lubomirsky, I.; Fleig, J.; Maier, J. Modeling of space-charge effects in nanocrystalline ceramics: The influence of geometry. J. Appl. Phys. 2007, 92, 6819. [Google Scholar] [CrossRef]
- Reddy, S.S.K.; Raju, N.; Reddy, C.G.; Reddy, P.Y.; Reddy, K.R.; Gupta, S.; Reddy, V.R. Study of Mn doped multiferroic DyFeO3 ceramics. Ceram. Int. 2017, 43, 6148–6155. [Google Scholar] [CrossRef]




| Level Doped (x) | Phase Space Group | % Phase | Lattice Parameters (Å) | Crystallite Size (Å) | µε (×10−4) | χ2 | Rwp | ||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | |||||||
| 0 | YFeO3 Pnma | 100 ± 0.7230 | 5.2683 ± 0.0005 | 5.5510 ± 0.0004 | 7.5764 ± 0.0005 | 911.11 ± 56.58 | 41.41 ± 0.0001 | 1.03 | 12.04 |
| 0.1 | YFeO3 Pnma | 100 ± 0.6692 | 5.2714 ± 0.0004 | 5.5664 ± 0.0004 | 7.5845 ± 0.0005 | 929.39 ± 31.46 | 16.24 ± 0.0006 | 1.16 | 17.49 |
| 0.2 | YFeO3 Pnma | 100 ± 0.5242 | 5.2974 ± 0.0002 | 5.5854 ± 0.0002 | 7.6224 ± 0.0003 | 1270.91 ± 31.25 | 11.77 ± 0.0001 | 1.20 | 16.34 |
| 0.3 | YFeO3 Pnma | 100 ± 0.9614 | 5.3117 ± 0.0002 | 5.5879 ± 0.0003 | 7.6411 ± 0.0004 | 1492.36 ± 55.61 | 10.27 ± 0.0004 | 1.23 | 16.81 |
| 0.4 | YFeO3 Pnma | 15.97 ± 0.3432 | 5.3139 ± 0.0010 | 5.5944 ± 0.0010 | 7.6461 ± 0.0009 | 952.63 ± 22.87 | 7.23 ± 0.0002 | 1.24 | 24.59 |
| Y3Fe5O12 I-a3d | 73.67 ± 0.2156 | 12.3835 ± 0.0003 | - | - | 1819.16 ± 126.78 | 8.68 ± 0.0008 | |||
| Bi25FeO40 I-23 | 10.36 ± 0.5221 | 10.0856 ± 0.0014 | - | - | 999.91 ± 257.04 | 29.35 ± 0.0003 | |||
| 0.5 | Y3Fe5O12 I-a3d | 86.68 ± 0.3264 | 12.3931 ± 0.0003 | - | - | 2768.51 ± 293.71 | 13.64 ± 0.0004 | 1.27 | 25.25 |
| Bi25FeO40 I-23 | 13.32 ± 0.5678 | 10.1573 ± 0.0003 | - | - | 1625.94 ± 107.13 | 4.59 ± 0.0001 | |||
| Bismuth Content (x) | Elem. | Occ. | Atomic Positions | Bond Angle (Degree) | Tilt Angle (Degree) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | θ1 | θ2 | φ1 | φ2 | t (adim) | |||
| 0 | Y | 1 | 0.06399 ± 0.0004 | 0.2500 ± 0.0000 | −0.0174 ± 0.0007 | 155.16 ± 0.10 | 142.12 ± 0.80 | 15.095 | 23.424 | 0.834 |
| Fe | 1 | 0.0000 | 0.0000 | 0.5000 | ||||||
| O1 | 1 | 0.4861 ± 0.0026 | 0.2500 ± 0.0000 | 0.0779 ± 0.0028 | ||||||
| O2 | 1 | −0.2975 ± 0.0022 | −0.0701 ± 0.0017 | 0.2986 ± 0.0027 | ||||||
| 0.1 | Y | 0.9 | 0.0625 ± 0.0005 | 0.2500 ± 0.0000 | −0.0118 ± 0.0010 | 152.51 ± 0.10 | 139.29 ± 0.80 | 16.667 | 25.215 | 0.839 |
| Bi | 0.1 | 0.0625 ± 0.0005 | 0.2500 ± 0.0000 | −0.0118 ± 0.0010 | ||||||
| Fe | 1 | 0.0000 | 0.0000 | 0.5000 | ||||||
| O1 | 1 | 0.4792 ± 0.0035 | 0.2500 ± 0.0000 | 0.0852 ± 0.0036 | ||||||
| O2 | 1 | −0.2967 ± 0.0029 | −0.0815 ± 0.0018 | 0.2951 ± 0.0029 | ||||||
| 0.2 | Y | 0.8 | 0.0587 ± 0.0004 | 0.2500 ± 0.0000 | −0.0102 ± 0.0007 | 149.71 ± 0.10 | 138.54 ± 0.80 | 18.340 | 25.691 | 0.843 |
| Bi | 0.2 | 0.0587 ± 0.0004 | 0.2500 ± 0.0000 | −0.0102 ± 0.0007 | ||||||
| Fe | 1 | 0.0000 | 0.0000 | 0.5000 | ||||||
| O1 | 1 | 0.4735 ± 0.0024 | 0.2500 ± 0.0000 | 0.09329 ± 0.0024 | ||||||
| O2 | 1 | −0.2983 ± 0.0023 | −0.0813 ± 0.0014 | 0.2973 ± 0.0023 | ||||||
| 0.3 | Y | 0.7 | 0.0538 ± 0.0004 | 0.2500 ± 0.0000 | −0.0062 ± 0.0007 | 149.13 ± 0.10 | 136.63 ± 0.80 | 18.683 | 26.907 | 0.848 |
| Bi | 0.3 | 0.0538 ± 0.0004 | 0.2500 ± 0.0000 | −0.0062 ± 0.0007 | ||||||
| Fe | 1 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 | 0.5000 ± 0.0000 | ||||||
| O1 | 1 | 0.4743 ± 0.0025 | 0.2500 ± 0.0000 | 0.0955 ± 0.0023 | ||||||
| O2 | 1 | −0.2935 ± 0.0024 | −0.0914 ± 0.0013 | 0.2881 ± 0.0024 | ||||||
| Bi3+ Doping Concentration (x) | Ms at 18 kOe (emu/g) | Mr (emu/g) | Hc (Oe) | χm (×10−7 m3/kg) |
|---|---|---|---|---|
| 0 | 0.34 | 0.02- | 710- | 2.34 |
| 0.1 | 0.21 | - | - | 1.66 |
| 0.2 | 0.22 | - | - | 1.54 |
| 0.3 | 0.38 | 0.06 | 495 | - |
| 0.4 | 4.42 | 1.18 | 251 | - |
| 0.5 | 14.72 | 3.24 | 202 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales-González, O.; Sánchez-De Jesús, F.; Pedro-García, F.; Cortés-Escobedo, C.A.; Ramírez-Cardona, M.; Bolarín-Miró, A.M. Enhanced Multiferroic Properties of YFeO3 by Doping with Bi3+. Materials 2019, 12, 2054. https://doi.org/10.3390/ma12132054
Rosales-González O, Sánchez-De Jesús F, Pedro-García F, Cortés-Escobedo CA, Ramírez-Cardona M, Bolarín-Miró AM. Enhanced Multiferroic Properties of YFeO3 by Doping with Bi3+. Materials. 2019; 12(13):2054. https://doi.org/10.3390/ma12132054
Chicago/Turabian StyleRosales-González, Omar, Félix Sánchez-De Jesús, Fernando Pedro-García, Claudia Alicia Cortés-Escobedo, Màrius Ramírez-Cardona, and Ana María Bolarín-Miró. 2019. "Enhanced Multiferroic Properties of YFeO3 by Doping with Bi3+" Materials 12, no. 13: 2054. https://doi.org/10.3390/ma12132054





