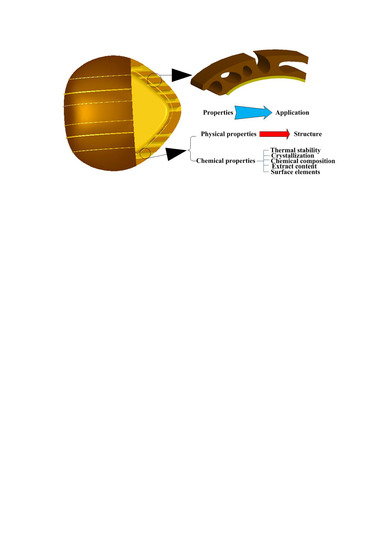
Study of Almond Shell Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Composition
2.3. Infrared Spectrum
2.4. X-ray Diffraction
2.5. Surface Element
2.6. Thermal Stability
2.7. Microscopic Morphology
3. Results and Discussion
3.1. Microscopic Morphology
3.2. Chemical Composition
3.3. Infrared Spectrum
3.4. X-ray Diffraction
3.5. X-ray Photoelectron Spectroscopy Characterization
3.6. Thermal Stability
4. Conclusions
- Observed by microscope and electron microscope, almond shells have the diameter of 300–500 μm for large holes, and 40–60 μm for small holes.
- The elements of almond shells include C (72.27%), O (22.88%), N (3.87%), and Si (0.87%). The main chemical constituents of cellulose, hemicellulose, and lignin account for 38.48%, 28.82% and 29.54%.
- The alkaline extract content of almond shells was 14.03%, and benzene alcohol extraction was 8.00%. The benzene alcohol extractives of almond shells mainly contain 17 types of organic compound including benzene ring, methylene, carbon three bond, and other multi-functional groups.
- Thermogravimetric analysis shows almond shells mainly lose weight at 260 °C and 335 °C. This shows that its pyrolysis temperature is lower than poplar; however, it is higher than pistachio shells and cashew nut shells.
- X-ray diffraction spectra showed that diffraction peaks of almond shells appeared near 16.6°, 21.48° and 34.67°. Cellulose crystals belong to cellulose I type. The crystallization of almond shells is 32.88%, which is lower than poplar; however, it is higher than chestnut shells, peanut shells, melon shells, macadamia shells, and walnut shells.
- X-ray photoelectron spectroscopy showed that almond shells contained more N than poplar and five other kinds of nut shell (chestnut shells, peanut shells, sunflower shells, Hawaii nut shells, and walnut shells). There was less Si in the almond shells compared to poplar.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Guo, S.; Xia, H.; Zhang, L.; Zhang, L.; Peng, J. Study on preparation of activated carbon from Hawaii nut shell via steam physical activation. In 7th International Symposium on High-Temperature Metallurgical Processing; Hwang, J.Y., Jiang, T., Pistorius, P.C., Alvear, G.R.F., Yücel, O., Cai, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 575–582. [Google Scholar] [CrossRef]
- Martínez, M.L.; Torres, M.M.; Guzmán, C.A.; Maestri, D.M. Preparation and characteristics of activated carbon from olive stones and walnut shells. Ind. Crops Prod. 2006, 23, 23–28. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Okutucu, C.; Duman, G.; Ucar, S.; Yasa, I.; Yanik, J. Production of fungicidal oil and activated carbon from pistachio shell. J. Anal. Appl. Pyrolysis 2011, 91, 140–146. [Google Scholar] [CrossRef]
- Wongcharee, S.; Aravinthan, V.; Erdei, L.; Sanongraj, W. Use of macadamia nut shell residues as magnetic nanosorbents. Int. Biodeterior. Biodegrad. 2017, 124, 276–287. [Google Scholar] [CrossRef]
- Pino, G.H.; Souza De Mesquita, L.M.; Torem, M.L.; Saavedra Pinto, G.A. Biosorption of cadmium by green coconut shell powder. Miner. Eng. 2006, 19, 380–387. [Google Scholar] [CrossRef]
- Alsaadi, M.; Erkliğ, A.; Albu-khaleefah, K. Effect of pistachio shell particle content on the mechanical properties of polymer composite. Arab. J. Sci. Eng. 2018, 43, 4689–4696. [Google Scholar] [CrossRef]
- Bae, J.; Su, S. Macadamia nut shell-derived carbon composites for post combustion CO2 capture. Int. J. Greenh. Gas Control 2013, 19, 174–182. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Barley husk and coconut shell reinforced polypropylene composites: The effect of fibre physical, chemical and surface properties. Compos. Sci. Technol. 2010, 70, 840–846. [Google Scholar] [CrossRef]
- Ayrilmis, N. Waste chestnut shell as a source of reinforcing fillers for polypropylene composites. J. Thermoplast. Compos. Mater. 2014, 27, 1054–1064. [Google Scholar] [CrossRef]
- Kasiraman, G.; Nagalingam, B.; Balakrishnan, M. Performance, emission and combustion improvements in a direct injection diesel engine using cashew nut shell oil as fuel with camphor oil blending. Energy 2012, 47, 116–124. [Google Scholar] [CrossRef]
- Bartocci, P.; Bidini, G.; Saputo, P.; Fantozzi, F. Biochar pellet carbon footprint. Chem. Eng. Trans. 2016, 50, 217–222. [Google Scholar] [CrossRef]
- Wang, L.; Buvarp, F.; Skreiberg, O.; Bartocci, P.; Fantozzi, F. A study on densification and CO2 gasification of biocarbon. Chem. Eng. Trans. 2018, 65, 145–150. [Google Scholar] [CrossRef]
- Ebringerová, A.; Hromádková, Z.; Zuzana, K.; Sasinková, V. Chemical valorization of agricultural by-products: Isolation and characterization of xylan-based antioxidants from almond shell biomass. Bioresources 2007, 3, 60–70. [Google Scholar]
- Rodríguez-Reinoso, F.; López-González, J.D.D.; Berenguer, C. Activated carbons from almond shells—II: Characterization of the pore structure. Carbon 1984, 22, 13–18. [Google Scholar] [CrossRef]
- Toles, C.A.; Marshall, W.E.; Johns, M.M.; Wartelle, L.H.; McAloon, A. Acid-activated carbons from almond shells: Physical, chemical and adsorptive properties and estimated cost of production. Bioresour. Technol. 2000, 71, 87–92. [Google Scholar] [CrossRef]
- Senturk, H.B.; Ozdes, D.; Duran, C. Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent. Desalination 2010, 252, 81–87. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Singh, V.K.; Alexandre-Franco, M.; Pittman, C.U. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. CHEM ENG J 2011, 172, 1111–1125. [Google Scholar] [CrossRef]
- Essabir, H.; Nekhlaoui, S.; Malha, M.; Bensalah, M.O.; Arrakhiz, F.Z.; Qaiss, A.; Bouhfid, R. Bio-composites based on polypropylene reinforced with almond shells particles: Mechanical and thermal properties. Mater. Des. 2013, 51, 225–230. [Google Scholar] [CrossRef]
- Lashgari, A.; Eshghi, A.; Farsi, M. A study on some properties of polypropylene based nanocomposites made using almond shell flour and organoclay. Asian J. Chem. 2013, 25, 1043–1049. [Google Scholar] [CrossRef]
- Caballero, J.A.; Font, R.; Marcilla, A. Comparative study of the pyrolysis of almond shells and their fractions, holocellulose and lignin. Thermochim. Acta 1996, 276, 57–77. [Google Scholar] [CrossRef]
- Elleuch, A.; Boussetta, A.; Yu, J.; Halouani, K.; Li, Y. Experimental investigation of direct carbon fuel cell fueled by almond shell biochar: Part I. Physico-chemical characterization of the biochar fuel and cell performance examination. Int. J. Hydrog. Energy 2013, 38, 16590–16604. [Google Scholar] [CrossRef]
- Sindhu, R.; Kuttiraja, M.; Binod, P.; Sukumaran, R.K.; Pandey, A. Physicochemical characterization of alkali pretreated sugarcane tops and optimization of enzymatic saccharification using response surface methodology. Renew. Energy 2014, 62, 362–368. [Google Scholar] [CrossRef]
- Xinyuan, J.; Yuanyuan, L.; Zhong, G.; An, M.; Zecai, H.; Suwen, Y. Pyrolysis characteristics and correlation analysis with the major components of seven kinds of nutshell. Scientia Silvae Sinicae 2015, 51, 79–86. [Google Scholar] [CrossRef]
- Wood Chemistry-Wood Extracts. Available online: https://wenku.baidu.com/view/ee830fe9524de518964b7d61.html (accessed on 13 September 2018). (In Chinese).
- Jian, L. Wood Spectroscopy; Science Press: Beijing, China, 2003. [Google Scholar]
- Hara, A.; Murakami, S.; Kitahara, K. Semiconductor Device with Polysilicon Layer of Good Crystallinity and Its Manufacture Method. U.S. Patent 5,970,369, 19 October 1999. [Google Scholar]
- Hu-xia, Y.; Yan, X.; Xing, F. Analysis on Element Composition Cellulose Content and Crystallinity of the Common Nut Shells. J. Anhui AGRI 2016, 44, 21–23. (In Chinese) [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]






| Sample | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| Almond shells | 38.47 ± 0.39 | 28.82 ± 0.25 | 29.54 ± 0.11 |
| Poplar | 44.12 ± 0.23 | 30.21 ± 0.11 | 21.24 ± 0.31 |
| Coconut shells | 34.12 ± 0.20 | 22.36 ± 1.47 | 28.04 ± 0.57 |
| Walnut shells | 36.38 ± 0.05 | 27.85 ± 0.31 | 43.70 ± 0.57 |
| Chestnut shells | 21.47 ± 0.27 | 16.28 ± 0.35 | 36.58 ± 0.26 |
| Pistachio shells | 43.08 ± 0.19 | 25.30 ± 0.46 | 16.33 ± 0.41 |
| Extraction Method | Almond Shells Extractive Content | Poplar Extractive Content |
|---|---|---|
| Cold water extraction | 3.14 | 0.11 |
| Hot water extraction | 4.64 | 8.64 |
| NaOH (1%) extraction | 14.03 | 20.56 |
| Benzene alcohol extraction | 8.00 | 7.50 |
| Chemical Compound | Molecular Formula | Structural Formula | Chemical Compound | Molecular Formula | Structural Formula |
|---|---|---|---|---|---|
| Cyclopentane, ethyl- | C7H14 |  | Cyclohexane,1,2-dimethyl-, cis- | C8H16 |  |
| Toluene | C7H8 |  | Cyclohexane, ethyl- | C8H16 |  |
| Cyclohexane, 1,3-dimethyl-, cis- | C8H16 |  | Cyclohexane, 1,1,3-trimethyl | C9H18 |  |
| Cyclohexane, 1,1-dimethyl- | C8H16 |  | Ethylbenzene | C8H10 |  |
| Cyclopentane,1-ethyl-3-methyl- | C8H16 |  | Cis-bicyclo[4.2.0]octane | C8H14 |  |
| (Z)-Hex-3-enyl (E)-2-methylbut-2-enoate | C11H18O2 |  | Hexane,3-methyl-4-methylene- | C8H16 |  |
| Cyclohexane,1,3-dimethyl-, cis- | C8H16 |  | Cyclohexane, ethenyl- | C8H14 |  |
| Butanoicacid,2-methyl-,1,2-dimethylpropyl ester | C10H20O2 |  | Cyclopentane, (1-methylethyl)- | C8H16 |  |
| Cyclohexane,1,4-dimethyl- | C8H16 |  |
| Wavenumber (cm−1) | Functional Group | Vibration Type | Cause |
|---|---|---|---|
| 3300~3500 | –OH | stretching vibration | cellulose, hemicellulose |
| 2900~2935 | –CH | stretching vibration | - |
| 1640~1735 | C=O | stretching vibration | lignin, hemicellulose |
| 1580~1605 | benzene ring | stretching vibration | lignin |
| 1455~1465 | –CH3O | stretching vibration | lignin |
| 1320~1430 | –CH | bending vibration | - |
| 1221~1230 | C–C C–O | stretching vibration | lignin |
| 1025~1035 | C–O | stretching vibration | cellulose, hemicellulose, and lignin |
| 885~895 | R2C=CH2 | bending vibration | - |
| 810~833 | benzene ring | disubstituted benzene | - |
| Sample Type | n C | n O | n si | n N |
|---|---|---|---|---|
| Chestnut shells | 80.26 | 17.28 | 0.85 | 1.61 |
| Peanut shells | 74.16 | 21.72 | 0.34 | 3.78 |
| Sunflower shells | 78.86 | 18.13 | 0.42 | 2.59 |
| Hawaii nut shells | 79.16 | 18.92 | 0.26 | 1.67 |
| Walnut shell | 78.84 | 19.32 | 0.16 | 1.67 |
| Poplar | 74.56 | 20.93 | 4.51 | - |
| Almond shells | 72.27 | 22.88 | 0.87 | 3.87 |
| Sample | Binding Energy (eV) | A (%) | ||||
|---|---|---|---|---|---|---|
| C1 1s | C2 1s | C3 1s | C1 1s | C2 1s | C3 1s | |
| Almond shells | 284.38 | 286.08 | 287.79 | 55.88 | 32.35 | 11.76 |
| Chestnut shells | 284.8 | 286.28 | 286.93 | 63.18 | 17.70 | 19.12 |
| Peanut shells | 284.8 | 286.24 | 286.79 | 48.93 | 16.34 | 34.73 |
| Sunflower shells | 284.8 | 286.25 | 288.07 | 61.99 | 25.94 | 12.07 |
| Hawaii nut shells | 284.8 | 286.25 | 287.70 | 54.58 | 30.85 | 14.57 |
| Walnut shells | 284.8 | 286.25 | 287.10 | 55.22 | 23.17 | 21.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Hao, J.; Wang, W. Study of Almond Shell Characteristics. Materials 2018, 11, 1782. https://doi.org/10.3390/ma11091782
Li X, Liu Y, Hao J, Wang W. Study of Almond Shell Characteristics. Materials. 2018; 11(9):1782. https://doi.org/10.3390/ma11091782
Chicago/Turabian StyleLi, Xuemin, Yinan Liu, Jianxiu Hao, and Weihong Wang. 2018. "Study of Almond Shell Characteristics" Materials 11, no. 9: 1782. https://doi.org/10.3390/ma11091782