Effect of Ti Transition Layer Thickness on the Structure, Mechanical and Adhesion Properties of Ti-DLC Coatings on Aluminum Alloys
Abstract
:1. Introduction
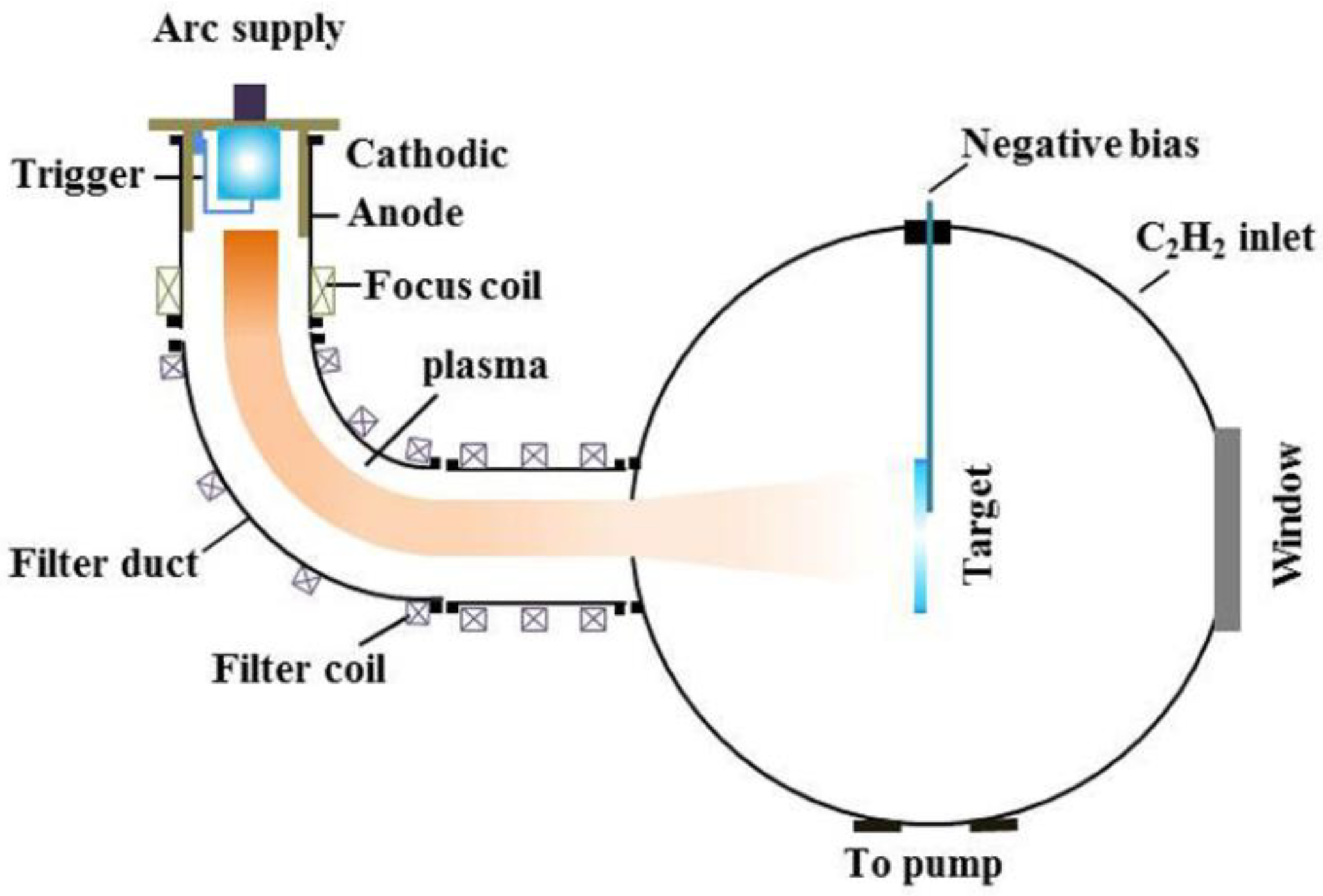
2. Experimental Details
2.1. Ti-DLC Coating Deposition
2.2. Ti-DLC Coating Characterization
3. Results and Discussion
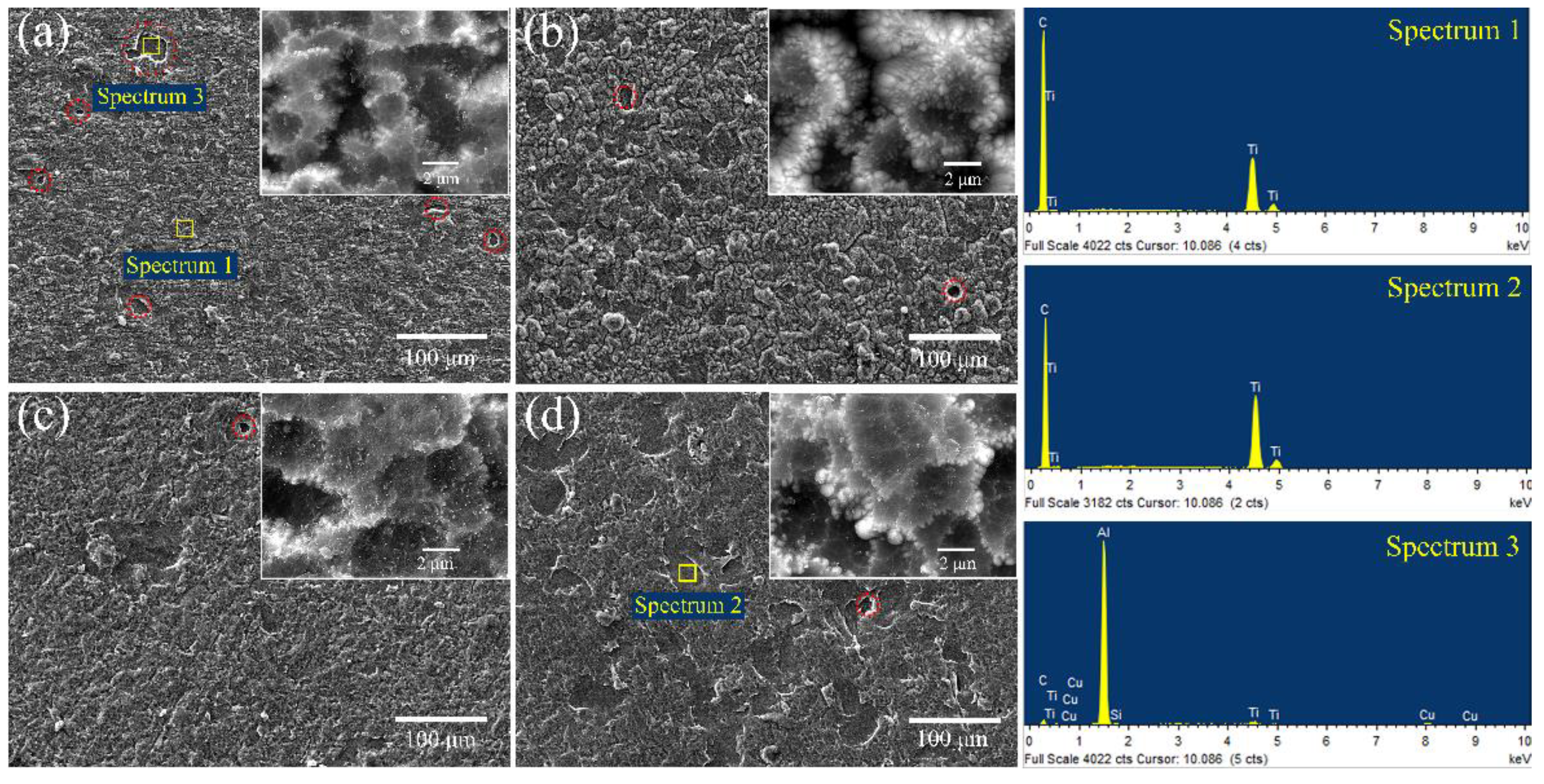
3.1. Morphology Analysis of Ti-DLC Coating
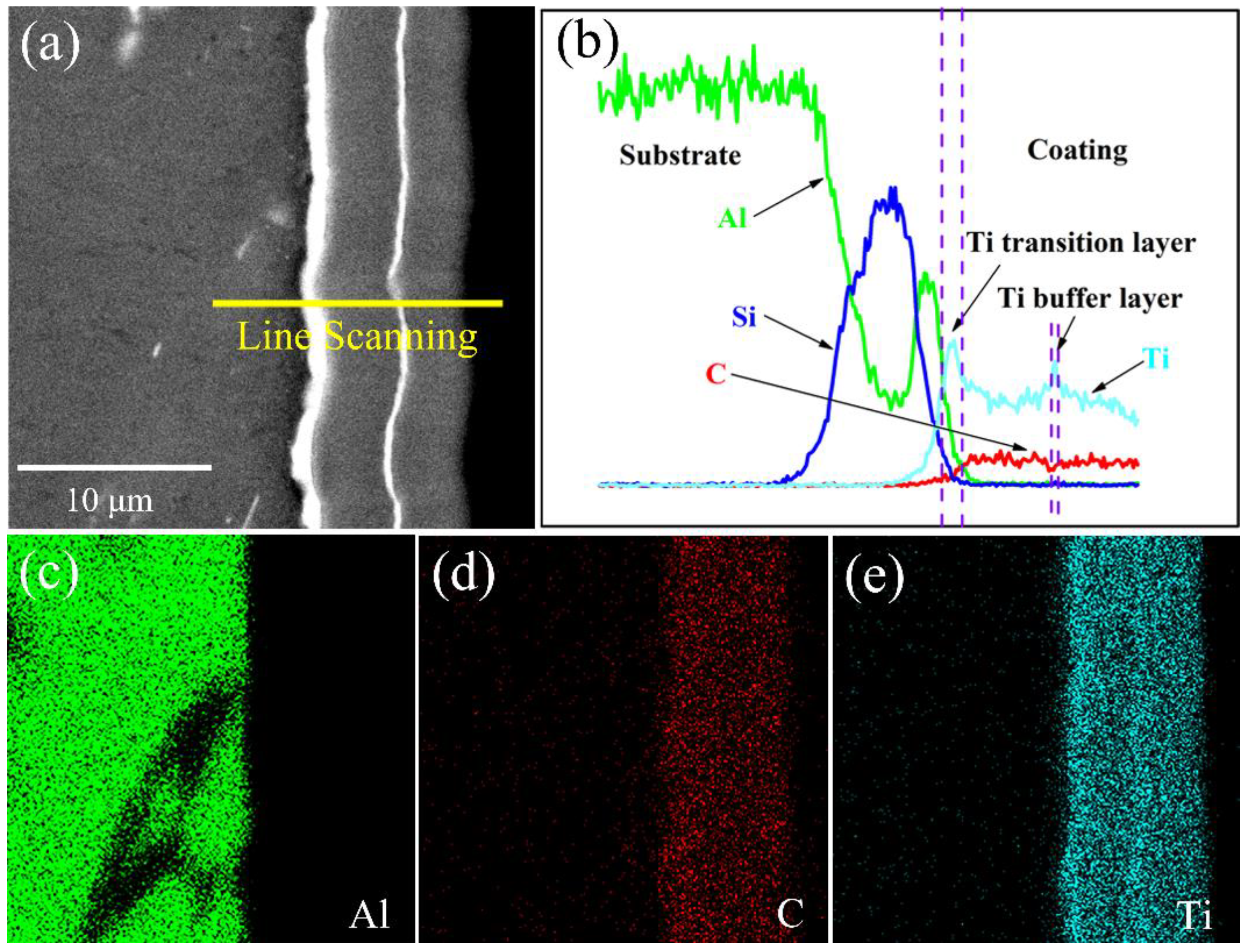
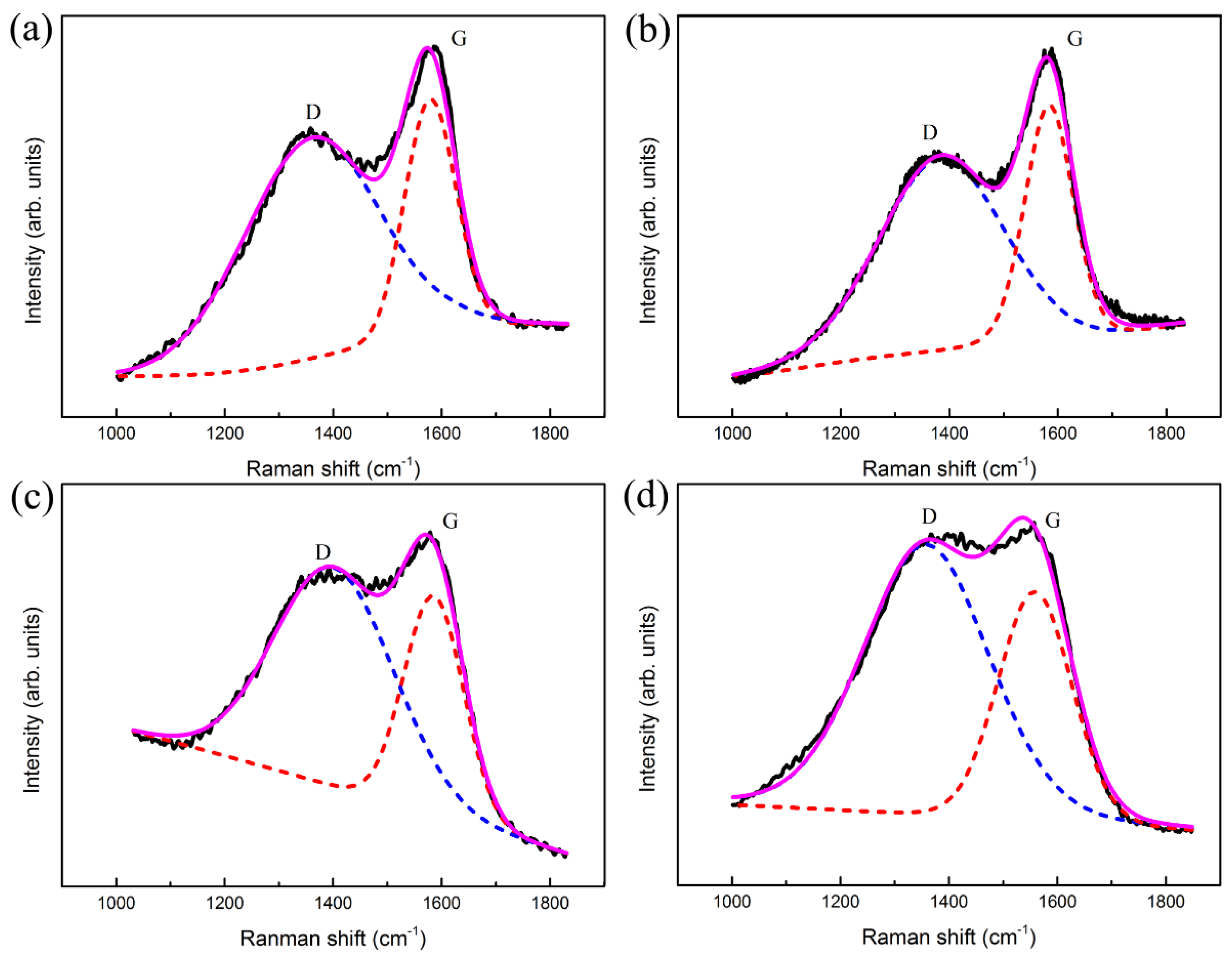
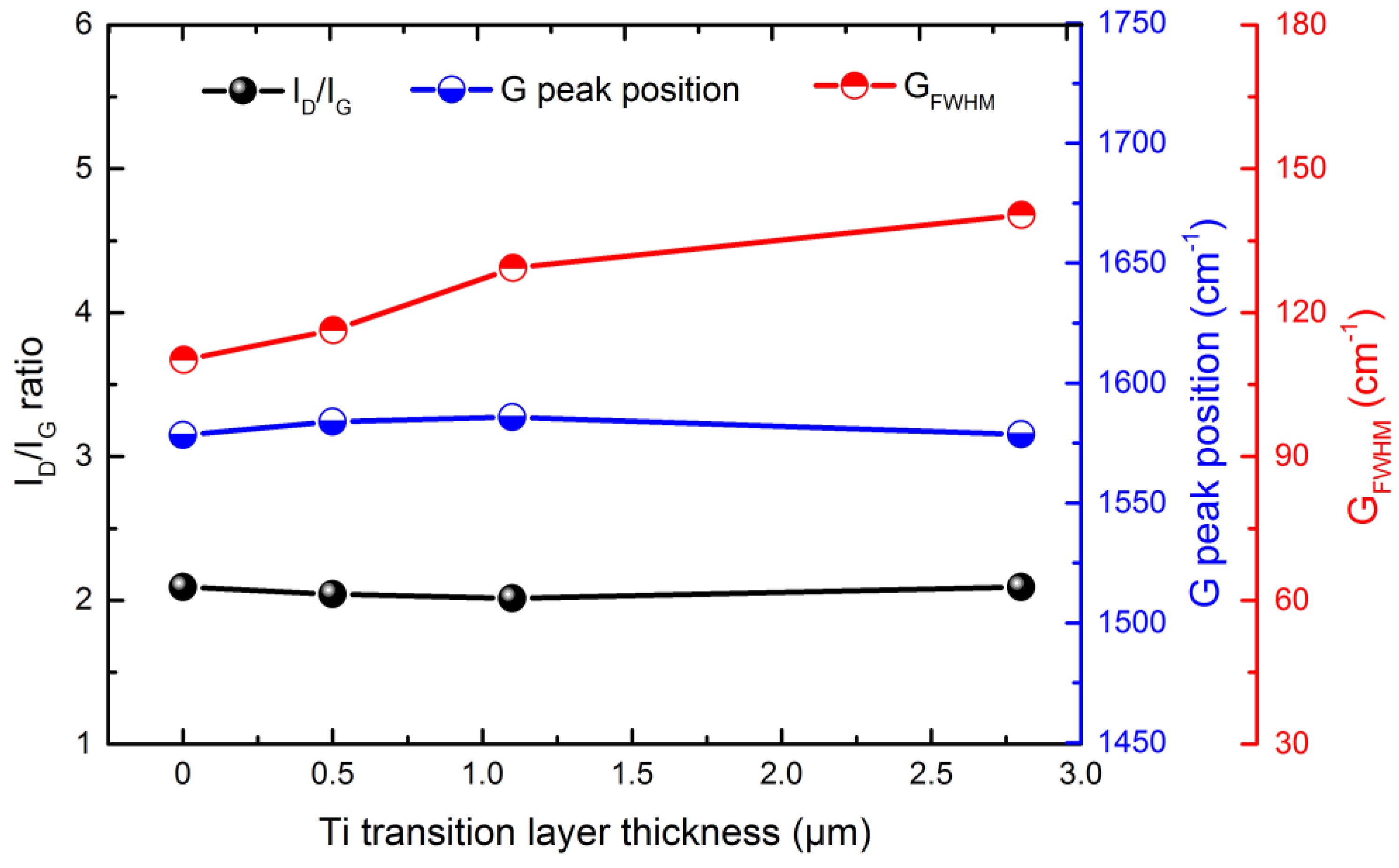
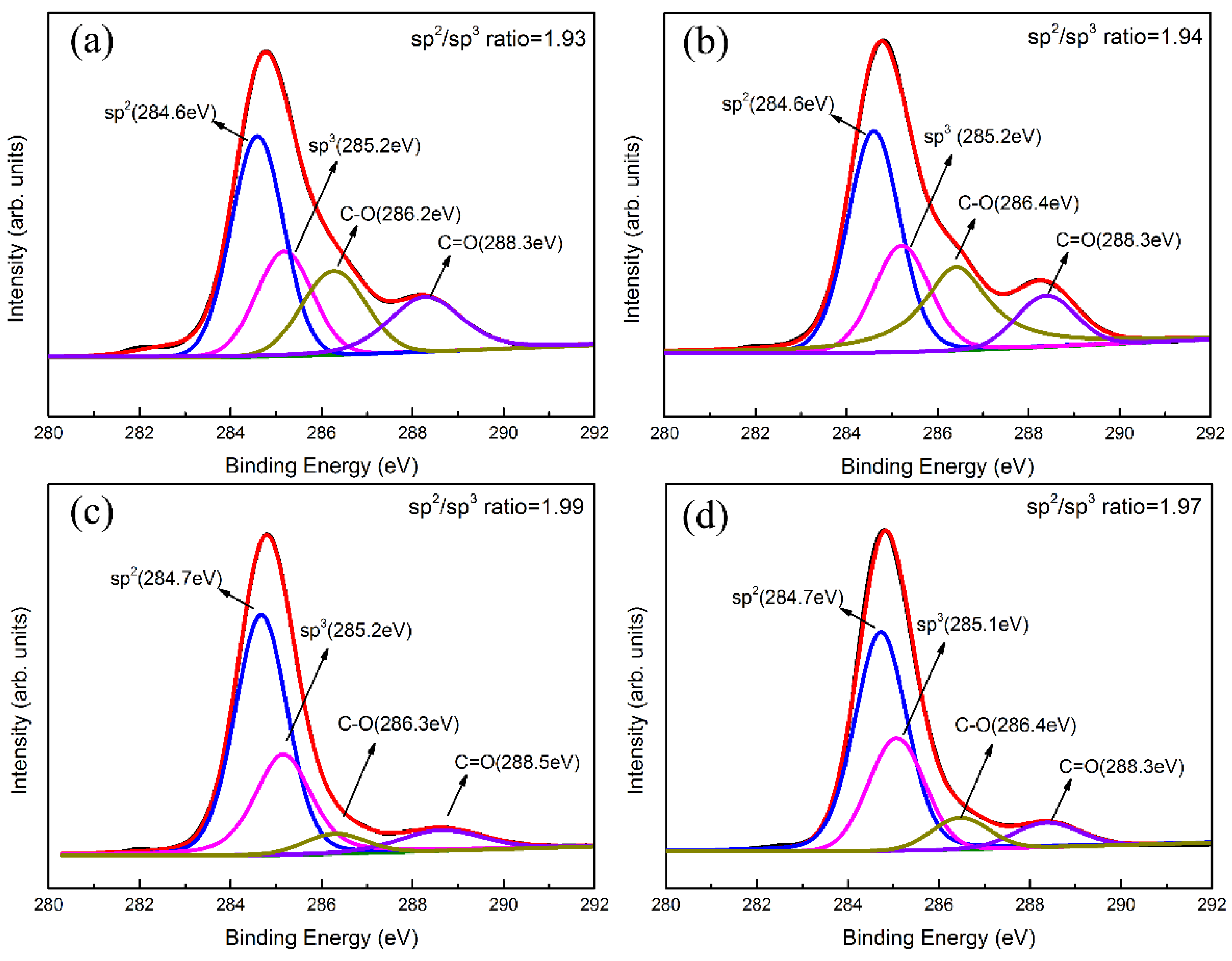
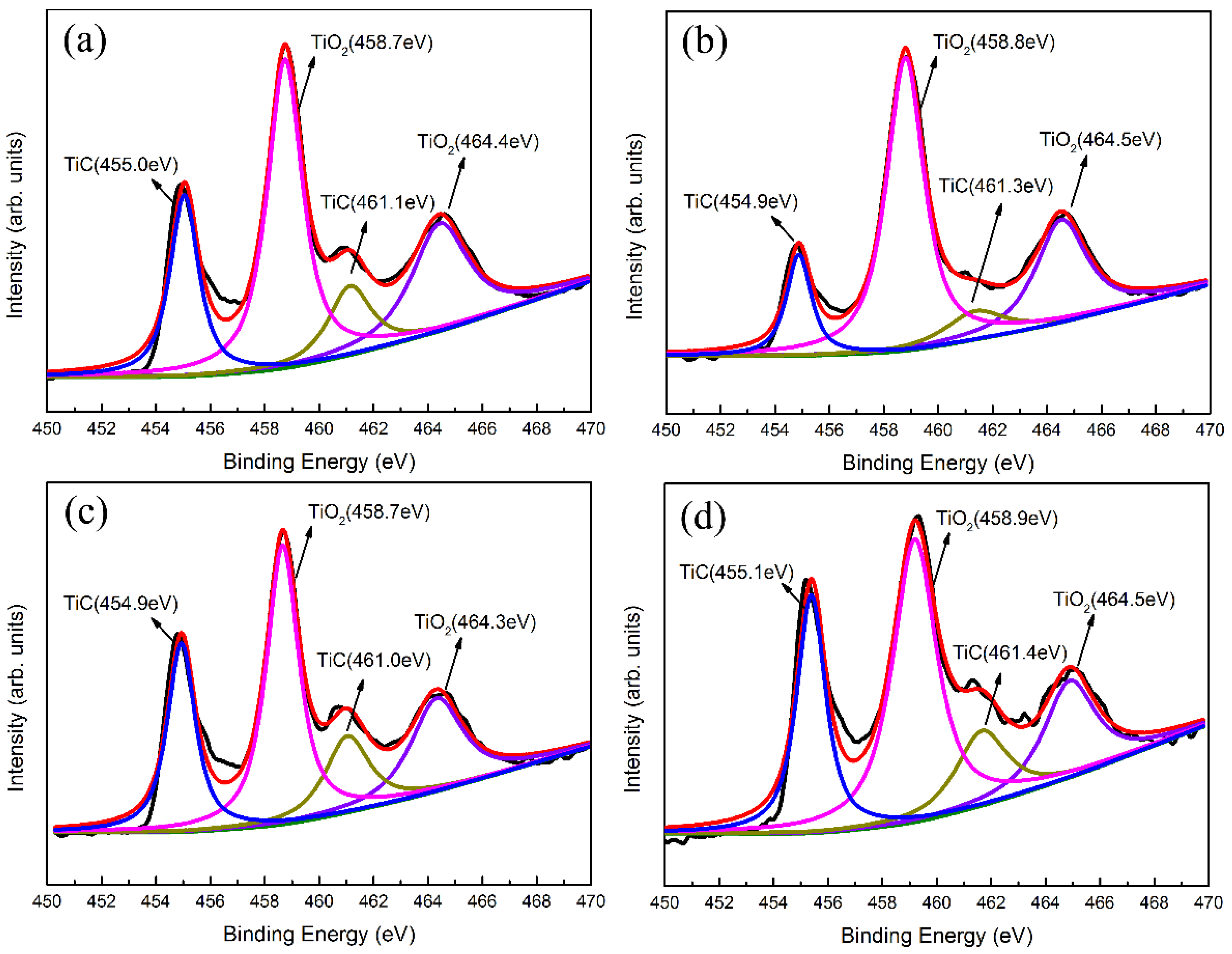
3.2. Composition and Structure Analysis of Ti-DLC Coating
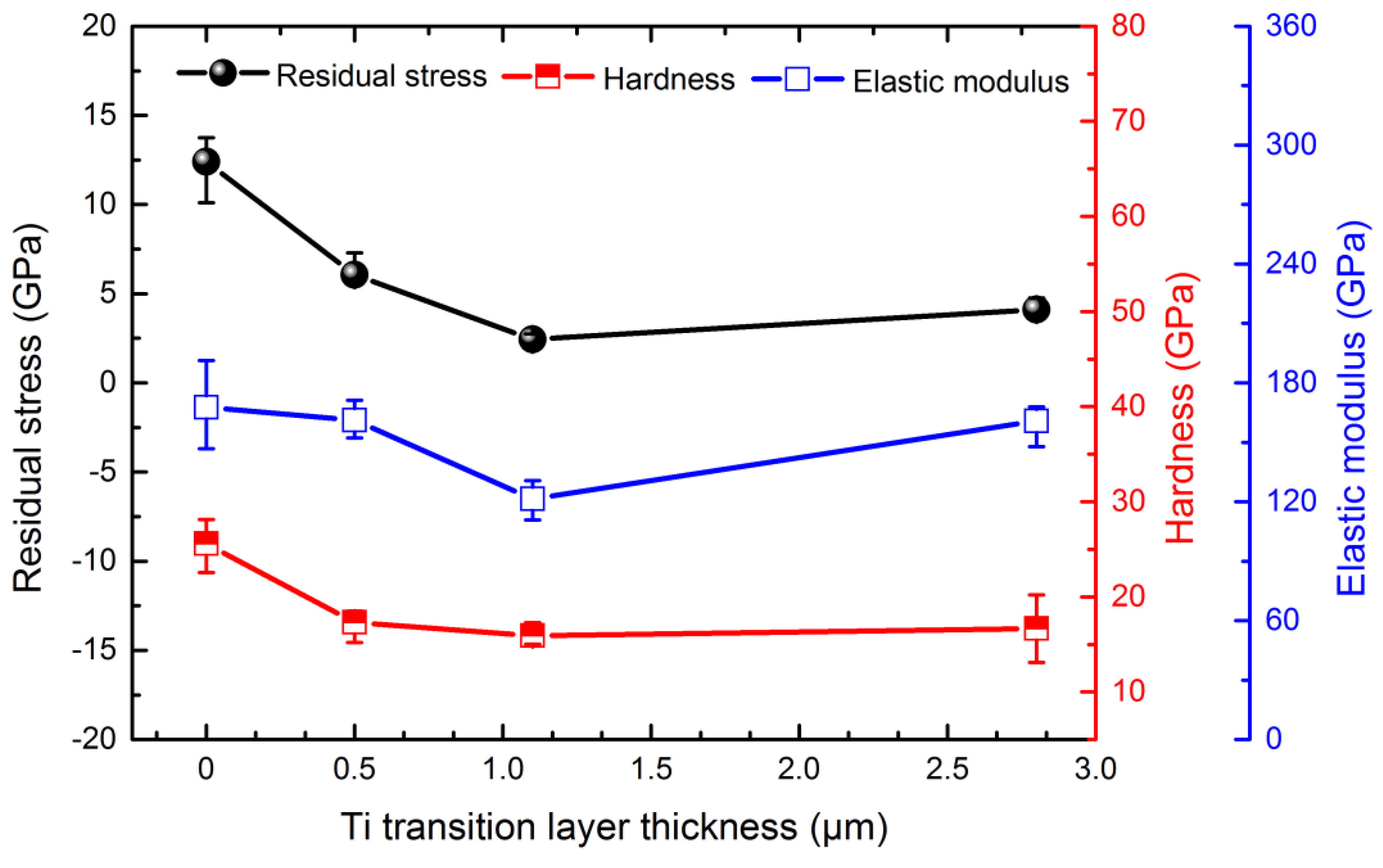
3.3. Effect of Ti Transition Layer Thickness on Mechanical Properties of Ti-DLC Coating
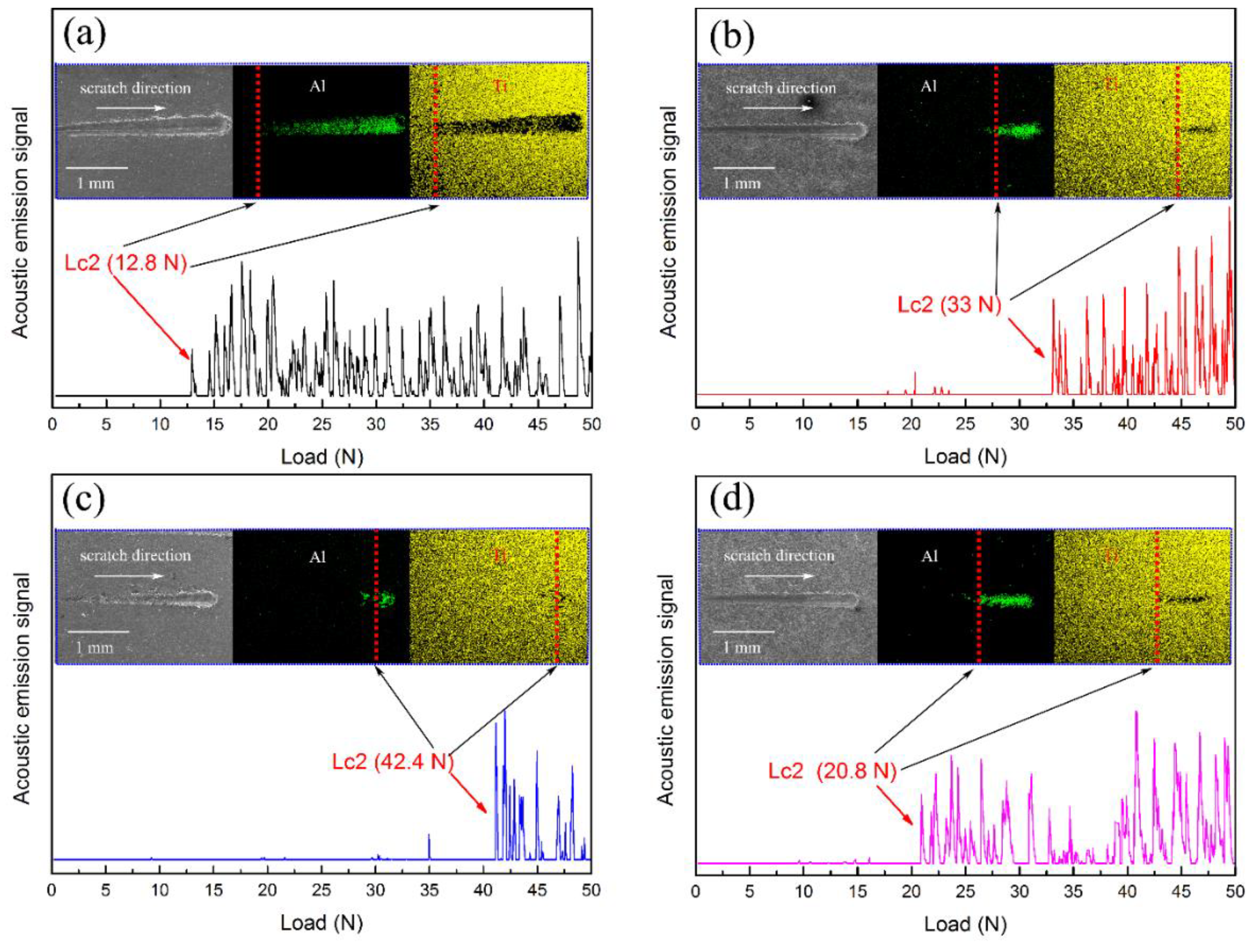
3.4. Effect of Ti Transition Layer Thickness on Adhesion of Ti-DLC Coating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashby, M.F.; Bréchet, Y.J.M.; Cebon, D.; Salvo, L. Selection strategies for materials and processes. Mater. Des. 2004, 25, 51–67. [Google Scholar] [CrossRef]
- Wang, L.; Wan, S.; Wang, S.C.; Wood, R.J.K.; Xue, Q.J. Gradient DLC-based nanocomposite coatings as a solution to improve tribological performance of aluminum alloy. Tribol. Lett. 2010, 38, 155–160. [Google Scholar] [CrossRef]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Gangopadhyay, A.K.; Willermet, P.A.; Vassell, W.C.; Tamor, M.A. Amorphous hydrogenated carbon films for tribological applications II. Films deposited on aluminium alloys and steel. Tribol. Int. 1997, 30, 19–31. [Google Scholar] [CrossRef]
- Ensinger, W.; Lensch, O.; Matsutani, T.; Kiuchi, M. Corrosion performance of thin amorphous carbon films on aluminum formed by ion beam-based coating techniques. Surf. Coat. Technol. 2005, 196, 231–235. [Google Scholar] [CrossRef]
- Malaczynski, G.W.; Hamdi, A.H.; Elmoursi, A.A.; Qiu, X. Diamond-like carbon coating for aluminum 390 alloy—Automotive applications. Surf. Coat. Technol. 1997, 93, 280–286. [Google Scholar] [CrossRef]
- Rangel, R.C.C.; Souza, M.E.P.; Schreiner, W.H.; Freire, C.M.A.; Rangel, E.C.; Cruz, N.C. Effect of the fluorination of dlc film on the corrosion protection of aluminum alloy (aa 5052). Surf. Coat. Technol. 2010, 204, 3022–3028. [Google Scholar] [CrossRef]
- Zou, Y.S.; Zhou, K.; Wu, Y.F.; Yang, H.; Cang, K.; Song, G.H. Structure, mechanical and tribological properties of diamond-like carbon films on aluminum alloy by arc ion plating. Vacuum 2012, 86, 1141–1146. [Google Scholar] [CrossRef]
- Ni, W.; Cheng, Y.T.; Weiner, A.M.; Perry, T.A. Tribological behavior of diamond-like-carbon (DLC) coatings against aluminum alloys at elevated temperatures. Surf. Coat. Technol. 2006, 201, 3229–3234. [Google Scholar] [CrossRef]
- Cicek, H.; Keles, A.; Totik, Y.; Efeoglu, I. Adhesion and multipass scratch characterization of ti:Ta-dlc composite coatings. Diamond Relat. Mater. 2018, 83, 80–86. [Google Scholar] [CrossRef]
- Ma, G.; Lin, G.; Sun, G.; Zhang, H.; Wu, H. Characteristics of DLC containing Ti and Zr films deposited by reactive magnetron sputtering. Phys. Procedia 2011, 18, 9–15. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and electrochemical properties of Ti-doped DLC films by a hybrid PVD/PECVD process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
- Chen, C.C.; Hong, C.N. Interfacial studies for improving the adhesion of diamond-like carbon films on steel. Appl. Surf. Sci. 2005, 243, 296–303. [Google Scholar] [CrossRef]
- Wei, C.; Yang, J.F.; Tai, F.C. The stress reduction effect by interlayer deposition or film thickness for diamond like carbon on rough surface. Diamond Relat. Mater. 2010, 19, 518–524. [Google Scholar] [CrossRef]
- Xu, M.; Cai, X.; Liu, Y.; Pu, S.; Chu, P.K. Tribological properties of graded diamond-like carbon films on Ti ion-implanted aluminum substrate. Diamond Relat. Mater. 2008, 17, 1844–1849. [Google Scholar] [CrossRef]
- Han, Z.; Li, H.; Lin, G.; Dong, C. Influence of nitrogen flow rate on the microstructure and properties of N and Me (Me = Cr, Zr) co-doped diamond-like carbon films. J. Mater. Sci. Technol. 2010, 26, 967–972. [Google Scholar] [CrossRef]
- Hee, A.C.; Zhao, Y.; Choudhury, D.; Ghosh, S.; Zhu, Q.; Zhu, H. Tribological behavior of hydrogenated diamond-like carbon on polished alumina substrate with chromium interlayer for biomedical application. Biotribology 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.J.; Liu, Z.K.; Mcnerny, C.; Mehrotra, P.; Inspektor, A. Investigations of the bonding layer in commercial CVD coated cemented carbide inserts. Surf. Coat. Technol. 2005, 198, 161–164. [Google Scholar] [CrossRef]
- Liao, J.X.; Xia, L.F.; Sun, M.R.; Liu, W.M.; Xu, T.; Xue, Q.J. The tribological properties of a gradient layer prepared by plasma-based ion implantation on 2024 aluminum alloy. Surf. Coat. Technol. 2004, 183, 157–164. [Google Scholar] [CrossRef]
- Bull, S.J. Failure mode maps in the thin film scratch adhesion test. Tribol. Int. 1997, 30, 491–498. [Google Scholar] [CrossRef]
- Lee, K.R.; Eun, K.Y.; Kim, I.; Kim, J. Design of w buffer layer for adhesion improvement of DLC films on tool steels. Thin Solid Films 2000, 377, 261–268. [Google Scholar] [CrossRef]
- Bhowmick, S.; Banerji, A.; Khan, M.Z.U.; Lukitsch, M.J.; Alpas, A.T. High temperature tribological behavior of tetrahedral amorphous carbon (ta-c) and fluorinated ta-c coatings against aluminum alloys. Surf. Coat. Technol. 2015, 284, 14–25. [Google Scholar] [CrossRef]
- Tsai, P.C.; Hwang, Y.F.; Chiang, J.Y.; Chen, W.J. The effects of deposition parameters on the structure and properties of titanium-containing DLC films synthesized by cathodic arc plasma evaporation. Surf. Coat. Technol. 2008, 202, 5350–5355. [Google Scholar] [CrossRef]
- Bhowmick, S.; Banerji, A.; Lukitsch, M.J.; Alpas, A.T. The high temperature tribological behavior of Si, O containing hydrogenated diamond-like carbon (a-C:H/a-Si:O) coating against an aluminum alloy. Wear 2015, 330–331, 261–271. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Ma, L.; Wang, C.B.; Hua, M. Influence of chromium buffer layer on Cr/Ta-C composite films. Surf. Eng. 2013, 29, 276–280. [Google Scholar] [CrossRef]
- Depla, D.; Gryse, R.D. Target poisoning during reactive magnetron sputtering: Part II: The influence of chemisorption and gettering. Surf. Coat. Technol. 2004, 183, 190–195. [Google Scholar] [CrossRef]
- WU, C.W. Effect of residual stress on spallation of nicrbsi coating; Springer International Publishing: Cham, Switzerland, 2014; pp. 229–234. [Google Scholar]
- Ferrari, A.C. Determination of bonding in diamond-like carbon by raman spectroscopy. Diamond Relat. Mater. 2002, 11, 1053–1061. [Google Scholar] [CrossRef]
- Panwar, O.S.; Tripathi, R.K.; Chockalingam, S. Improved nanomechanical properties of hydrogenated tetrahedral amorphous carbon films measured with ultra low indentation load. Mater. Express 2015, 5, 410–418. [Google Scholar] [CrossRef]
- Habibi, A.; Khoie, S.M.M.; Mahboubi, F.; Urgen, M. Raman spectroscopy of thin dlc film deposited by plasma electrolysis process. Surf. Coat. Technol. 2016, 309, 945–950. [Google Scholar] [CrossRef]
- Pardo, A.; Gómez-Aleixandre, C.; Orwa, J.O.; Cimmino, A.; Prawer, S. Modification of characteristics of diamond-like carbon thin films by low chromium content addition. Diamond Relat. Mater. 2012, 26, 39–44. [Google Scholar] [CrossRef]
- Long, F.; Wei, Q.; Yu, Z.M.; Luo, J.; Zhang, X.; Long, H.; Wu, X. Effects of temperature and Mo2C layer on stress and structural properties in CVD diamond film grown on Mo foil. J. Alloys Compd. 2013, 579, 638–645. [Google Scholar] [CrossRef]
- Lesiak, B.; Zemek, J.; Houdkova, J.; Kromka, A.; Józwik, A. Electron spectra line shape analysis of highly oriented pyrolytic graphite and nanocrystalline diamond. Anal. Sci. 2010, 26, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.C.; Lee, S.C.; Wei, C.H.; Tyan, S.L. Correlation between id/ig ratio from visible raman spectra and sp2/sp3 ratio from xps spectra of annealed hydrogenated dlc film. Mater. Transac. 2006, 47, 1847–1852. [Google Scholar] [CrossRef]
- Mani, A.; Aubert, P.; Mercier, F.; Khodja, H.; Berthier, C.; Houdy, P. Effects of residual stress on the mechanical and structural properties of TiC thin films grown by RF sputtering. Surf. Coat. Technol. 2005, 194, 190–195. [Google Scholar] [CrossRef]
- Krzanowski, J.E.; Leuchtner, R.E. Chemical, mechanical, and tribological properties of pulsed-laser-deposited titanium carbide and vanadium carbide. J. Am. Ceram. Soc. 2010, 80, 1277–1280. [Google Scholar] [CrossRef]
- Obrosov, A.; Gulyaev, R.; Ratzke, M.; Volinsky, A.A.; Bolz, S.; Naveed, M.; Weiß, S. XPS and AFM investigations of Ti-Al-N coatings fabricated using DC magnetron sputtering at various nitrogen flow rates and deposition temperatures. Met. Open Access Metall. J. 2017, 7, 52. [Google Scholar] [CrossRef]
- Diebold, U.; Madey, T.E. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227–231. [Google Scholar] [CrossRef]
- Schroeder, A.; Francz, G.; Bruinink, A.; Hauert, R.; Mayer, J.; Wintermantel, E. Titanium containing amorphous hydrogenated carbon films (a-C:H/Ti): Surface analysis and evaluation of cellular reactions using bone marrow cell cultures in vitro. Biomaterials 2000, 21, 449–456. [Google Scholar] [CrossRef]
- Shum, P.W.; Xu, Y.F.; Zhou, Z.F.; Li, K.Y. Effects of carbon and nitrogen ion implantations on surface and tribological properties of Ti-Al-Si-N coatings. Surf. Eng. 2012, 28, 149–154. [Google Scholar] [CrossRef]
- Dalibón, E.L.; Escalada, L.; Simison, S.; Forsich, C.; Heim, D.; Brühl, S.P. Mechanical and corrosion behavior of thick and soft DLC coatings. Surf. Coat. Technol. 2016, 312, 101–109. [Google Scholar] [CrossRef]
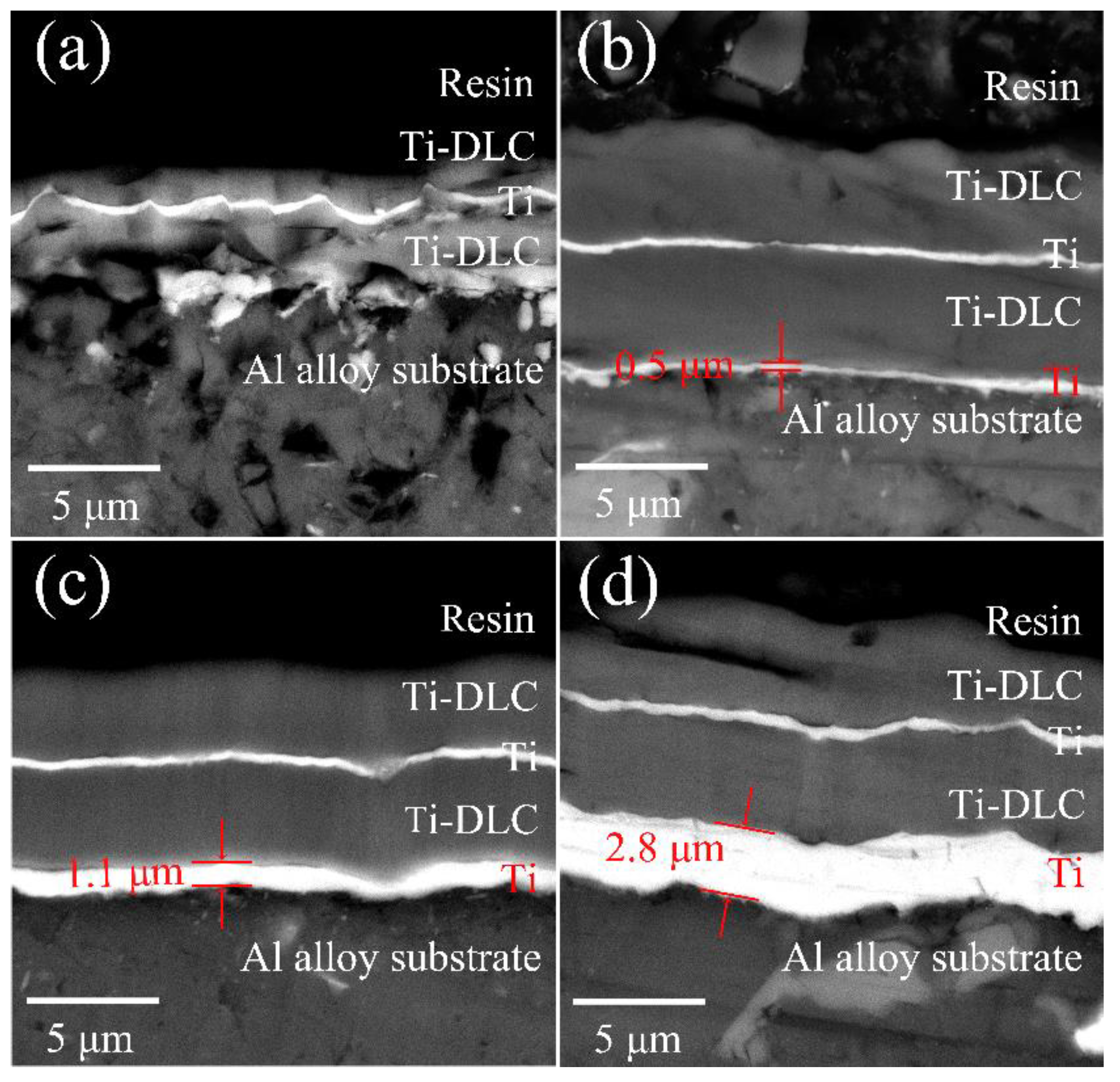
| Sample No. | T (min) | t0 (μm) | tf (μm) |
|---|---|---|---|
| 1 | 0 | 0 | 5.3 ± 0.62 |
| 2 | 5 | 0.5 ± 0.15 | 9.1 ± 0.58 |
| 3 | 10 | 1.1 ± 0.19 | 9.5 ± 0.73 |
| 4 | 20 | 2.8 ± 0.17 | 10.1 ± 0.62 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Qi, F.; Ouyang, X.; Zhao, N.; Zhou, Y.; Li, B.; Luo, W.; Liao, B.; Luo, J. Effect of Ti Transition Layer Thickness on the Structure, Mechanical and Adhesion Properties of Ti-DLC Coatings on Aluminum Alloys. Materials 2018, 11, 1742. https://doi.org/10.3390/ma11091742
Cao H, Qi F, Ouyang X, Zhao N, Zhou Y, Li B, Luo W, Liao B, Luo J. Effect of Ti Transition Layer Thickness on the Structure, Mechanical and Adhesion Properties of Ti-DLC Coatings on Aluminum Alloys. Materials. 2018; 11(9):1742. https://doi.org/10.3390/ma11091742
Chicago/Turabian StyleCao, Hongshuai, Fugang Qi, Xiaoping Ouyang, Nie Zhao, Yun Zhou, Beibei Li, Wenzhong Luo, Bin Liao, and Jun Luo. 2018. "Effect of Ti Transition Layer Thickness on the Structure, Mechanical and Adhesion Properties of Ti-DLC Coatings on Aluminum Alloys" Materials 11, no. 9: 1742. https://doi.org/10.3390/ma11091742





