Calcined Mussel Shell Powder (CMSP) via Modification with Surfactants: Application for Antistatic Oil-Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
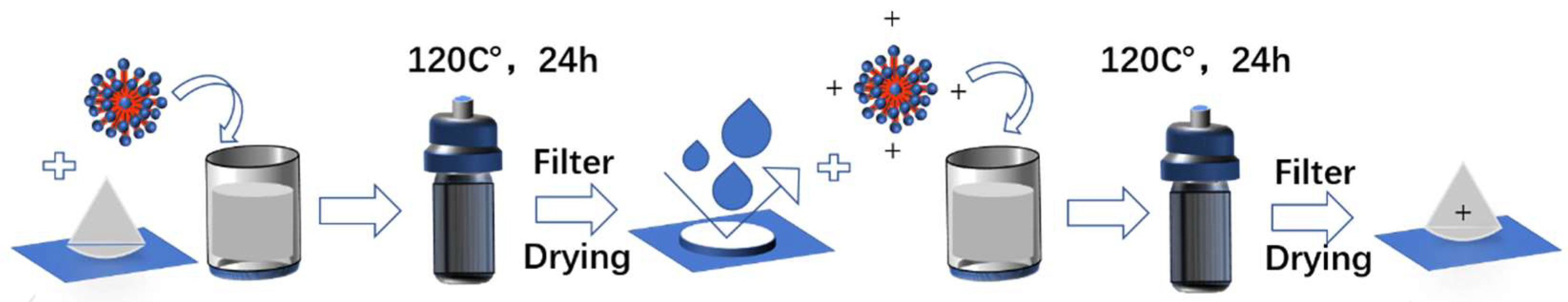
2.2. Preparation of CMSP
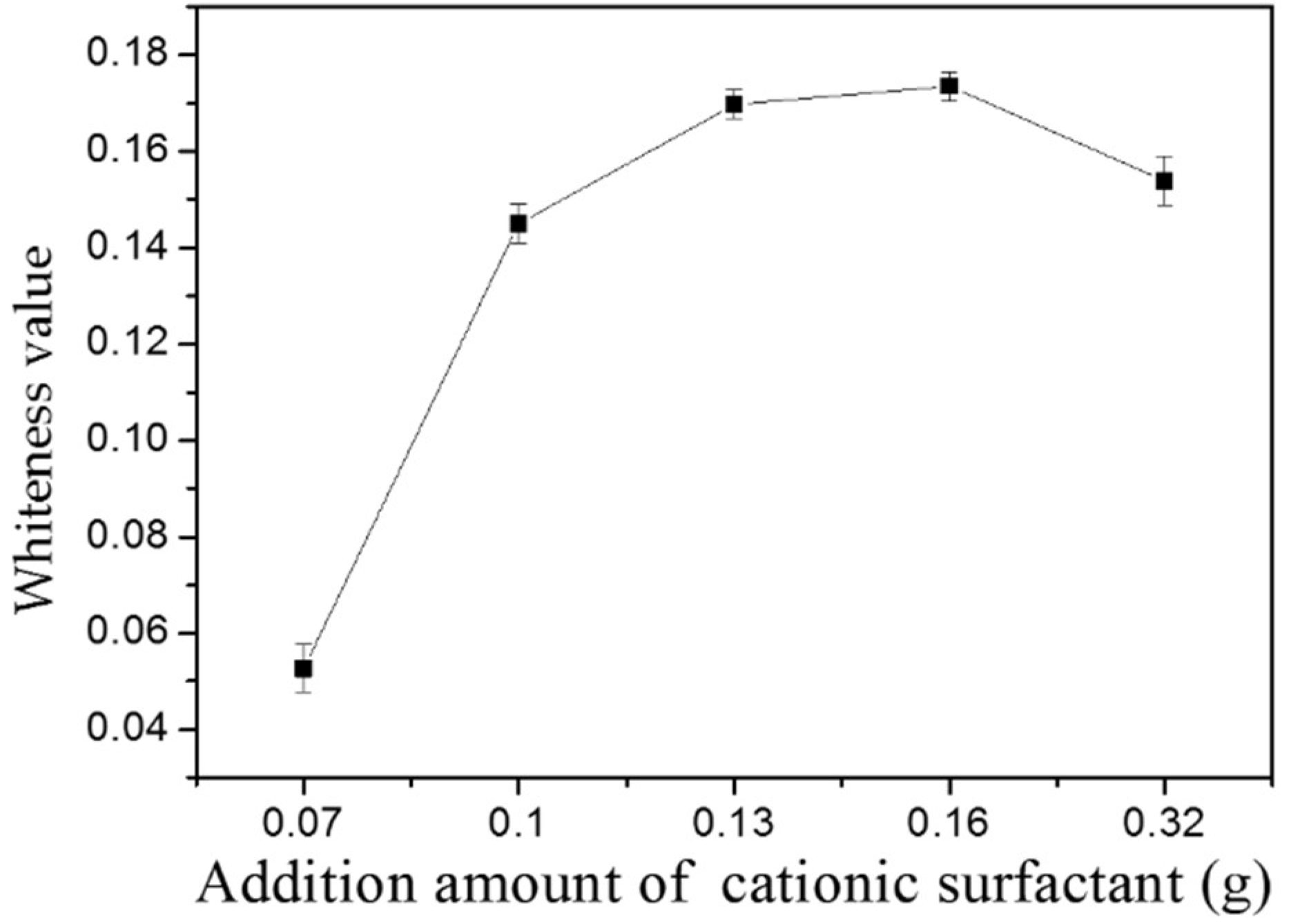
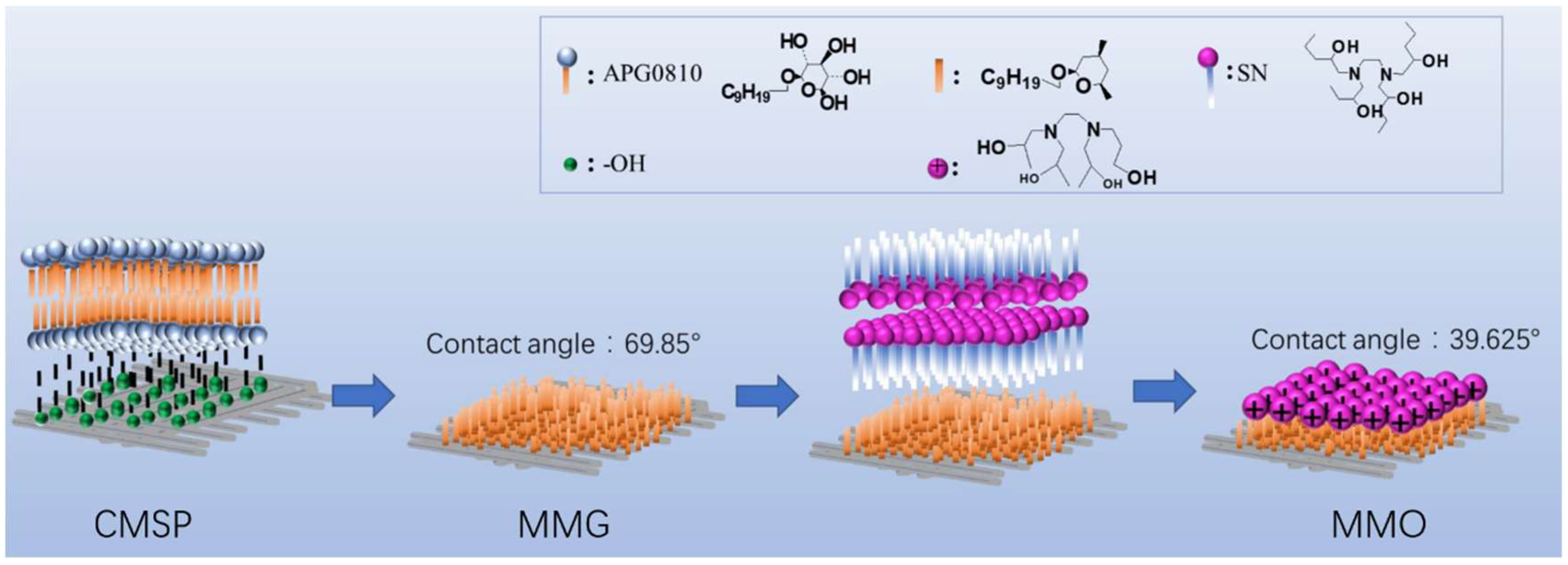
2.3. Modification of Shell Powder by Surfactants
2.4. Research Method
2.5. Determination of Oil Removal Rate
2.6. Statistical Analysis
3. Results and Discussion
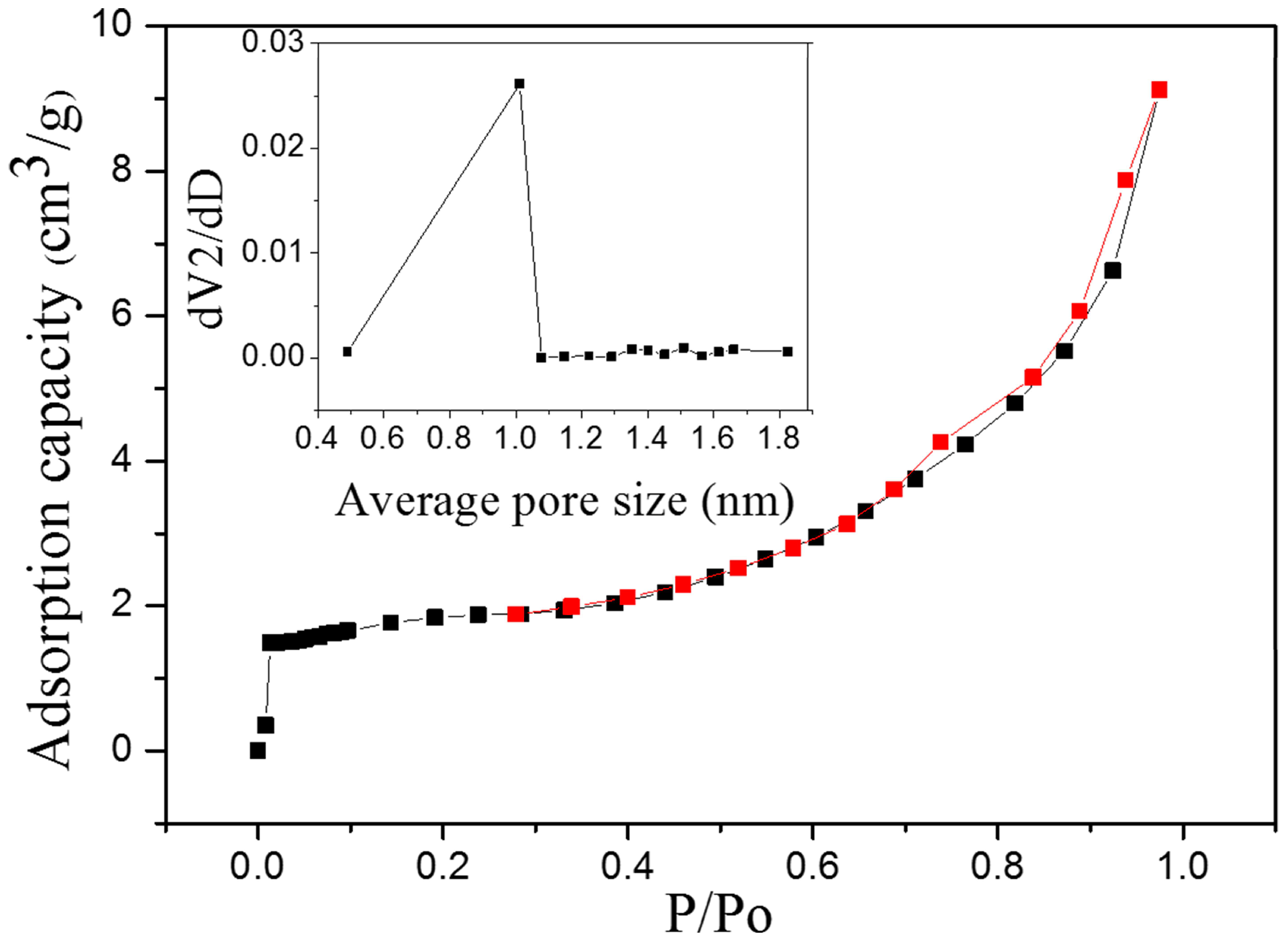
3.1. BET Measurement
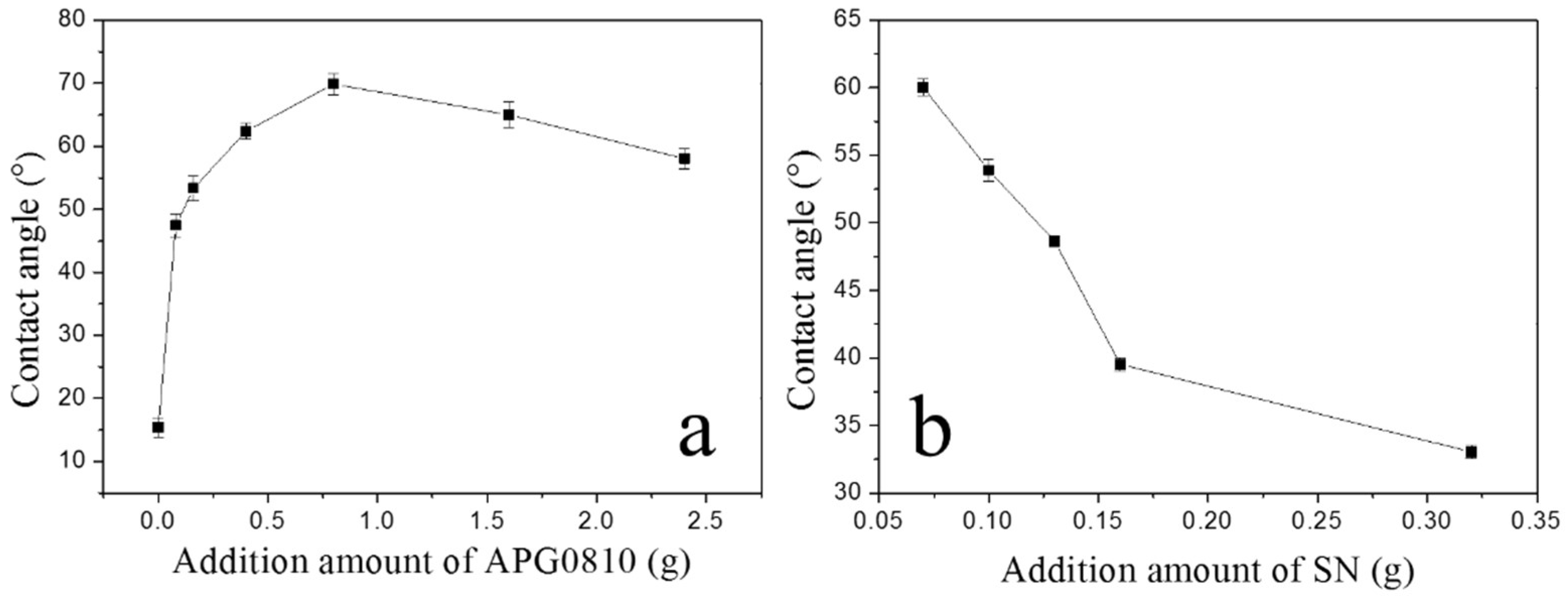
3.2. Contact Angle Measurement
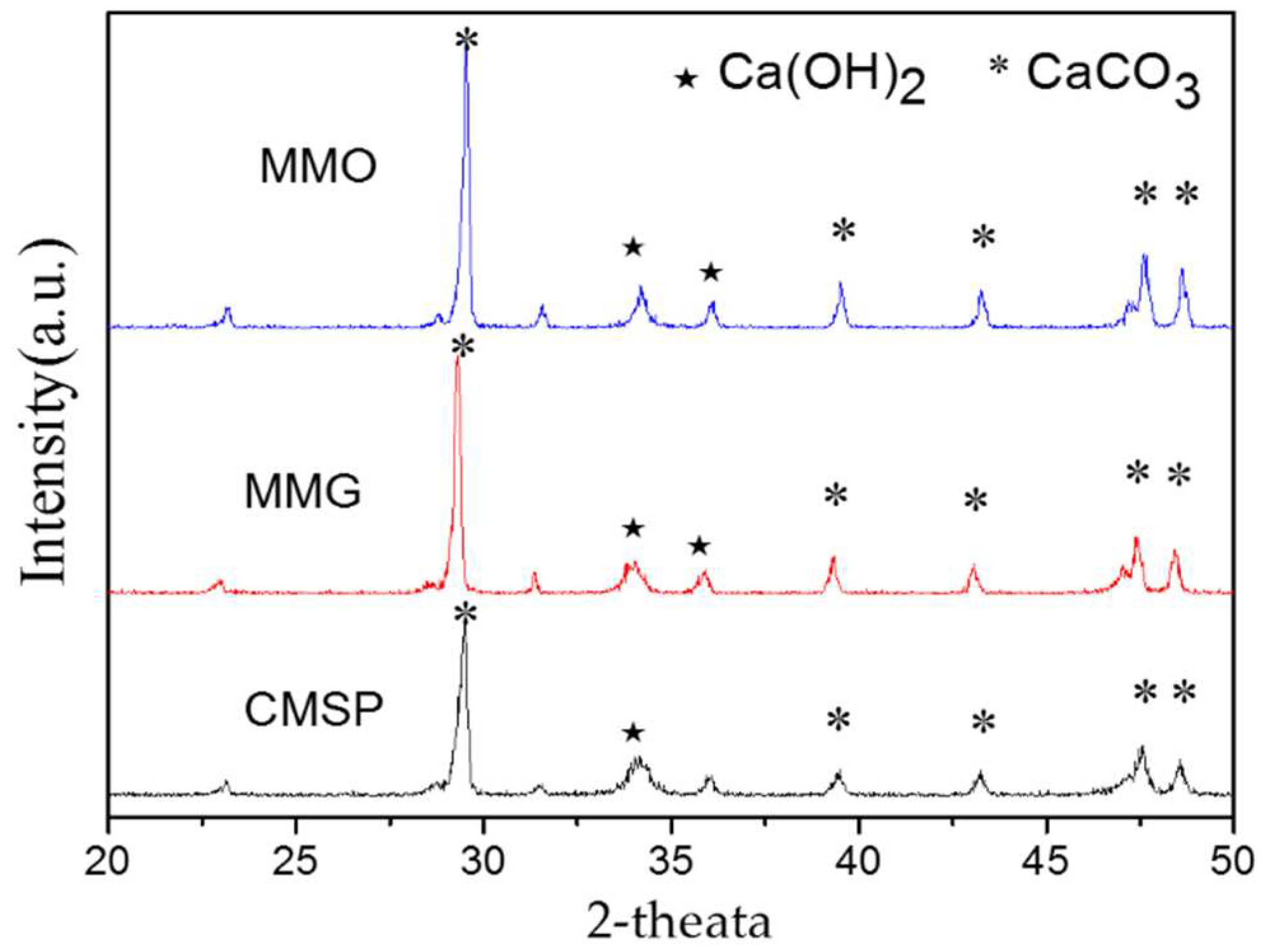
3.3. XRD Characterization
3.4. FTIR Analysis
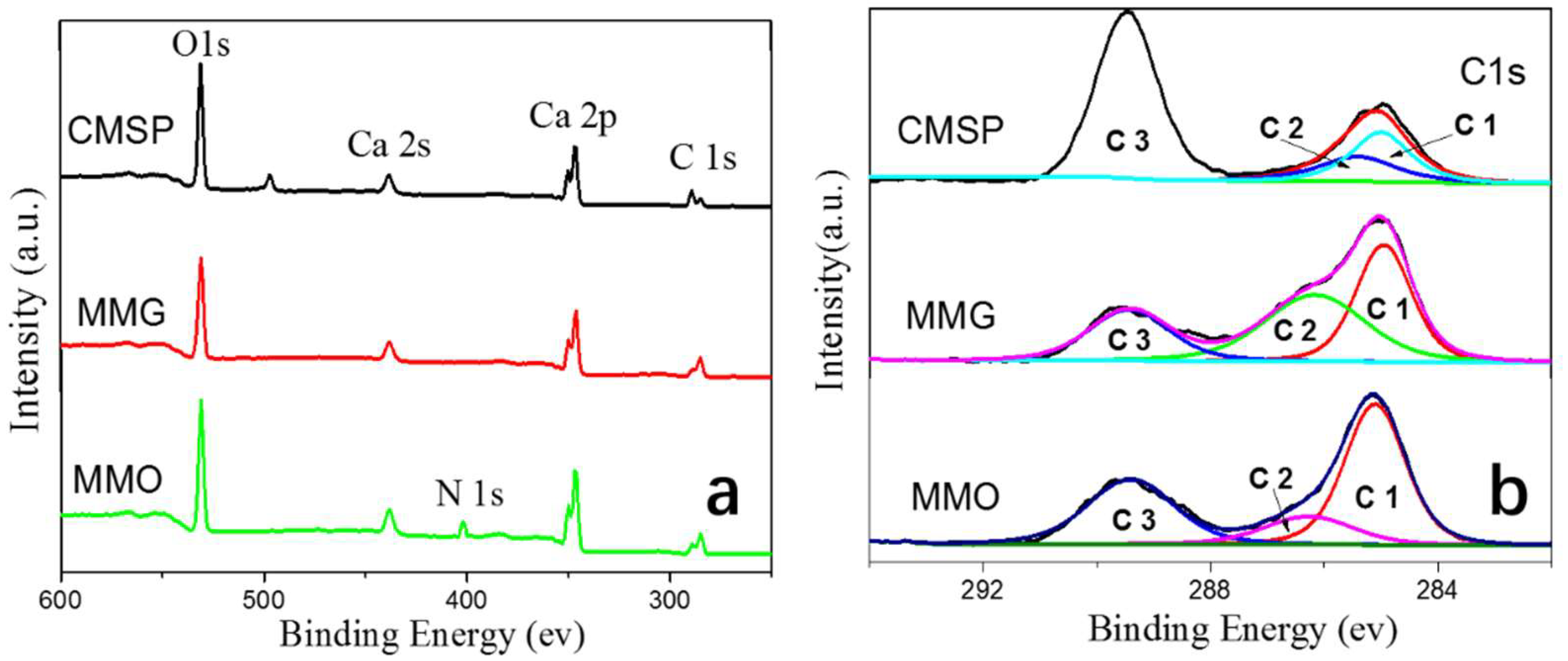
3.5. XPS Characterization
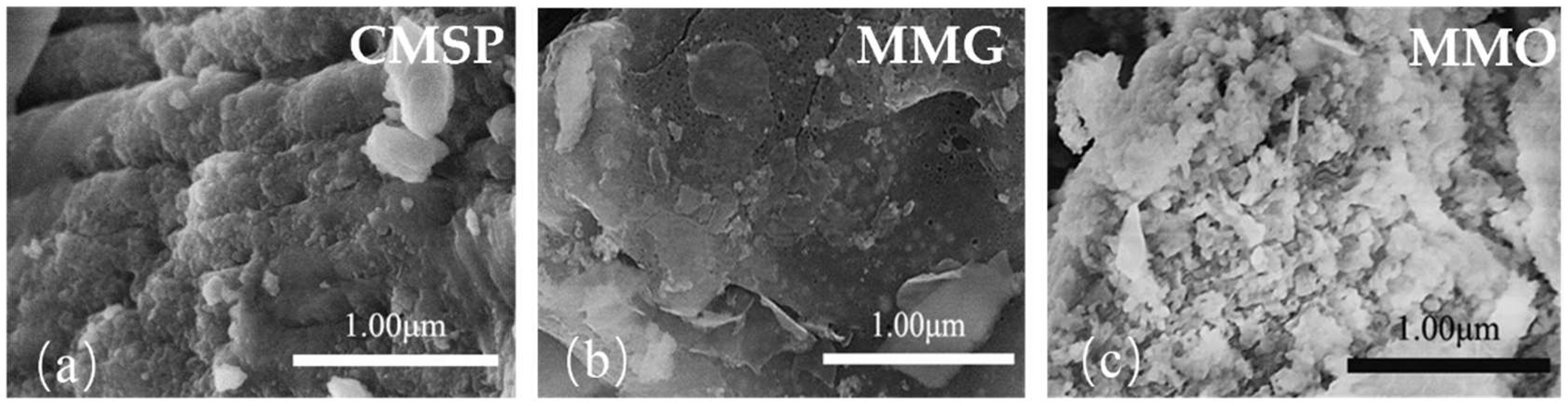
3.6. SEM Characterization
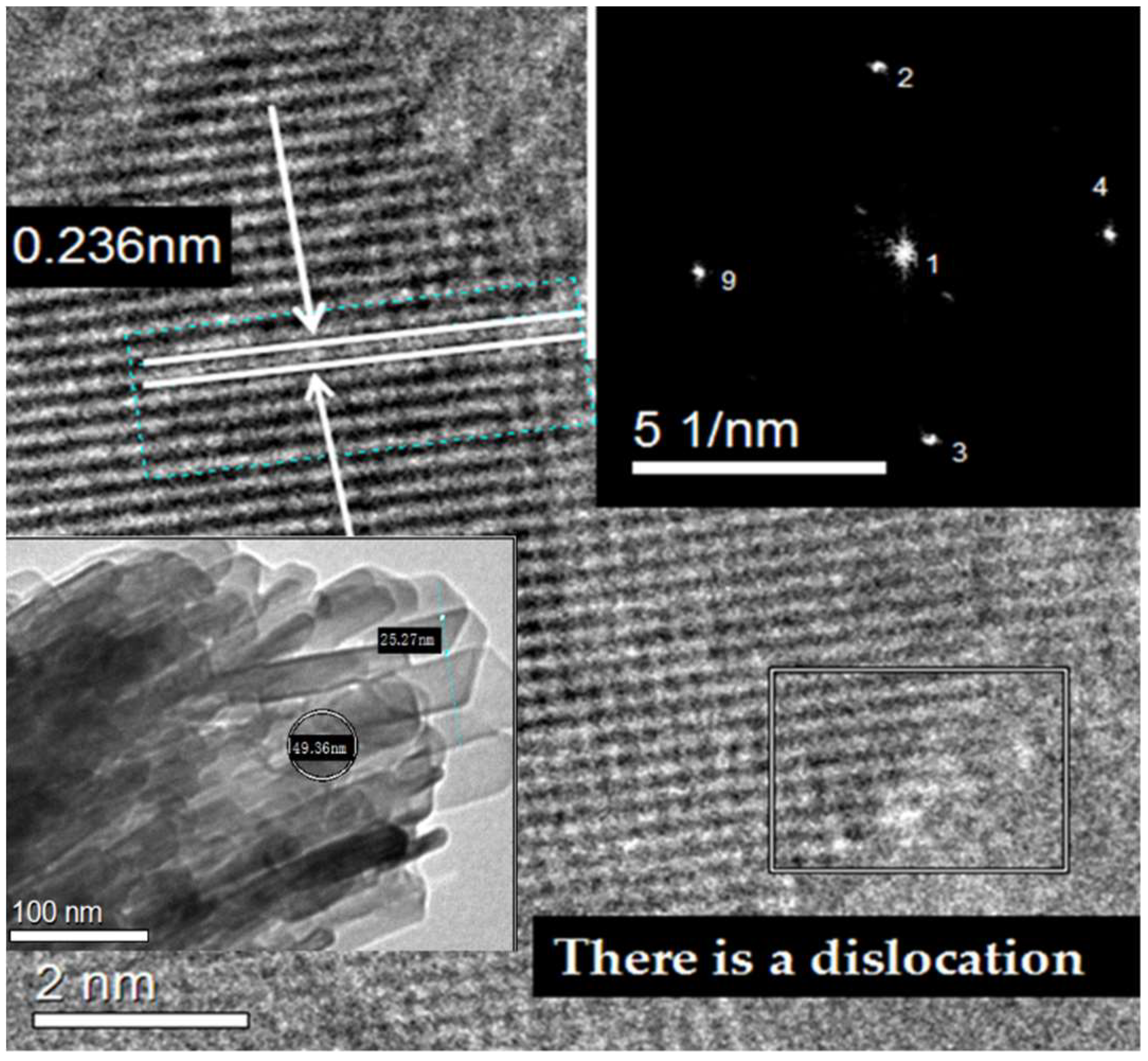
3.7. TEM Characterization
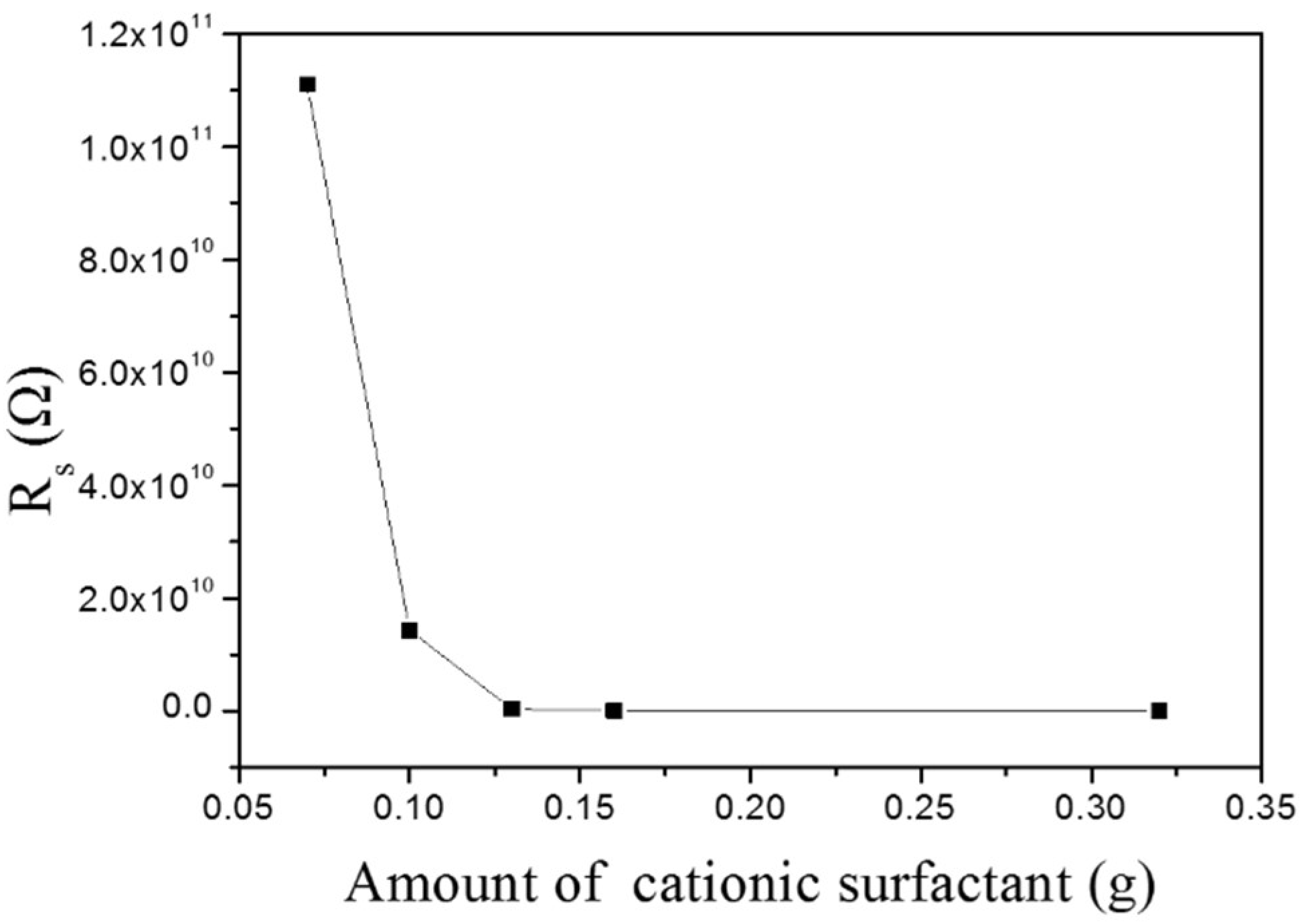
3.8. Surface Resistance of MMO
3.9. Detergency Rate of MMO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Argillier, J.F.; Henaut, I.; Gateau, P.; Heraud, J.P.; Glenat, P. Heavy-Oil Dilution. In Heavy Crude Oils: From Geology to Upgrading: An Overview; IFP Energies Nouvelles: Paris, France, 2005; pp. 503–509. [Google Scholar]
- Wang, S.; Liu, Q.; Tan, X.; Xu, C.; Gray, M.R. Adsorption of asphaltenes on kaolinite as an irreversible process. Colloids Surf. A 2016, 504, 280–286. [Google Scholar] [CrossRef]
- Chung, Y.L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M. A Renewable Lignin–Lactide Copolymer and Application in Biobased Composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V. Nanotechnology-based remediation of petroleum impurities from water. J. Petrol. Sci. Eng. 2014, 122, 705–718. [Google Scholar] [CrossRef]
- Khan, E.; Virojnagud, W.; Ratpukdi, T. Use of biomass sorbents for oil removal from gas station runoff. Chemosphere 2004, 57, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Q.; Mao, D.; Bai, N.; Dong, H. A versatile bio-based material for efficiently removing toxic dyes, heavy metal ions and emulsified oil droplets from water simultaneously. Biores. Technol. 2017, 245, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.R.; Chen, L.; Guo, X.; Xiao, Z.; Liu, R. Biodegradable lignocellulosic porous materials: Fabrication, characterization and its application in water processing. Int. J. Biol. Macromol. 2018, 115, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Wen, Z.; Chen, J.; Li, Q.; Ding, S.; Zhang, Q. Effects for rapid conversion from abalone shell to hydroxyapaptite nanosheets by ionic surfactants. Mater. Sci. Eng. C 2017, 77, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, S.M.; Higgins, B.; Xu, Y.; Brittainet, W.J. Hyperbranched polyol/carbon nanofiber composites. Polymer 2007, 48, 1500–1509. [Google Scholar] [CrossRef]
- Li, C.; Liang, T.; Lu, W.; Tang, C.; Hu, X.; Cao, M.; Liang, J. Improving the antistatic ability of polypropylene fibers by inner antistatic agent filled with carbon nanotubes. Compos. Sci. Technol. 2004, 64, 2089–2096. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Hou, J.; Zhang, X.; Zhu, W.; Xu, M. Polypyrrole-polypropylene composite films: preparation and properties. Polymer 1996, 37, 793–798. [Google Scholar] [CrossRef]
- Hausmann, K. Permanent antistatic agent offers long term performance for films and containers. Plast. Addit. Compd. 2007, 9, 40–42. [Google Scholar] [CrossRef]
- Zheng, A.; Xu, X.; Xiao, H.; Li, N.; Guan, Y.; Li, S. Antistatic modification of polypropylene by incorporating Tween/modified Tween. Appl. Surf. Sci. 2012, 258, 8861–8866. [Google Scholar] [CrossRef]
- Currey, J.D. Mechanical Properties of Mother of Pearl in Tension. Proc. R. Soc. Lond. Ser. A 1977, 196, 443–463. [Google Scholar] [CrossRef]
- Okumura, K.; De Gennes, P.G. Why is nacre strong? Elastic theory and fracture mechanics for biocomposites with stratified structures. Eur. Phys. J. E 2001, 4, 121–127. [Google Scholar] [CrossRef]
- Currie, J.A.; Harrison, N.R.; Wang, L.; Jones, M.I.; Brooks, M.S. A preliminary study of processing seafood shells for eutrophication control. Asia-Pac. J. Chem. Eng. 2007, 2, 460–467. [Google Scholar] [CrossRef]
- Lowe, C.R. Nanobiotechnology: the fabrication and applications of chemical and biological nanostructures. Curr. Opin. Struct. Biol. 2000, 10, 428–434. [Google Scholar] [CrossRef]
- Peña-Rodríguez, S.; Bermúdez-Couso, A.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Mercury removal using ground and calcined mussel shell. J. Environ. Sci. 2013, 25, 2476–2486. [Google Scholar] [CrossRef]
- Lu, C.; Gong, J.; Liu, J.; Zhang, H.; Song, W.; Ji, L. Facile preparation of nano-Bi2MoO6/Diatomite composite for enhancing photocatalytic performance under visible light irradiation. Materials 2018, 11, 267. [Google Scholar] [CrossRef]
- Paradelo, R.; Conde-Cid, M.; Cutillas-Barreiro, L.; Núñez-Delgado, A. Phosphorus removal from wastewater using mussel shell: Investigation on retention mechanisms. Ecol. Eng. 2016, 97, 558–566. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P.D. Fixed-bed adsorption of Malachite Green onto binary solid mixture of adsorbents: seashells and eggshells. Toxicol. Environ. Chem. 2012, 94, 1272–1282. [Google Scholar] [CrossRef]
- Hsu, T.C. Experimental assessment of adsorption of Cu2+, and Ni2+, from aqueous solution by oyster shell powder. J. Hazard. Mater. 2009, 171, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Whang, J.H.; Kim, J.M.; Suh, H.J. The effect of oyster shell powder on the extension of the shelf-life of kimchi. Food Control 2006, 17, 695–699. [Google Scholar] [CrossRef]
- Peña-Rodríguez, S.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Kinetics of Hg (II) adsorption and desorption in calcined mussel shells. J. Hazard. Mater. 2010, 180, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Jiang, Y.; Niu, T.; Huang, J. Cellulose-based material with amphiphobicity to inhibit bacterial adhesion by surface modification. J. Mater. Chem. 2012, 22, 12562–12567. [Google Scholar] [CrossRef]
- Xiao, W.; Hu, H.; Huang, J. Colorimetric detection of cysteine by surface functionalization of natural cellulose substance. Sens. Actuators B Chem. 2012, 171–172, 878–885. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, J. Hierarchical-structured anatase-titania/cellulose composite sheet with high photocatalytic performance and antibacterial activity. Chem. Eur. J. 2015, 21, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J. Guidelines to measurements of reproducible contact angles using a sessile-drop technique. Surf. Innov. 2013, 1, 248–254. [Google Scholar] [CrossRef]
- Marmur, A.; Volpe, C.D.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: towards common and accurate terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.C.; Han, D.S. Synthesis of dialkylamidoamine oxide surfactant and characterization of its dual function of detergency and softness. Colloids. Surf. A 2011, 389, 166–174. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic−inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Wang, X.Q.; Jiang, D.G. Modification of nanometer calcium carbonate for water-borne architectural coatings. J. China Univ. Min. Technol. 2008, 18, 76–81. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.J.; Chen, F. Microwave hydrothermal transformation of amorphous calcium carbonate nanospheres and application in protein adsorption. ACS Appl. Mater. Interfaces 2014, 6, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Military Handbook DOD-HDBK-263. Available online: http://www.barringer1.com/mil_files/MIL-HDBK-263B.pdf (accessed on 21 January 2018).
- Salman, A.A.; Tabandeh, M.; Heidelberg, T.; Hussen, R.S.; Ali, H.M. Alkyl-imidazolium glycosides: non-ionic-cationic hybrid surfactants from renewable resources. Carbohydr. Res. 2015, 412, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.C.; Magán, A.; Valiño, S.; Bello, P.M.; Casares, J.J.; Blanco, J.M. Identification of best available techniques in the seafood industry: A case study. J. Clean. Prod. 2009, 17, 391–399. [Google Scholar] [CrossRef]
- Gao, J.; Song, X.; Huang, X.; Wang, L.; Li, B.; Xue, H. Facile preparation of polymer microspheres and fibers with a hollow core and porous shell for oil adsorption and oil/water separation. Appl. Surf. Sci. 2018, 439, 394–404. [Google Scholar] [CrossRef]
- Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A.M. Neodymium Recovery by Chitosan/Iron (III) Hydroxide [ChiFer (III)] Sorbent Material: Batch and Column Systems. Polymers 2018, 10, 204. [Google Scholar] [CrossRef]
- Rahmani, Z.; Rashidi, A.M.; Samadi, M.T.; Rahmani, A.R. N-doped reduced graphene oxide aerogel for the selective adsorption of oil pollutants from water: Isotherm and kinetic study. J. Ind. Eng. Chem. 2018, 61, 416–426. [Google Scholar] [CrossRef]
- Du, Z.; Mao, X.; Tai, X.; Wang, G.; Liu, X. Preparation and properties of microemulsion detergent with linear medium chain fatty alcohols as oil phase. J. Mol. Liq. 2016, 223, 805–810. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Yu, H.; Lim, J. Synthesis of environment friendly biosurfactants and characterization of interfacial properties for cosmetic and household products formulations. Colloids Surf. A 2018, 536, 224–233. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Yu, H.; Lim, J. Synthesis of environment friendly nonionic surfactants from sugar base and characterization of interfacial properties for detergent application. J. Ind. Eng. Chem. 2016, 38, 157–166. [Google Scholar] [CrossRef]

| Temperature (°C) | Specific Surface Area (m2/g) | Average Pore Size (nm) |
|---|---|---|
| blank | 1.120 | 1.350 |
| 300 | 2.516 | 3.826 |
| 500 | 1.308 | 3.330 |
| 800 | 5.539 | 10.615 |
| 1000 | 6.004 | 9.404 |
| Degreasing Mechanism | Sorbent | Degreasing Effect | Action Object | Ref. |
|---|---|---|---|---|
| Adsorption | Polymethylmethacrylate | 55 g/g. | motor oil | [38] |
| (ChiFer(III)) | 0.0138 g/g | Neodymium | [39] | |
| N-doped reduced graphene oxide aerogel | 210 g/g. | Crude oil | [40] | |
| Emulsification | (SDS) + isomeric alcohol ethoxylate (IC13EO6) | 20.27% | Standard carbon black oil cloth JB-02 | [41] |
| LP(A) | 95.00%. | The artificial soil | [42] | |
| SA08-07(C28H53O13) | 91.60% | The artificial soil | [43] | |
| MMO | 17.35% | Standard carbon black oil cloth JB-02 | In this work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Calcined Mussel Shell Powder (CMSP) via Modification with Surfactants: Application for Antistatic Oil-Removal. Materials 2018, 11, 1410. https://doi.org/10.3390/ma11081410
Wei D, Zhang H, Cai L, Guo J, Wang Y, Ji L, Song W. Calcined Mussel Shell Powder (CMSP) via Modification with Surfactants: Application for Antistatic Oil-Removal. Materials. 2018; 11(8):1410. https://doi.org/10.3390/ma11081410
Chicago/Turabian StyleWei, Danyi, Hailong Zhang, Lu Cai, Jian Guo, Yaning Wang, Lili Ji, and Wendong Song. 2018. "Calcined Mussel Shell Powder (CMSP) via Modification with Surfactants: Application for Antistatic Oil-Removal" Materials 11, no. 8: 1410. https://doi.org/10.3390/ma11081410





