Reduced Graphene Oxide and Its Modifications as Catalyst Supports and Catalyst Layer Modifiers for PEMFC
Abstract
:1. Introduction
2. Materials and Methods
2.1. RGO Synthesis and Modification
2.2. Pt-Based Catalyst Synthesis
2.3. RGO and Pt/RGO Characterization
2.4. Catalysts Electrochemical Characterization
2.5. Membrane Electrode Assemblies (MEAs) Preparation and Testing
3. Results and Discussion
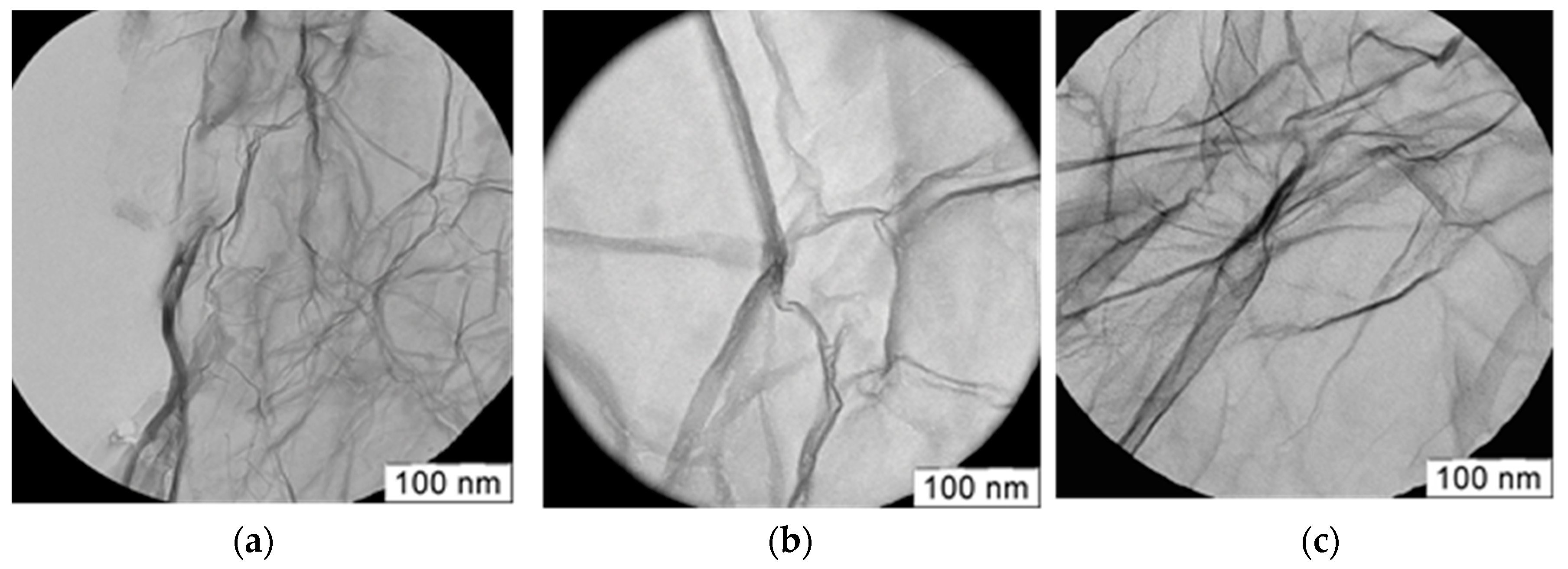
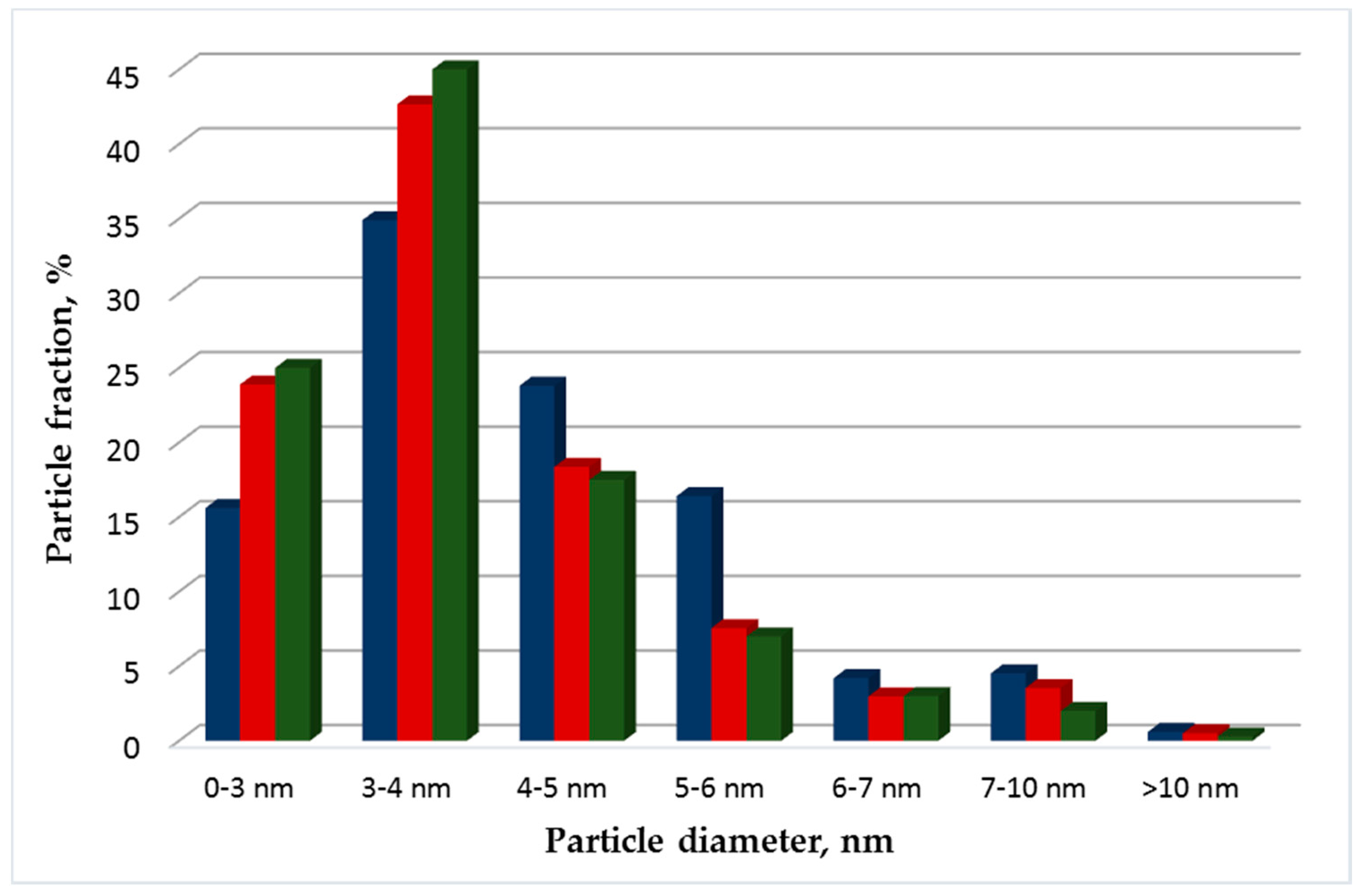
3.1. Structural Studies of Pt Electrocatalysts Based on RGO
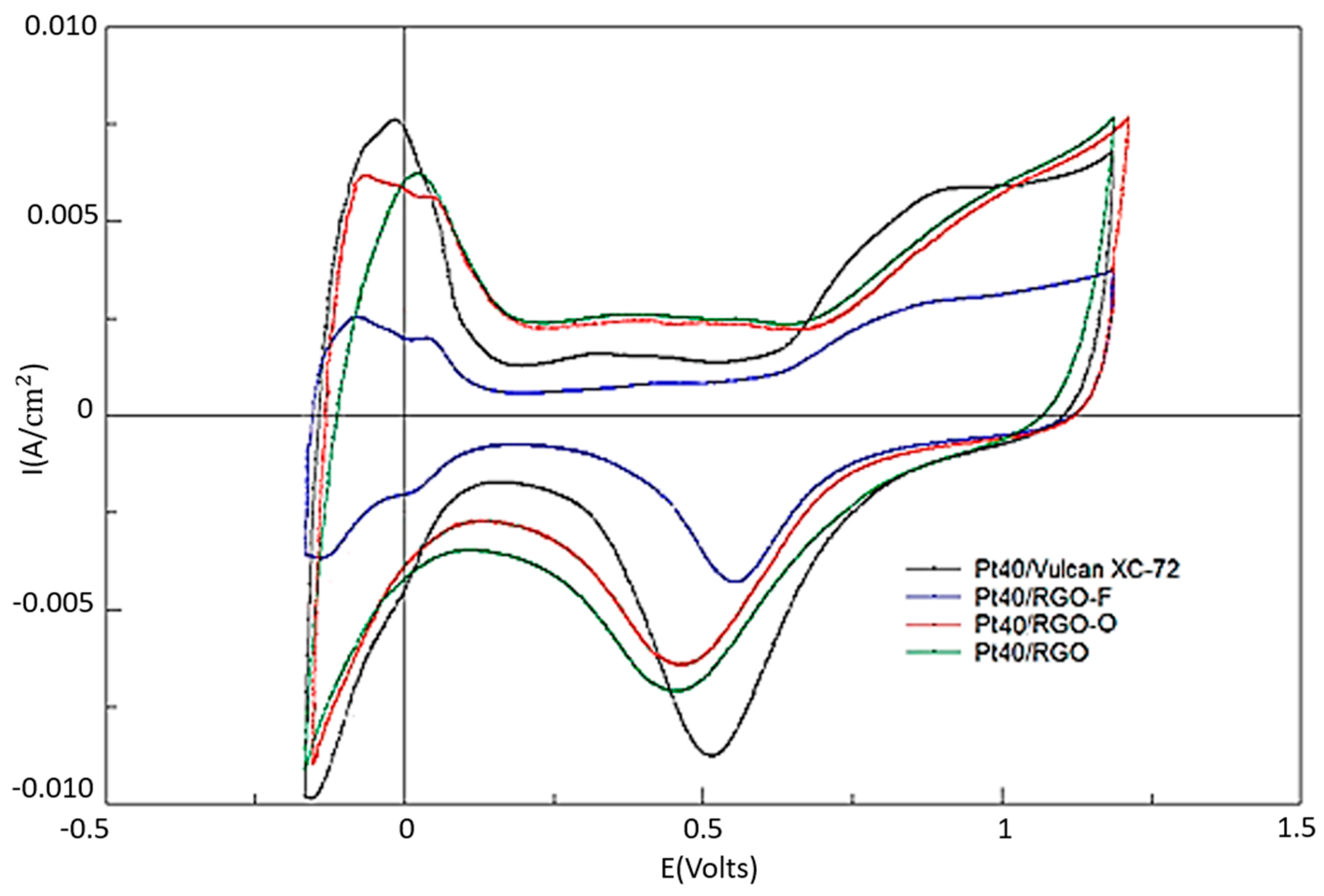
3.2. Electrochemical Studies of Pt Electrocatalysts Based on RGO
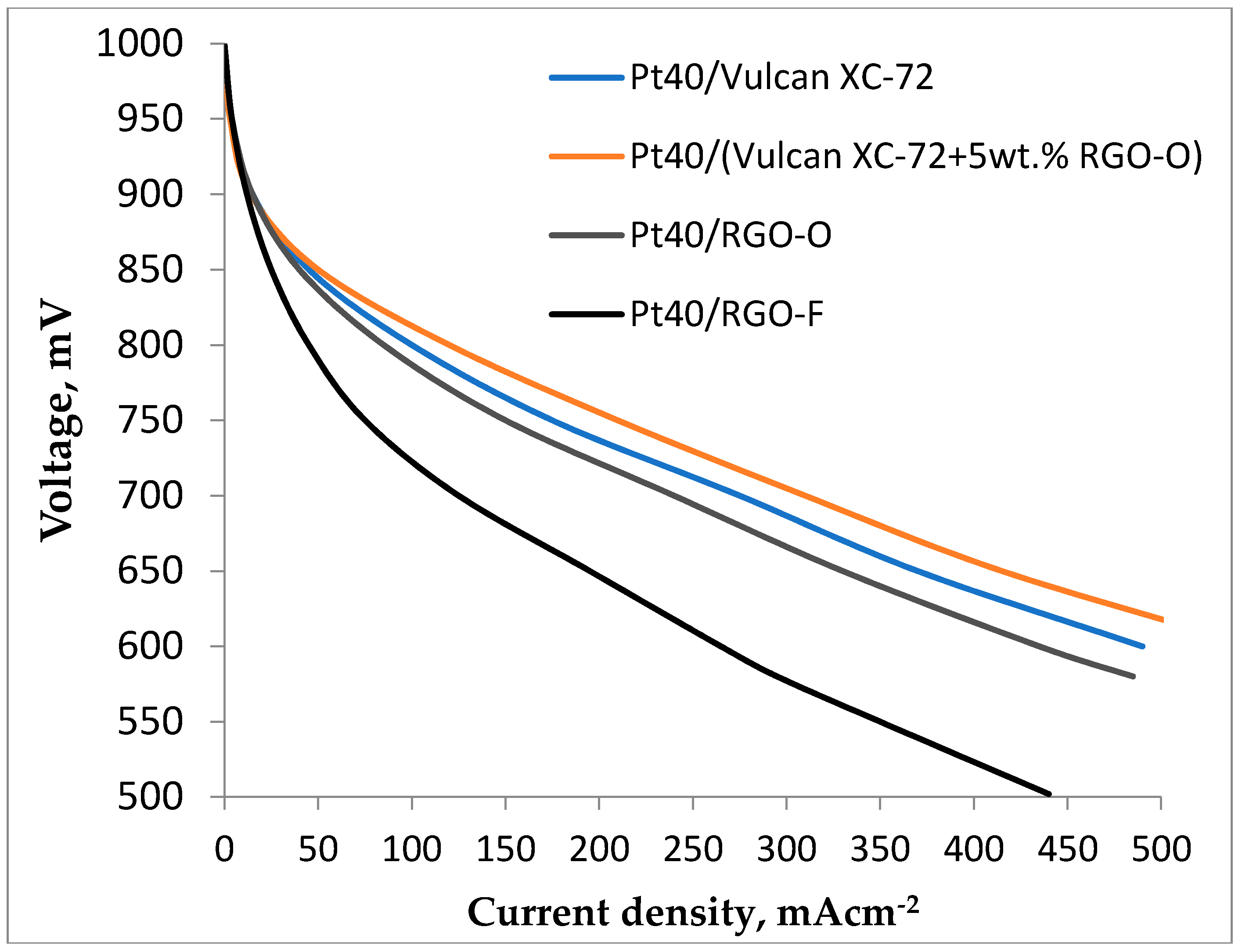
3.3. Fuel Cell Testing of RGO-Supported Pt Electrocatalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Doucet, G.; Etievant, C.; Puyenchet, C.; Grigoriev, S.; Millet, P. Hydrogen-based PEM auxiliary power unit. Int. J. Hydrog. Energy 2009, 34, 4983–4989. [Google Scholar] [CrossRef]
- De Jong, K.P.; Geus, J.W. Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev. Sci. Eng. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Kvande, I.; Hammer, N.; Rønning, M.; Chen, D.; Briskeby, S.T.; Tsypkin, M.; Sunde, S.; Tunold, R. On the preparation of carbon nanofiber-supported Pt-catalyst. Top. Catal. 2007, 45, 81–85. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Pushkarev, A.S.; Kalinichenko, V.N.; Pushkareva, I.V.; Presnyakov, M.Yu.; Fateev, V.N. Electrocatalytic layers based on reduced graphene oxide for fabrication of low temperature fuel cells. Kinet. Catal. 2015, 56, 689–693. [Google Scholar] [CrossRef]
- Yılmaz, M.S.; Kaplan, B.Y.; Metin, Ö.; Gürsel, S.A. A facile synthesis and assembly of ultrasmall Pt nanoparticles on reduced graphene oxide-carbon black hybrid for enhanced performance in PEMFC. Mater. Des. 2018, 151, 29–36. [Google Scholar] [CrossRef]
- Devrim, Y.; Arıca, E.D.; Albostan, A. Graphene based catalyst supports for high temperature PEM fuel cell application. Int. J. Hydrog. Energy 2018, 43, 11820–11829. [Google Scholar] [CrossRef]
- Şanlı, L.I.; Bayram, V.; Ghobadi, S.; Düzen, N.; Gürsel, S.A. Engineered catalyst layer design with graphene-carbon black hybrid supports for enhanced platinum utilization in PEM fuel cell. Int. J. Hydrog. Energy 2017, 42, 1085–1092. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Graphene as a new carbon support for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2012, 123–124, 52–68. [Google Scholar] [CrossRef]
- Pushkarev, A.S.; Pushkareva, I.V.; Grigoriev, S.A.; Kalinichenko, V.N.; Presniakov, M.Yu.; Fateev, V.N. Electrocatalytic layers modified by reduced graphene oxide for PEM fuel cells. Int. J. Hydrog. Energy 2015, 40, 14492–14497. [Google Scholar] [CrossRef]
- Ticianelli, E.A.; Derouin, C.R.; Redondo, A.; Srinivasan, S. Methods to advance technology of proton exchange membrane fuel cells. J. Electrochem. Soc. 1998, 135, 2209–2214. [Google Scholar] [CrossRef]
- Baranov, I.E.; Nikolaev, I.I.; Pushkarev, A.S.; Pushkareva, I.V.; Kalinnikov, A.A.; Fateev, V.N. Numerical modeling of polymer electrolyte fuel cell catalyst layer with different carbon supports. Int. J. Electrochem. Sci. 2018, in press. [Google Scholar]
- Park, S.; Shao, Y.; Wan, H.; Peter, C.R.; Vilayanur, V.V.; Silas, A.T.; Laxmikant, V.S. Design of graphene sheets-supported Pt catalyst layer in PEM fuel cells. Electrochem. Commun. 2011, 13, 258–261. [Google Scholar] [CrossRef]
- Nechitailov, A.A.; Glebova, N.V. Mechanism of the effect of oxygen-modified carbon nanotubes on the kinetics of oxygen electroreduction on platinum. Russ. J Electrochem. 2014, 50, 751–755. [Google Scholar] [CrossRef]
- Glebova, N.V.; Nechitailov, A.A.; Gurin, V.N. Specific features of oxygen electroreduction on platinized carbon black—Functionalized carbon nanotube composite. Tech. Phys. Lett. 2011, 37, 661–663. [Google Scholar] [CrossRef]
- Nechitailov, A.A.; Glebova, N.V.; Koshkina, D.V.; Tomasov, A.A.; Zelenina, N.K.; Terukova, E.E. Specific features of operation of a membrane-electrode assembly of an air-hydrogen fuel cell. Tech. Phys. Lett. 2013, 39, 762–766. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Du, Z.; Fan, Z.; Jiang, J.; Wang, Y.; Zhang, H.; Xie, L.; Huang, W.; Yu, T. Chemically engineered graphene oxide as high performance cathode materials for Li-ion batteries. CARBON 2014, 76, 148–154. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, M.; Chen, L.; Zhou, B.; Shan, M.; Niu, J.; Li, B.; Qian, X. A facile preparation of edge etching, porous and highly reactive graphene nanosheets via ozone treatment at a moderate temperature. Chem. Eng. J. 2014, 240, 187–194. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Vikkisk, M.; Kruusenberg, I.; Joost, U.; Shulga, E.; Kinkb, I.; Tammeveski, K. Electrocatalytic oxygen reduction on nitrogen-doped graphene in alkaline media. Appl. Catal. B Environ. 2014, 147, 369–376. [Google Scholar] [CrossRef]
- Maiti, U.N.; Lee, W.J.; Lee, J.M.; Oh, Y.; Kim, J.Y.; Kim, J.E.; Shi, J.; Han, T.H.; Kim, S.O. 25th anniversary article: Chemically modified/doped carbon nanotubes & graphene for optimized nanostructures & nanodevices. Adv. Mater. 2014, 26, 40–67. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc 1958, 80, 1339. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Fateev, V.N. Evaluation of carbon supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. J. Power Sources 2008, 177, 281–285. [Google Scholar] [CrossRef]
- Damaskin, B.B.; Petriy, O.A. Introduction to Electrochemical Kinetics, 2nd ed.; Vysshaya Shkola: Moscow, Russia, 1983; p. 400. (In Russian) [Google Scholar]
- Grigor'ev, S.A.; Lyutikova, E.K.; Pritulenko, E.G.; Samsonov, D.P.; Fateev, V.N. Synthesis and test of palladium-based nanostructured anodic electrocatalysts for hydrogen fuel cells with solid polymer electrolyte. Russ. J. Electrochem. 2006, 42, 1251–1254. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhang, H.; Zhong, H.; Lu, Q.; Wang, Y.; Su, D. Effect of particle size on the activity and durability of the Pt/C electrocatalyst for proton exchange membrane fuel cells. Appl. Catal. B Environ. 2012, 111–112, 264–270. [Google Scholar] [CrossRef]
- Shao, M.; Peles, A.; Shoemaker, K. Electrocatalysis on platinum nanoparticles: Particle size effect on oxygen reduction reaction activity. Nano Lett. 2011, 11, 3714–3719. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Groom, D.J.; Wang, X.; Yang, Z.; Gummalla, M.; Ball, S.C.; Myers, D.J.; Ferreira, P.J. Degradation mechanisms of platinum nanoparticle catalysts in proton exchange membrane fuel cells: The role of particle size. Chem. Mater. 2014, 26, 5540–5548. [Google Scholar] [CrossRef]
- Sanli, L.I.; Bayram, V.; Yarar, B.; Ghobadi, S.; Gürsel, S.A. Development of graphene supported platinum nanoparticles for polymer electrolyte membrane fuel cells: Effect of support type and impregnation–reduction methods. Int. J. Hydrog. Energy 2016, 41, 3414–3427. [Google Scholar] [CrossRef]
- Marinkas, A.; Arena, F.; Mitzel, J.; Prinz, G.M.; Heinzel, A.; Peinecke, V.; Natter, H. Graphene as catalyst support: The influences of carbon additives and catalyst preparation methods on the performance of PEM fuel cells. Carbon 2013, 58, 139–150. [Google Scholar] [CrossRef]
- Hoe, L.P.; Boaventura, M.; Lagarteira, T.; Shyuan, L.K.; Mendes, A. Polyol synthesis of reduced graphene oxide supported platinum electrocatalysts for fuel cells: Effect of Pt precursor, support oxidation level and pH. Int. J. Hydrog. Energy 2018. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Hsu, M.C.; Liu, W.L.; Chen, W.J. Study of Pt catalyst on graphene and its application to fuel cell. Appl. Surf. Sci. 2013, 277, 223–230. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J.W. Graphene—Metal particle nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Sung, C.-C.; Liu, C.-Y.; Cheng, C.C.J. Durability improvement at high current density by graphene networks on PEM fuel cell. Int. J. Hydrogen Energy 2014, 39, 11706–11712. [Google Scholar] [CrossRef]
- Heaney, M.B. Electrical Conductivity and Resistivity. In Electrical Measurement, Signal Processing, and Displays; Webster, J.G., Ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]

| Material | RGO | RGO-F | RGO-O |
|---|---|---|---|
| Element | wt % | ||
| C | 97.15 | 91.45 | 91.63 |
| O | 2.85 | 1.95 | 8.37 |
| F | 0.00 | 6.60 | 0.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, S.A.; Fateev, V.N.; Pushkarev, A.S.; Pushkareva, I.V.; Ivanova, N.A.; Kalinichenko, V.N.; Yu. Presnyakov, M.; Wei, X. Reduced Graphene Oxide and Its Modifications as Catalyst Supports and Catalyst Layer Modifiers for PEMFC. Materials 2018, 11, 1405. https://doi.org/10.3390/ma11081405
Grigoriev SA, Fateev VN, Pushkarev AS, Pushkareva IV, Ivanova NA, Kalinichenko VN, Yu. Presnyakov M, Wei X. Reduced Graphene Oxide and Its Modifications as Catalyst Supports and Catalyst Layer Modifiers for PEMFC. Materials. 2018; 11(8):1405. https://doi.org/10.3390/ma11081405
Chicago/Turabian StyleGrigoriev, Sergey A., Vladimir N. Fateev, Artem S. Pushkarev, Irina V. Pushkareva, Natalia A. Ivanova, Valery N. Kalinichenko, Mikhail Yu. Presnyakov, and Xing Wei. 2018. "Reduced Graphene Oxide and Its Modifications as Catalyst Supports and Catalyst Layer Modifiers for PEMFC" Materials 11, no. 8: 1405. https://doi.org/10.3390/ma11081405





