The Formation of Nanoparticles and Their Competitive Interaction with Twins during Eutectic Si Growth
Abstract
:1. Introduction
2. Materials and Methods
3. Results
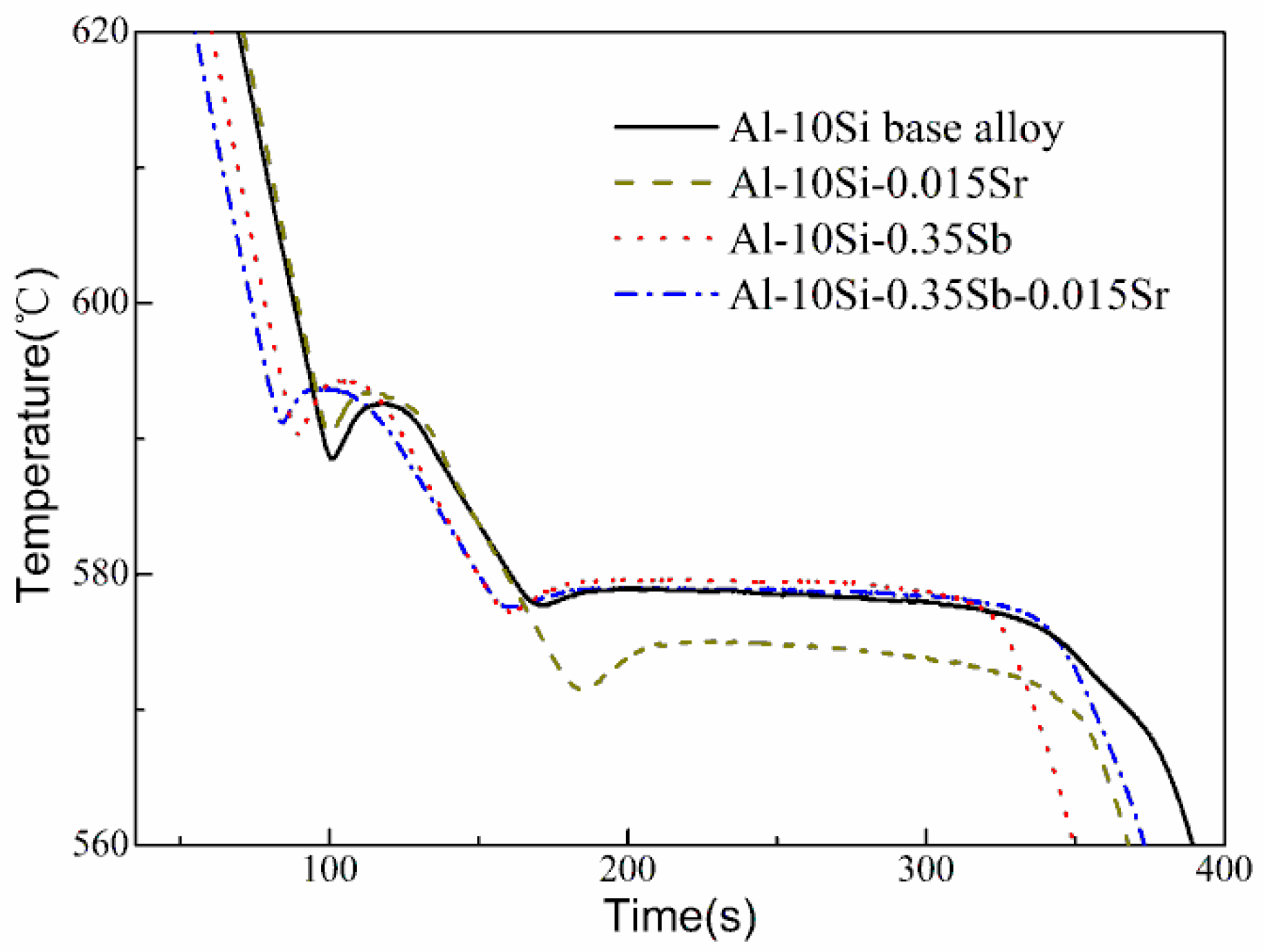
3.1. Cooling Curves
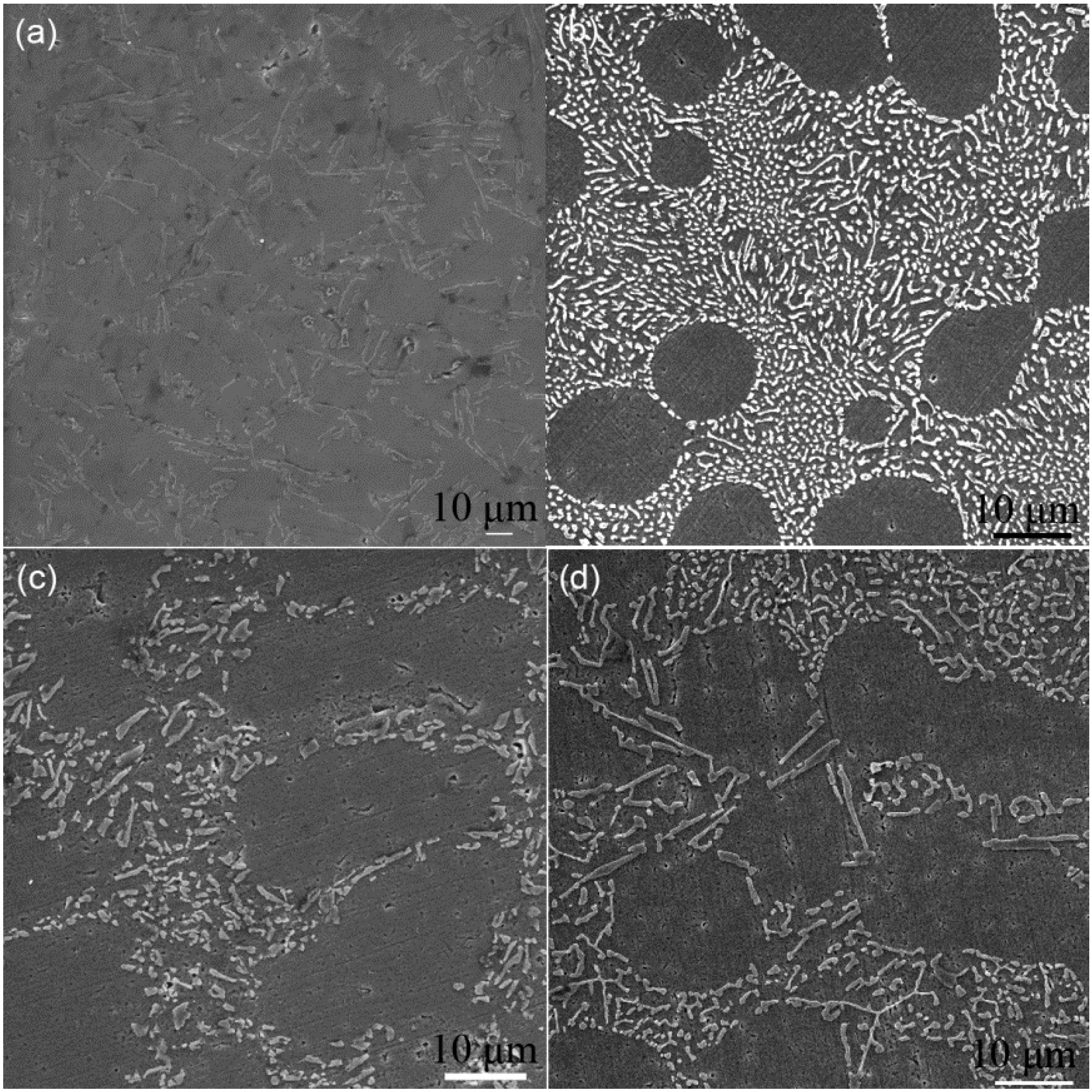
3.2. Eutectic Si Morphology
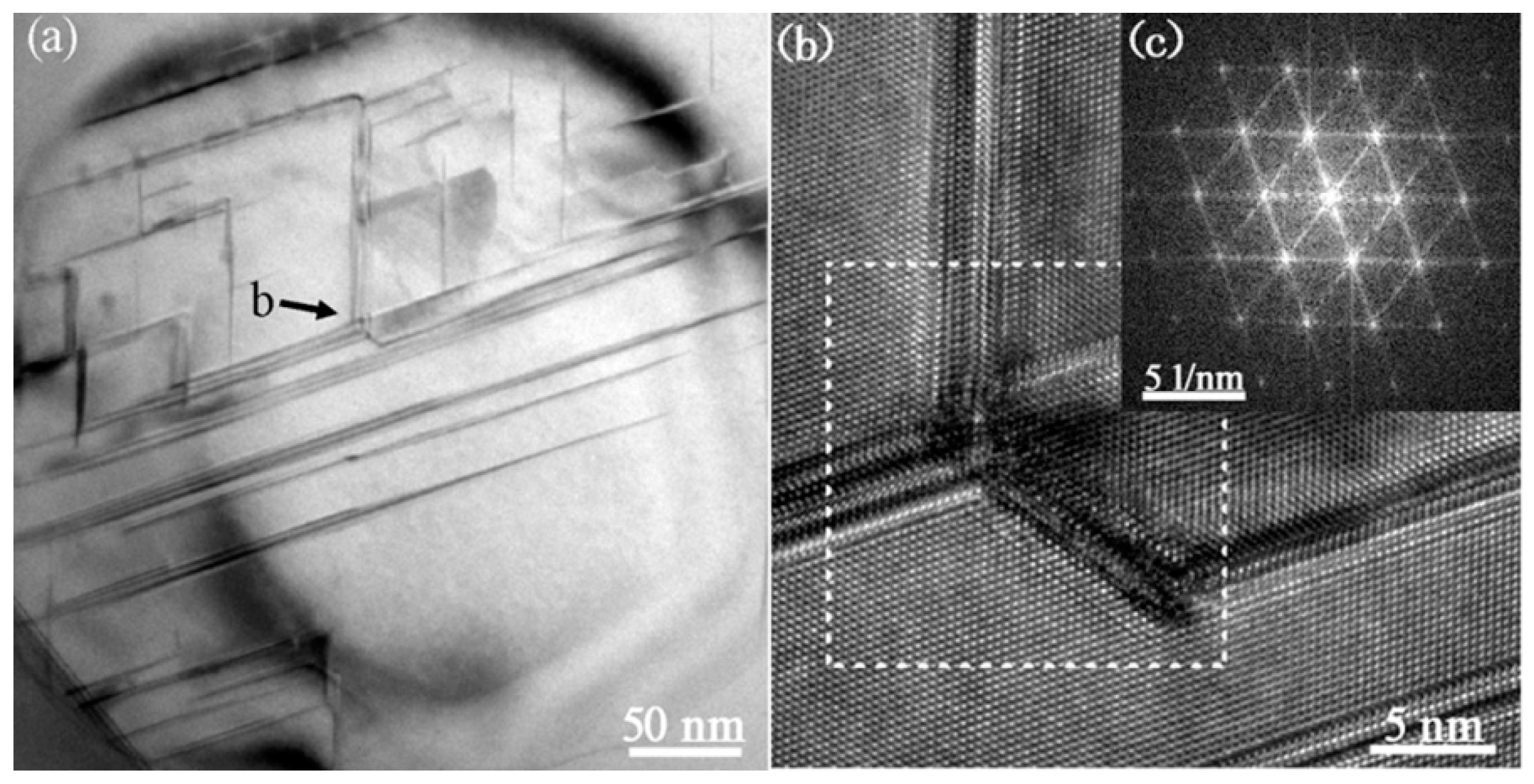
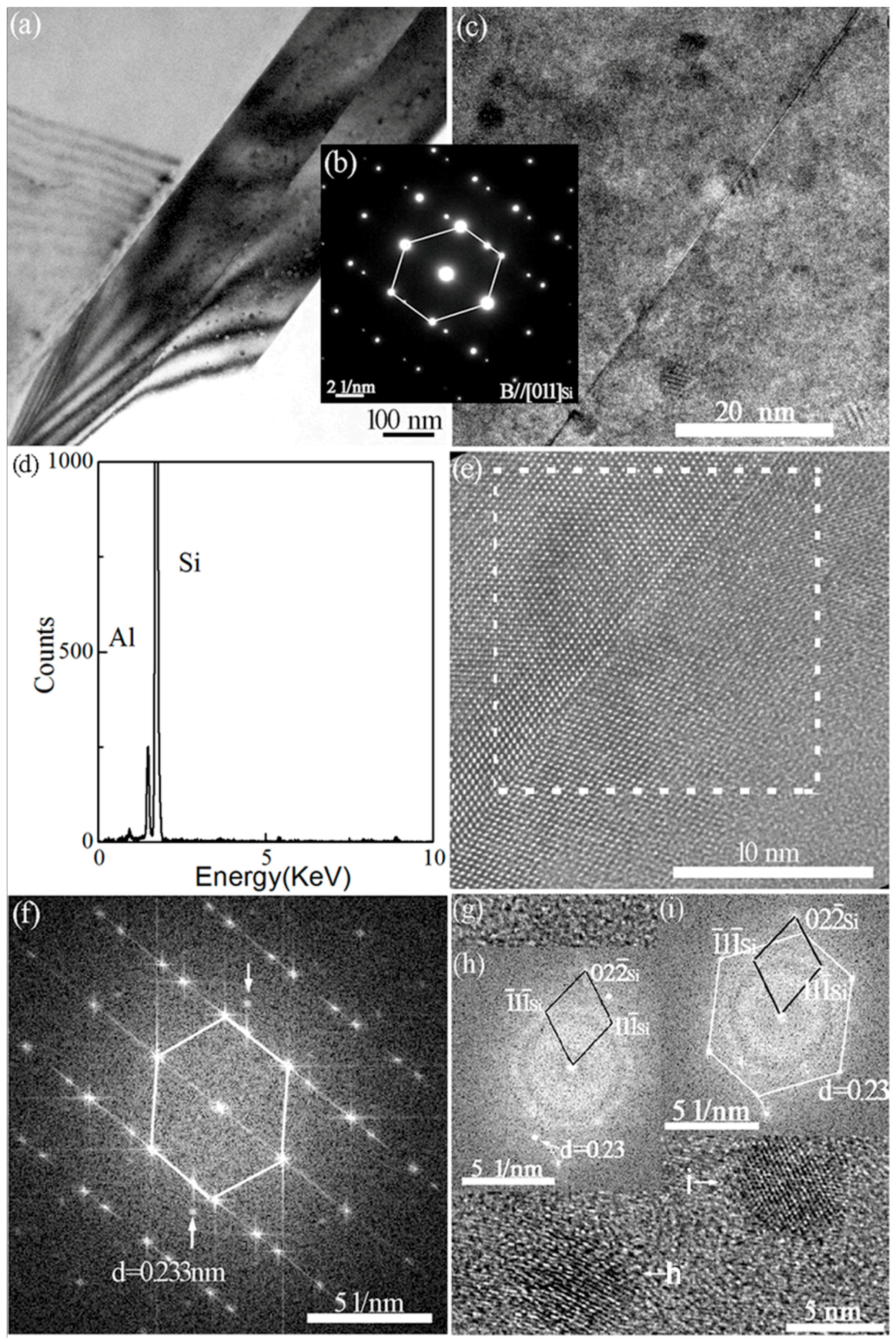
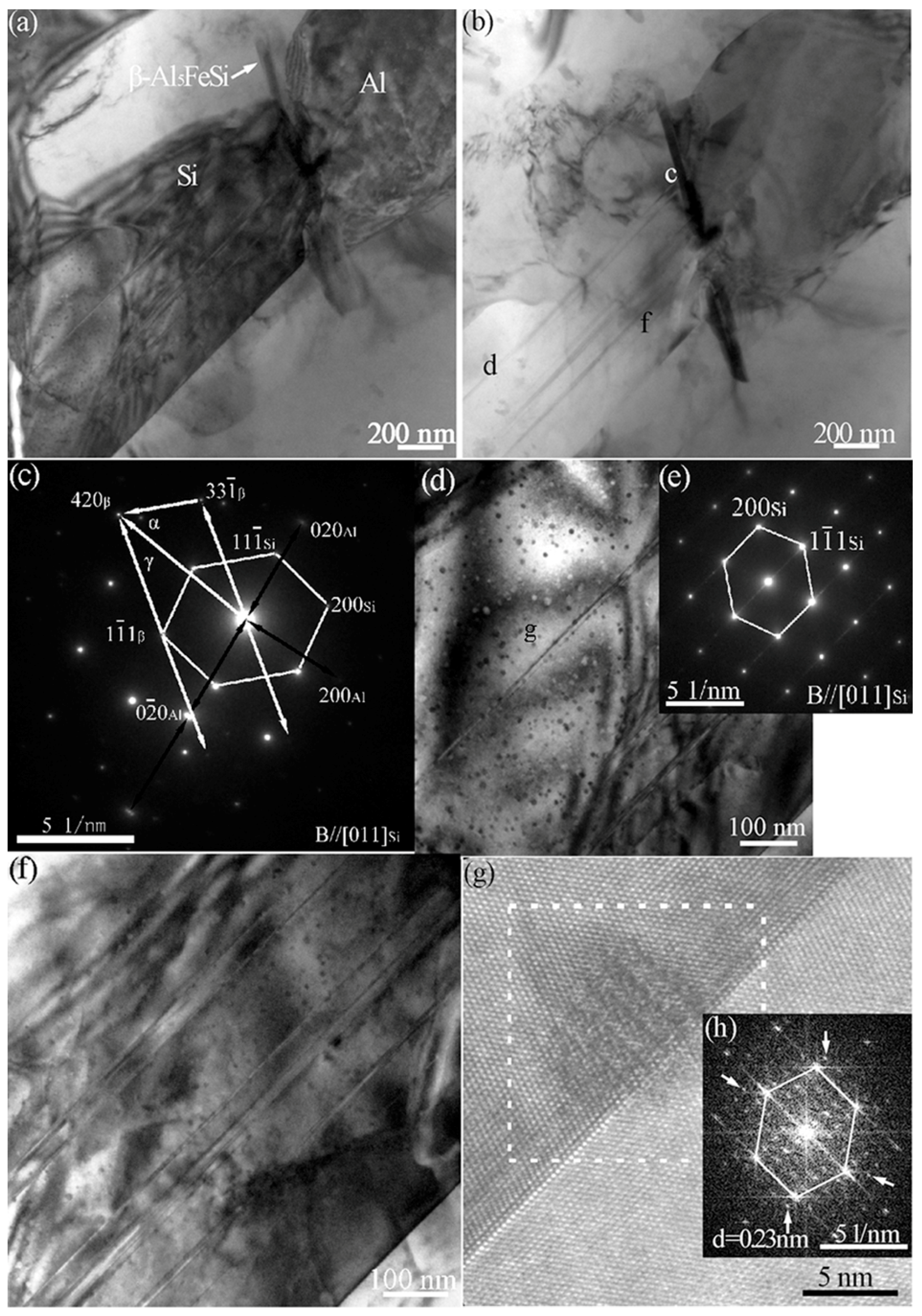
3.3. Cystal Defects by TEM
3.4. Interaction of Nanoparticles with Twins
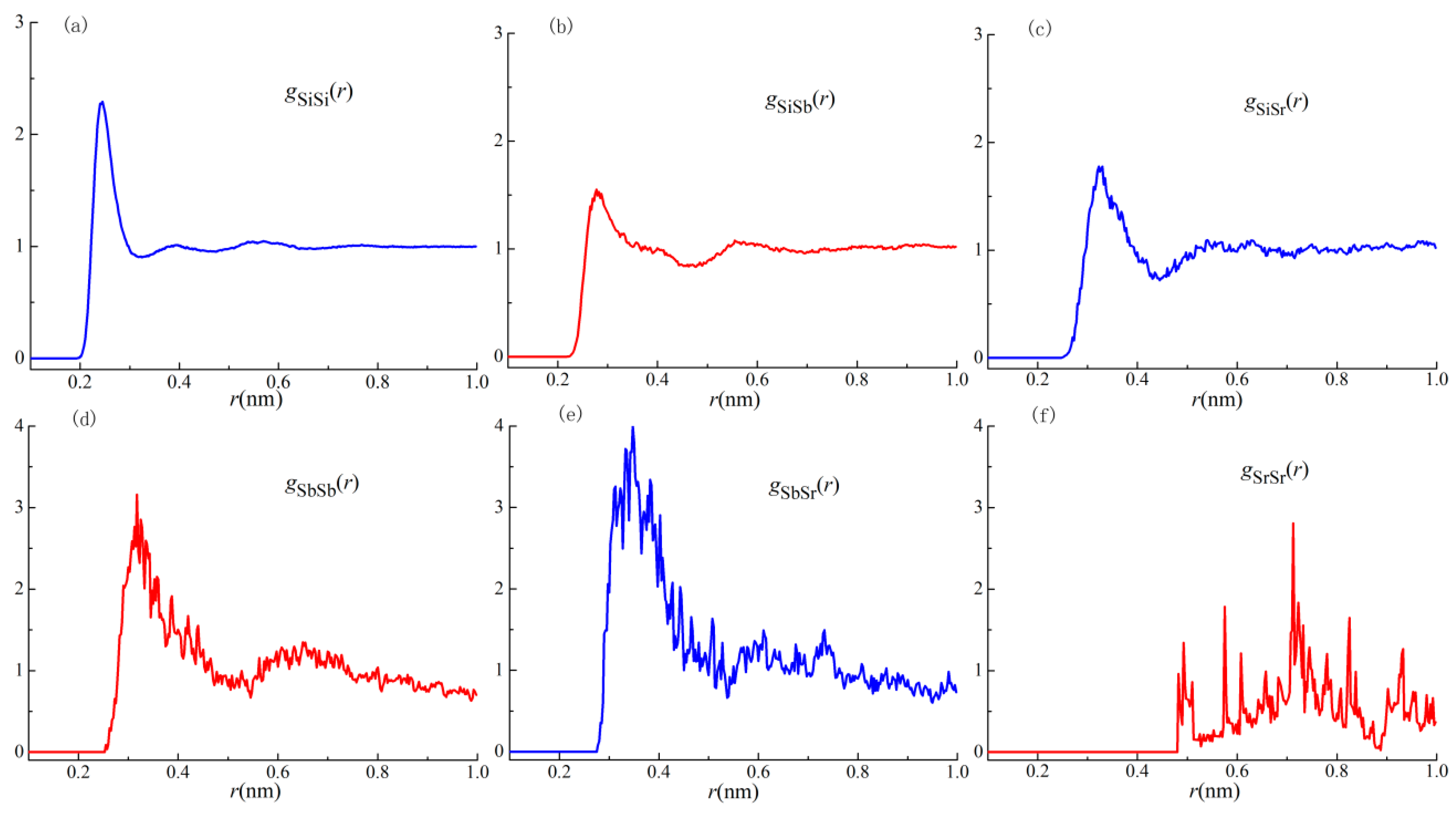
3.5. Ab initio Molecular Dynamics Simulation
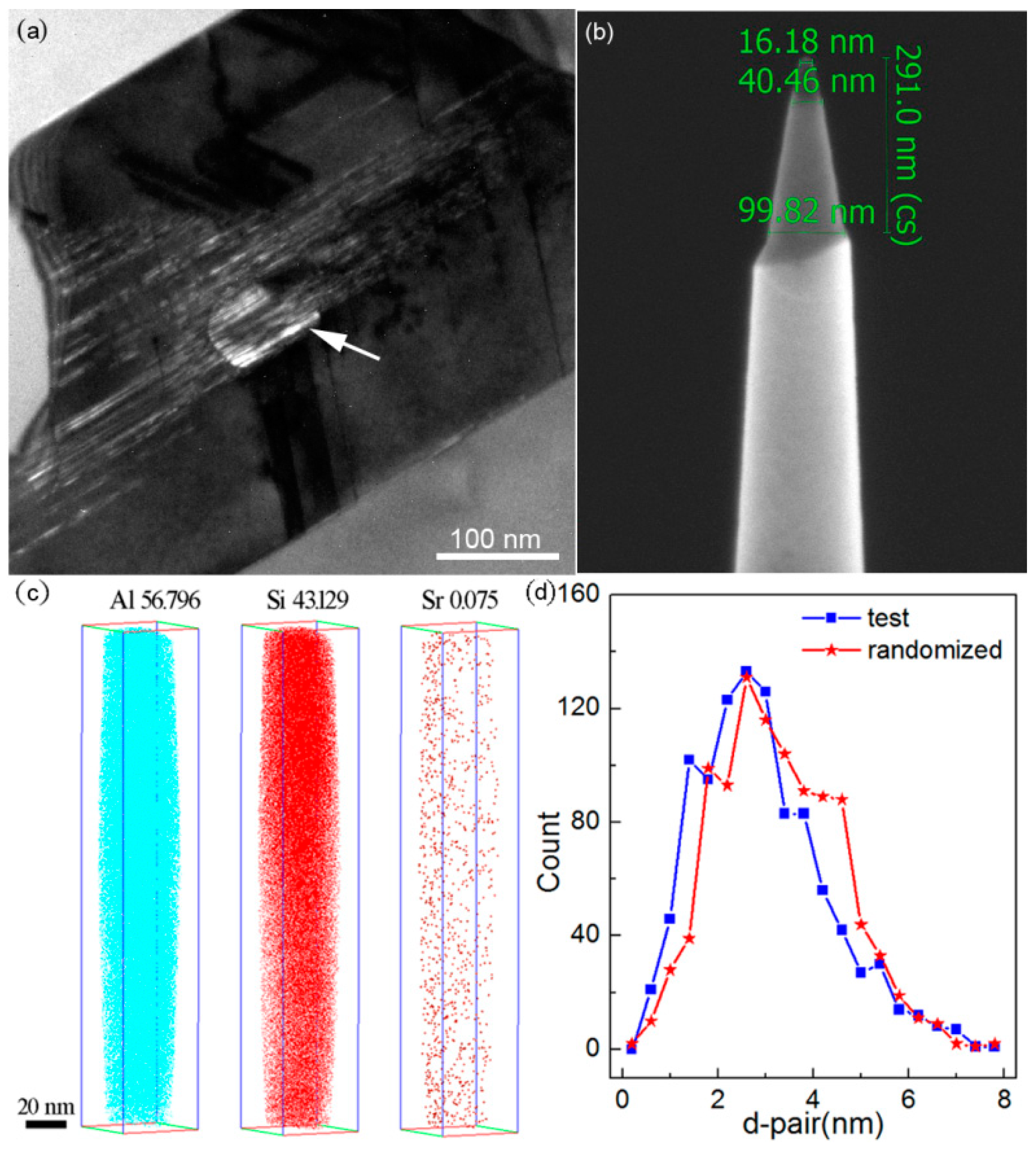
3.6. Atom Probe Tomography
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mazahery, A.; Shabani, M.O. Modification mechanism and microstructural characteristics of eutectic Si in casting Al-Si Alloys: A review on experimental and numerical studies. JOM J. Min. Met. Mater. Soc. 2014, 66, 726–738. [Google Scholar] [CrossRef]
- Bahmani, A.; Hatami, N.; Varahram, N.; Davami, P.; Shabani, M.O. A mathematical model for prediction of microporosity in aluminum alloy A356. Int. J. Adv. Manuf. Technol. 2013, 64, 1313–1321. [Google Scholar] [CrossRef]
- Gong, C.; Tu, H.; Wu, C.; Wang, J.; Su, X. Study on microstructure and mechanical properties of hypereutectic Al–18Si alloy modified with Al–3B. Materials 2018, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Dong, Y.; Zhao, D.; Wang, Y.; Teng, X. Study on the anti-poison performance of Al–Y–P master alloy for impurity Ca in aluminum alloys. Materials 2017, 10, 1356. [Google Scholar]
- Anilchandra, A.R.; Arnberg, L.; Bonollo, F.; Fiorese, E.; Timelli, G. Evaluating the tensile properties of aluminum foundry alloys through reference castings. Materials 2017, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.; Schaefer, A.; Chikova, O.; Borodianskiy, K. Study of Al-Si alloy oxygen saturation on its microstructure and mechanical properties. Materials 2017, 10, 786. [Google Scholar] [CrossRef] [PubMed]
- Selivorstov, V.; Dotsenko, Y.; Borodianskiy, K. Influence of low-frequency vibration and modification on solidification and mechanical properties of Al-Si casting alloy. Materials 2017, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.D. The effect of internal stresses due to precipitates on twin growth in magnesium. Acta Mater. 2016, 121, 277–287. [Google Scholar] [CrossRef]
- Robson, J.D.; Stanford, N.; Barnett, M. Effect of precipitate shape on slip and twinning in magnesium alloys. Acta Mater. 2011, 59, 1945–1956. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, X.D.; Ludwig, T.H.; Tsunekawa, Y.; Arnberg, L.; Jiang, J.Z.; Schumacher, P. Modification of eutectic Si in Al–Si alloys with Eu addition. Acta Mater. 2015, 84, 153–163. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, H.; Fan, G.; Li, J.; Lu, Y.; Jie, J.; Zhang, Y.; Li, T.; Jian, X.; Wang, T. Grain refinement of hypoeutectic Al-Si alloys with B. Acta Mater. 2016, 120, 168–178. [Google Scholar] [CrossRef]
- Barrirero, J.; Li, J.; Engstler, M.; Ghafoor, N.; Schumacher, P.; Odén, M.; Mücklich, F. Cluster formation at the Si/liquid interface in Sr and Na modified Al-Si alloys. Scr. Mater. 2016, 117, 16–19. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Kang, S.B.; Cho, J.H.; Min, G.; Stetsenko, V.Y. Effect of fine-grained structural Al–12%Si materials on morphologies and crystal defects of eutectic Si in HCC Al–12%Si alloy billets. J. Alloy. Compd. 2012, 541, 157–162. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.Y.; Jia, Y.W.; Zhou, H.; Wang, Q.D.; Ye, B.; Ding, W.J. Nanoparticle-inhibited growth of primary aluminum in Al–10Si alloys. Acta Mater. 2016, 103, 252–263. [Google Scholar] [CrossRef]
- Lu, S.Z.; Hellawell, A. The mechanism of silicon modification in aluminum-silicon alloys: Impurity induced twinning. Metall. Trans. A 1987, 18, 1721–1733. [Google Scholar] [CrossRef]
- Wanger, R.S. On the growth of germanium dendrites. Acta Metall. 1960, 8, 57–60. [Google Scholar]
- Hamilton, R.D.; Seidensticker, R.G. Propagation Mechanism of Germanium Dendrites. J. Appl. Phys. 1960, 31, 1165–1168. [Google Scholar] [CrossRef]
- Li, J.H.; Albu, M.; Hofer, F.; Schumacher, P. Nucleation kinetics of entrained eutectic Si in Al–5Si alloys. Acta Mater. 2014, 72, 80–98. [Google Scholar] [CrossRef]
- Timpel, M.; Wanderka, N.; Schlesiger, R.; Yamamoto, T.; Lazarev, N.; Isheim, D.; Schmitz, G.; Matsumura, S.; Banhart, J. Effect of fine-grained structural Al–12%Si materials on morphologies and crystal defects of eutectic Si in HCC Al–12%Si alloy billets. Acta Mater. 2012, 60, 3920–3928. [Google Scholar] [CrossRef]
- Barrirero, J.; Engstler, M.; Ghafoor, N.; de Jonge, N.; Odén, M.; Mücklich, F. Comparison of segregations formed in unmodified and Sr-modified Al–Si alloys studied by atom probe tomography and transmission electron microscopy. J. Alloy. Compd. 2014, 611, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zheng, H.; Liu, Y.; Shi, L.; Xu, R.; Tian, X. Cluster-assisted nucleation of silicon phase in hypoeutectic Al–Si alloy with further inoculation. Acta Mater. 2014, 70, 162–173. [Google Scholar] [CrossRef]
- Guo, F.; Wang, W.; Yu, W.; Zhang, Y.; Pan, S.; Zhou, Z.; Liu, D.; Qin, J.; Wang, Y.; Tian, X. Enhanced nucleation and refinement of eutectic Si by high number-density nano-particles in Al–10Si–0.5Sb alloys. Mater. Des. 2017, 117, 382–389. [Google Scholar] [CrossRef]
- Farahany, S.; Idris, M.H.; Ourdjini, A. Evaluations of antimony and strontium interaction in an Al–Si–Cu–Zn die cast alloy. Thermochim. Acta 2014, 584, 72–78. [Google Scholar] [CrossRef]
- Zhao, D.; Bao, W.; Li, H.; Zheng, S.; Chou, K.C. Cluster-assisted nucleation mechanism of titanium oxides in Fe-Ti supercooled alloys. J. Alloy. Compd. 2018, 744, 797–800. [Google Scholar] [CrossRef]
- Yi, G.; Sun, B.; Poplawsky, J.D.; Zhu, Y.; Free, M.L. Investigation of pre-existing particles in Al 5083 alloys. J. Alloy. Compd. 2018, 740, 461–469. [Google Scholar] [CrossRef]
- Yue, G.; Wu, S.; Shen, B.; Wang, S.; Wang, C.; Ho, K.; Kramer, M.; Chen, L. Effects of strontium impurity on the structure and dynamics of Al88Si12 liquid. J. Phys. Condens. Matter 2013, 24, 245102. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Feng, S.; Qiao, J.; Dong, B.; Qin, J. The atomic structure of liquid Fe–C alloys. J. Alloy. Compd. 2015, 648, 178–183. [Google Scholar] [CrossRef]
- Makhlouf, M.M.; Guth, H.V. The Al-Si eutectic reaction: Mechanisms and crystallography. J. Light Met. 2001, 1, 199–218. [Google Scholar] [CrossRef]
- Guo, F.; Zheng, H.; Qin, J.; Qin, X.; Lv, T.; Jia, Y.; Xu, R.; Tian, X. Medium-range order and physical properties of Cu–20at.% Sb melts. J. Non-Cryst. Solids 2012, 358, 3327–3331. [Google Scholar] [CrossRef]
- Li, J.H.; Albu, M.; Hofer, F.; Schumacher, P. Solute adsorption and entrapment during eutectic Si growth in A–Si-based alloys. Acta Mater. 2015, 83, 187–202. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.Y.; Wang, Q.D.; Ding, W.J. Nanoparticle-induced nucleation of eutectic silicon in hypoeutectic Al-Si alloy. Mater. Charact. 2016, 117, 41–46. [Google Scholar] [CrossRef]
- Zuo, M.; Liu, X.F.; Sun, Q.Q.; Jiang, K. Effect of rapid solidification on the microstructure and refining performance of an Al–Si–P master alloy. J. Mater. Process. Technol. 2009, 209, 5504–5508. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yu, W.; Wang, X.; Zheng, H.; Tian, X. Pre-nucleation clusters mediated crystallization in Al–Si melts. Scr. Mater. 2016, 110, 87–91. [Google Scholar] [CrossRef]
- Terzi, S.; Taylor, J.A.; Cho, Y.H.; Salvo, L.; Suery, M.; Boller, E.; Dahle, A.K. In situ study of nucleation and growth of the irregular α-Al/β-Al5FeSi eutectic by 3-D synchrotron X-ray microtomography. Acta Mater. 2010, 58, 5370–5380. [Google Scholar] [CrossRef]
- Timpel, M.; Wanderka, N.; Schlesiger, R.; Yamamoto, T.; Isheim, D.; Schmitz, G.; Matsumura, S.; Banhart, J. Sr–Al–Si co-segregated regions in eutectic Si phase of Sr-modified Al–10Si alloy. Ultramicroscopy 2013, 132, 216–221. [Google Scholar] [CrossRef] [PubMed]

| Alloys | Al | Si | Fe | Mn | Ni | Ti | Na | P | Sb | Sr |
|---|---|---|---|---|---|---|---|---|---|---|
| Al–10Si | 89.844 | 10.010 | 0.141 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0 | 0 |
| Al–10Si–0.015Sr | 90.133 | 9.720 | 0.125 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 | 0 | 0.015 |
| Al–10Si–0.35Sb–0.015Sr | 89.552 | 9.940 | 0.140 | 0.001 | 0.003 | 0.005 | 0.001 | 0.001 | 0.344 | 0.013 |
| Al–10Si–0.35Sb | 89.409 | 10.100 | 0.148 | 0.001 | 0.003 | 0.006 | 0.001 | 0.001 | 0.331 | 0 |
| TN,α-Al (°C) | TM,α-Al (°C) | TN,E (°C) | TM,E (°C) | TG,E (°C) | |
|---|---|---|---|---|---|
| Al–10Si | 589.3 | 588.6 | 578.4 | 577.7 | 578.8 |
| Al–10Si–0.015Sr | 591.1 | 590.4 | 572.6 | 571.5 | 575.1 |
| Al–10Si–0.35Sb | 591.0 | 590.4 | 578.3 | 577.2 | 579.6 |
| Al–10Si–0.35Sb–0.015Sr | 592.0 | 591.2 | 578.3 | 577.6 | 578.9 |
| Ni-j | j = Si | j = Sb | j = Sr | CSROi-Si | CSROi-Sb | CSROi-Sr |
|---|---|---|---|---|---|---|
| i = Si | 6.668 | 0.887 | 0.130 | 0.077 | −1.308 | −0.700 |
| i = Sb | 16.673 | 1.573 | 0.452 | 0.051 | −0.682 | −1.410 |
| i = Sr | 12.250 | 2.258 | 0.023 | 0.103 | −2.108 | 0.840 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Guo, F.; Gai, Z.; Zhang, T.; Tang, J.; Tian, X.; Liu, W. The Formation of Nanoparticles and Their Competitive Interaction with Twins during Eutectic Si Growth. Materials 2018, 11, 1404. https://doi.org/10.3390/ma11081404
Wang W, Guo F, Gai Z, Zhang T, Tang J, Tian X, Liu W. The Formation of Nanoparticles and Their Competitive Interaction with Twins during Eutectic Si Growth. Materials. 2018; 11(8):1404. https://doi.org/10.3390/ma11081404
Chicago/Turabian StyleWang, Wei, Fengxiang Guo, Zhigang Gai, Tao Zhang, Jianguo Tang, Xuelei Tian, and Wenqing Liu. 2018. "The Formation of Nanoparticles and Their Competitive Interaction with Twins during Eutectic Si Growth" Materials 11, no. 8: 1404. https://doi.org/10.3390/ma11081404




