Composites of Laponite and Cu–Mn Hopcalite-Related Mixed Oxides Prepared from Inverse Microemulsions as Catalysts for Total Oxidation of Toluene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
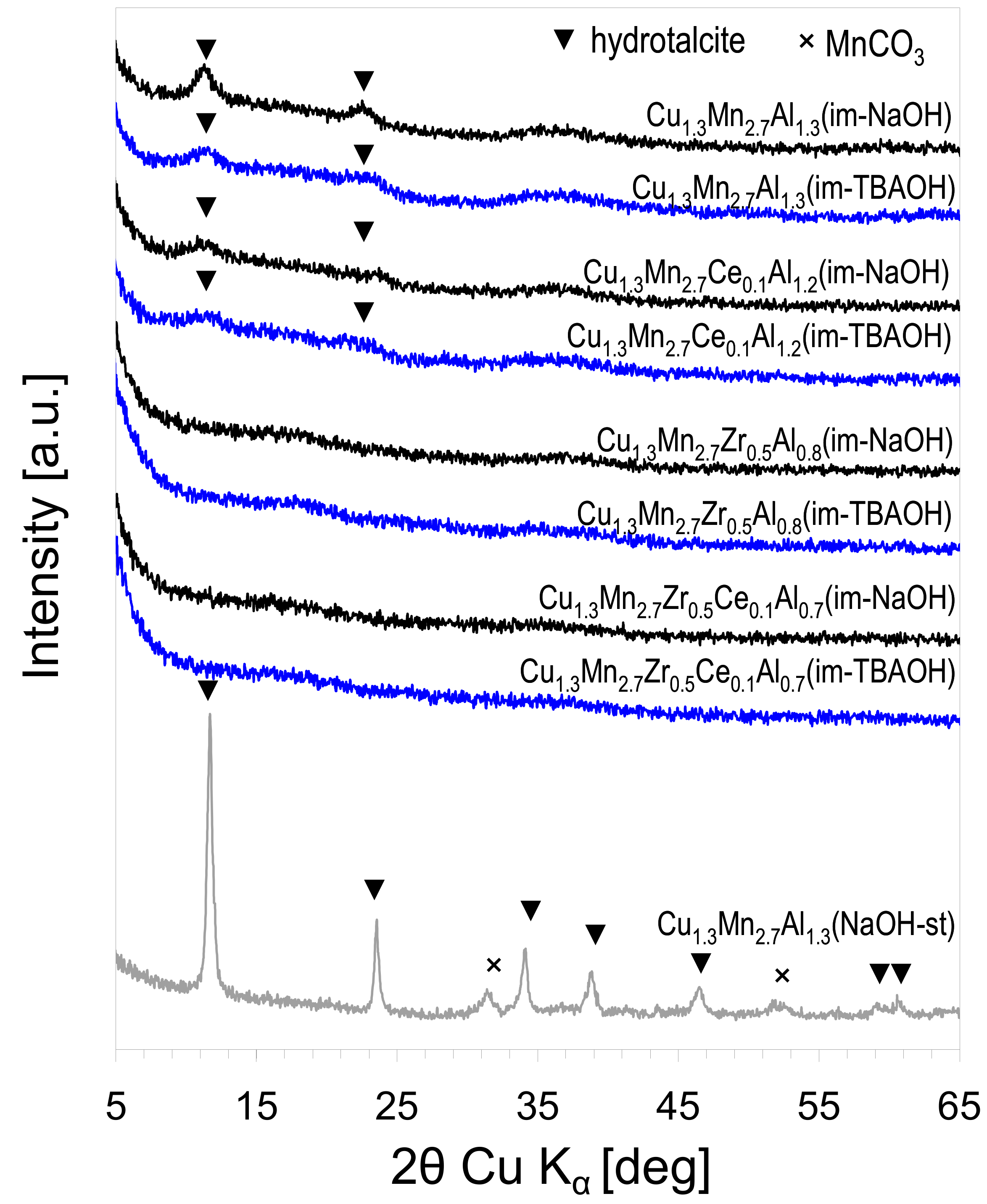
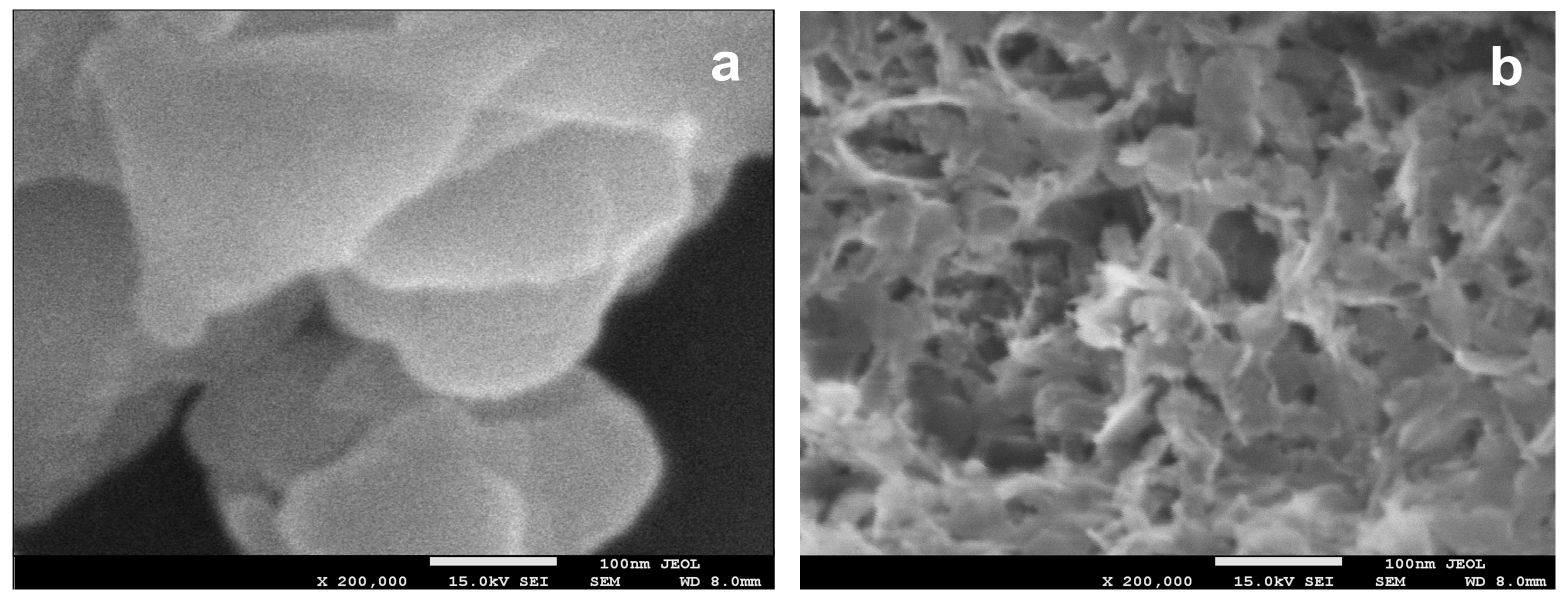
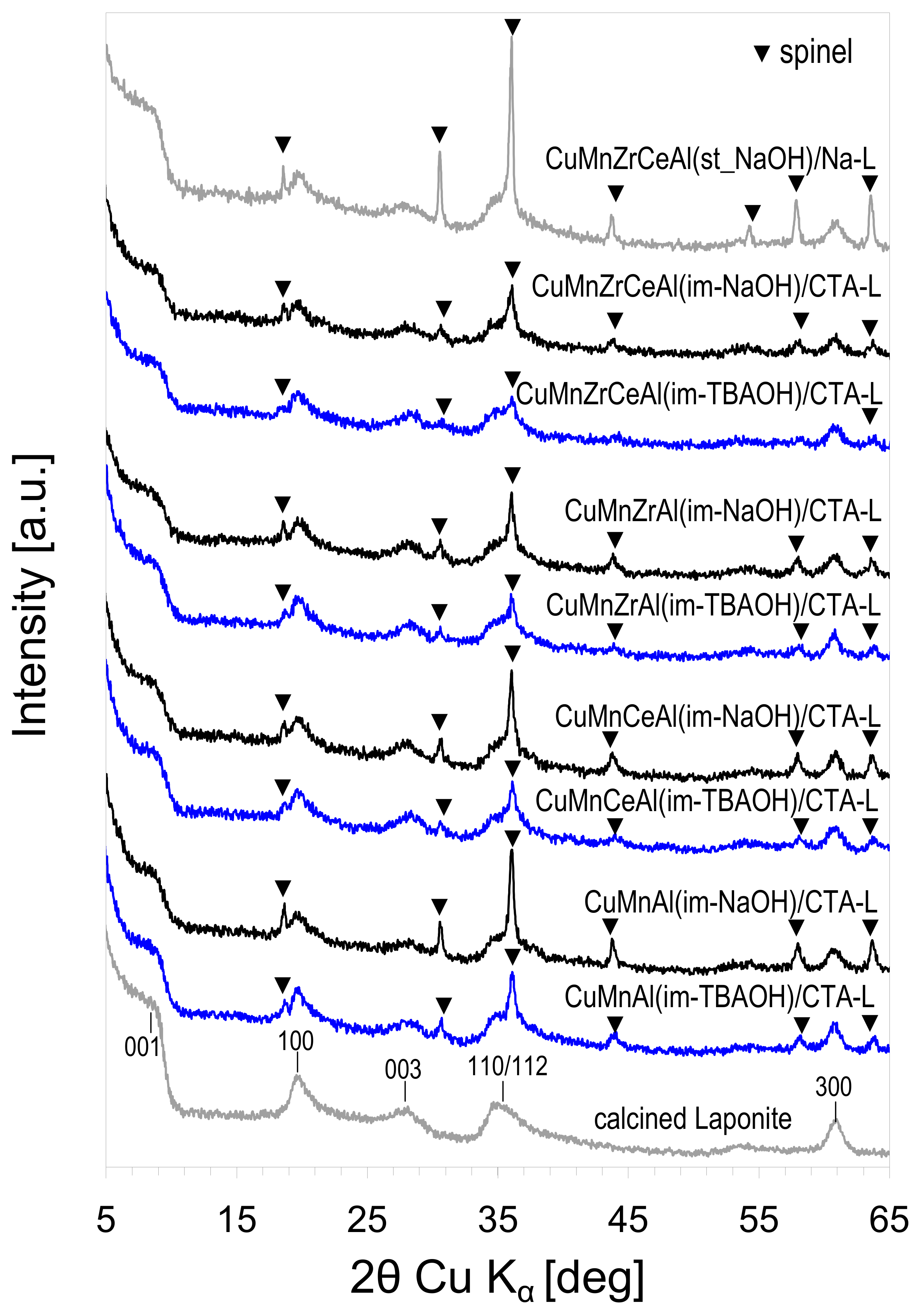
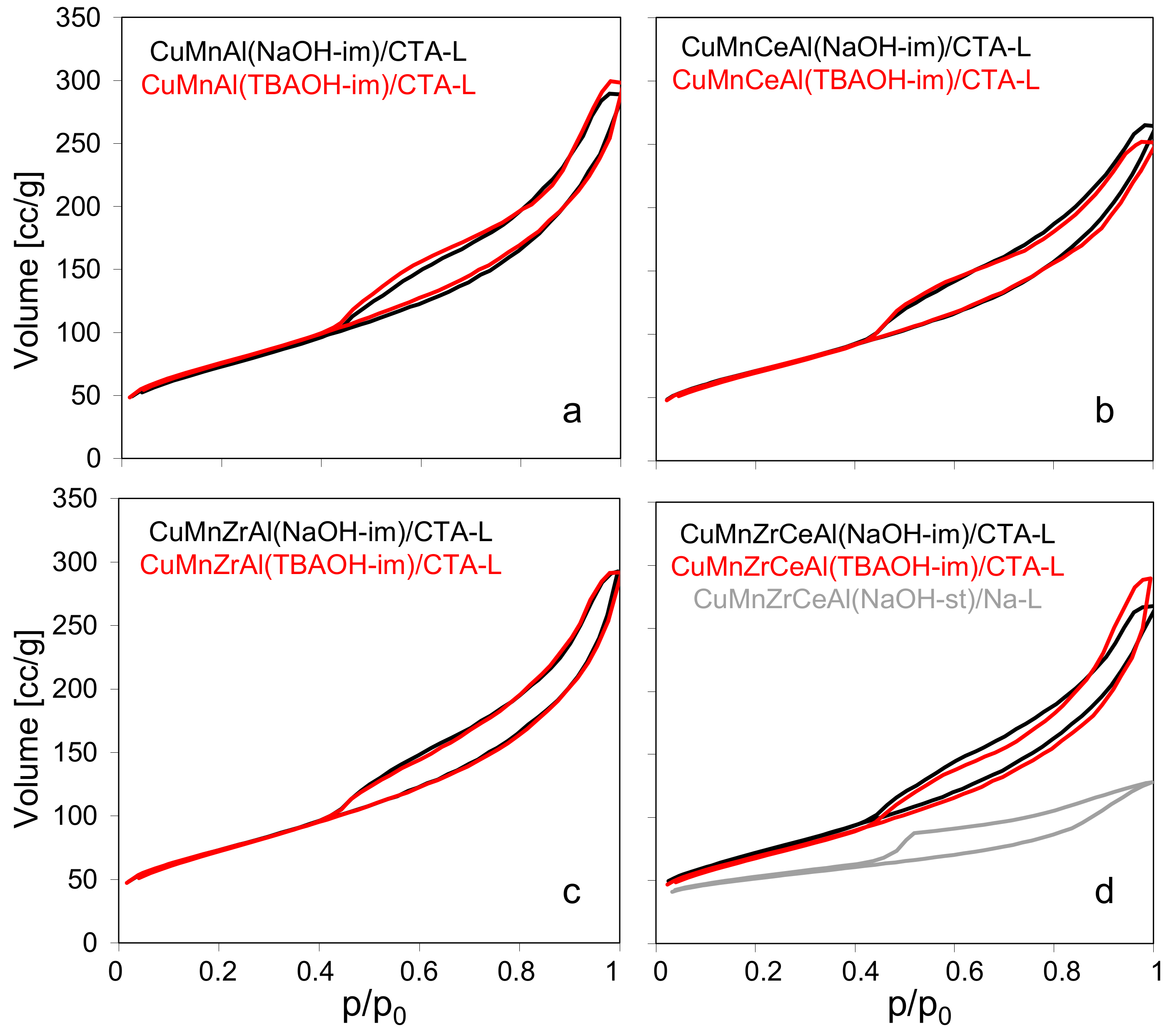
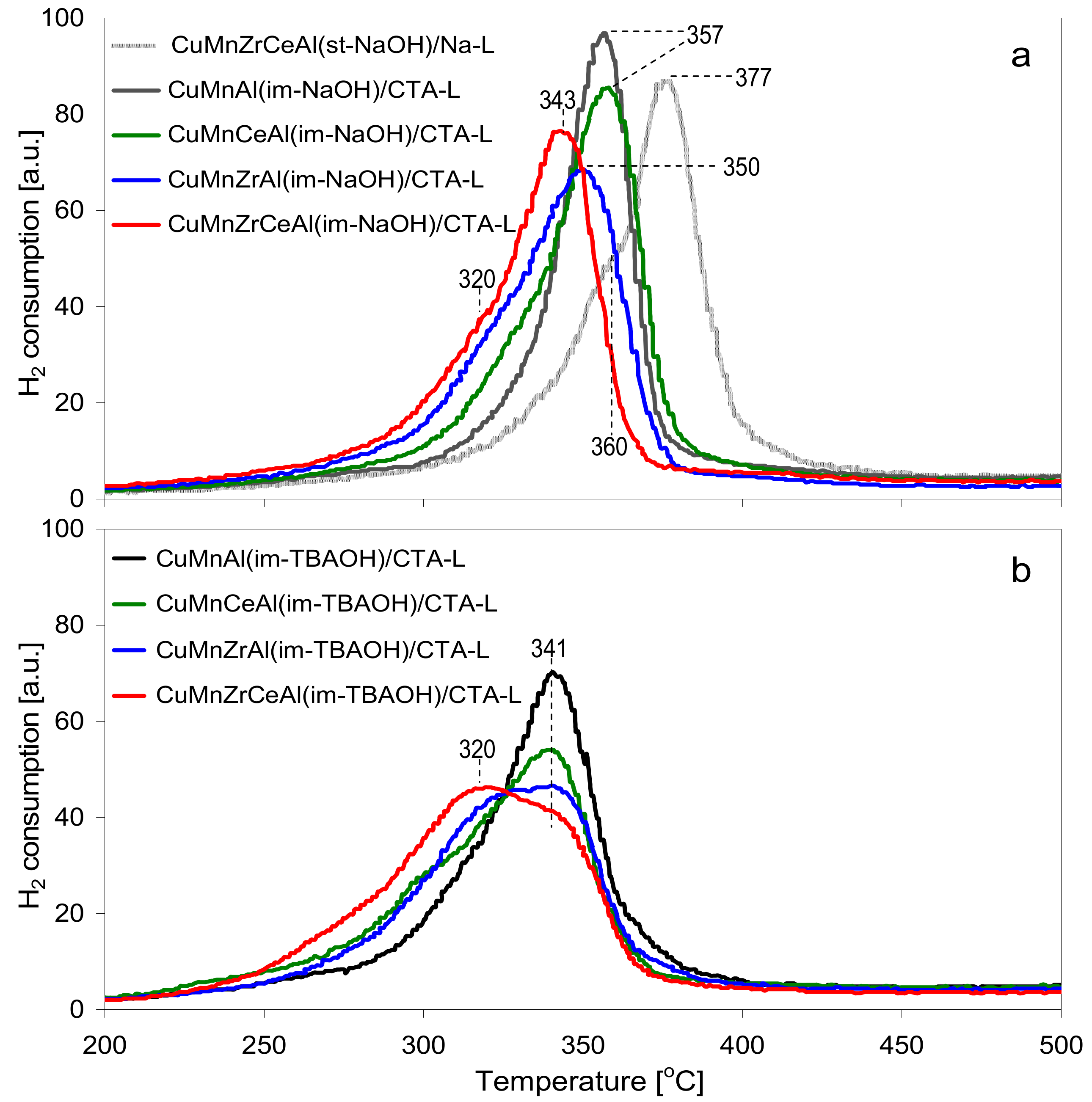
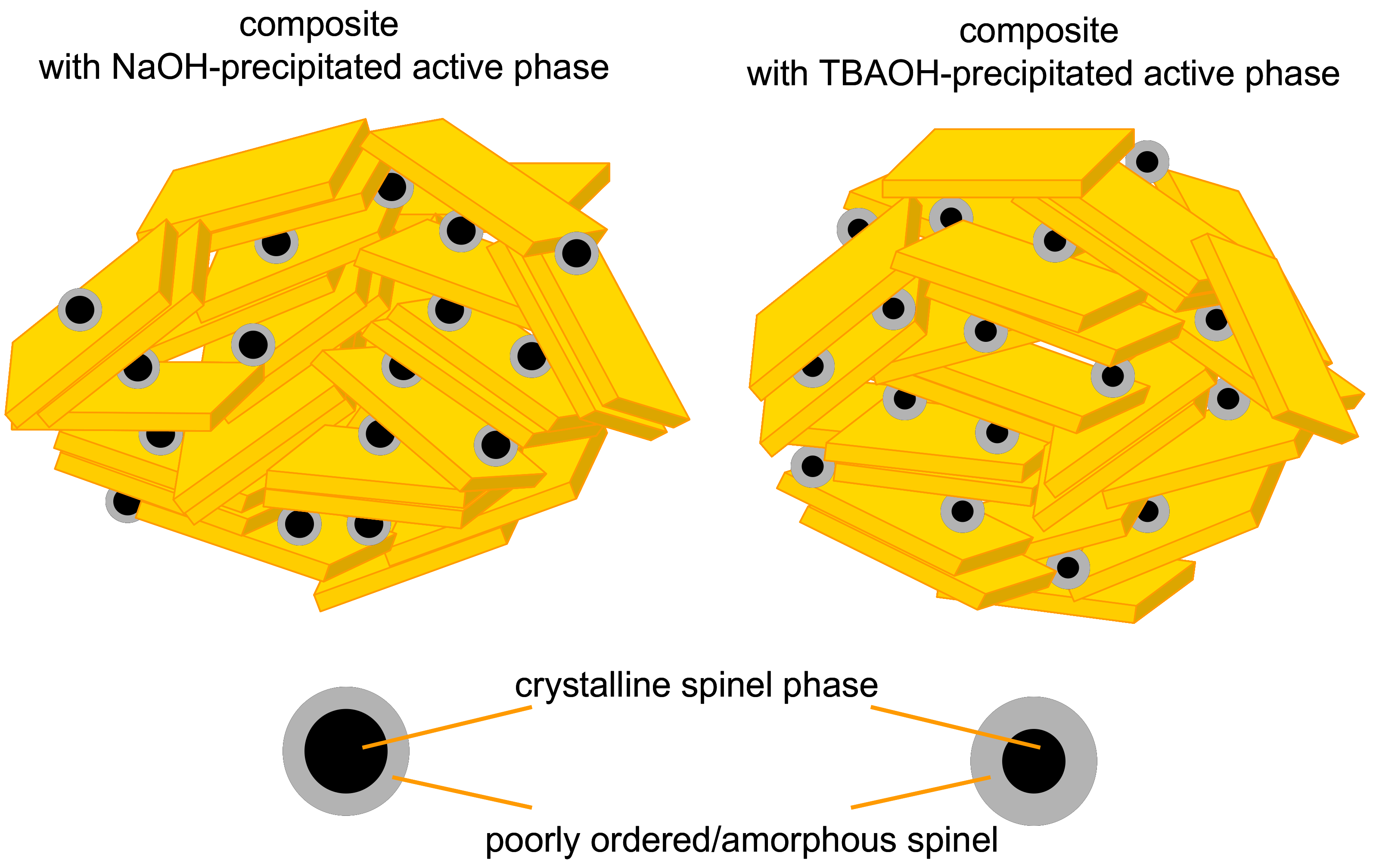
3.1. Characterization of Composites
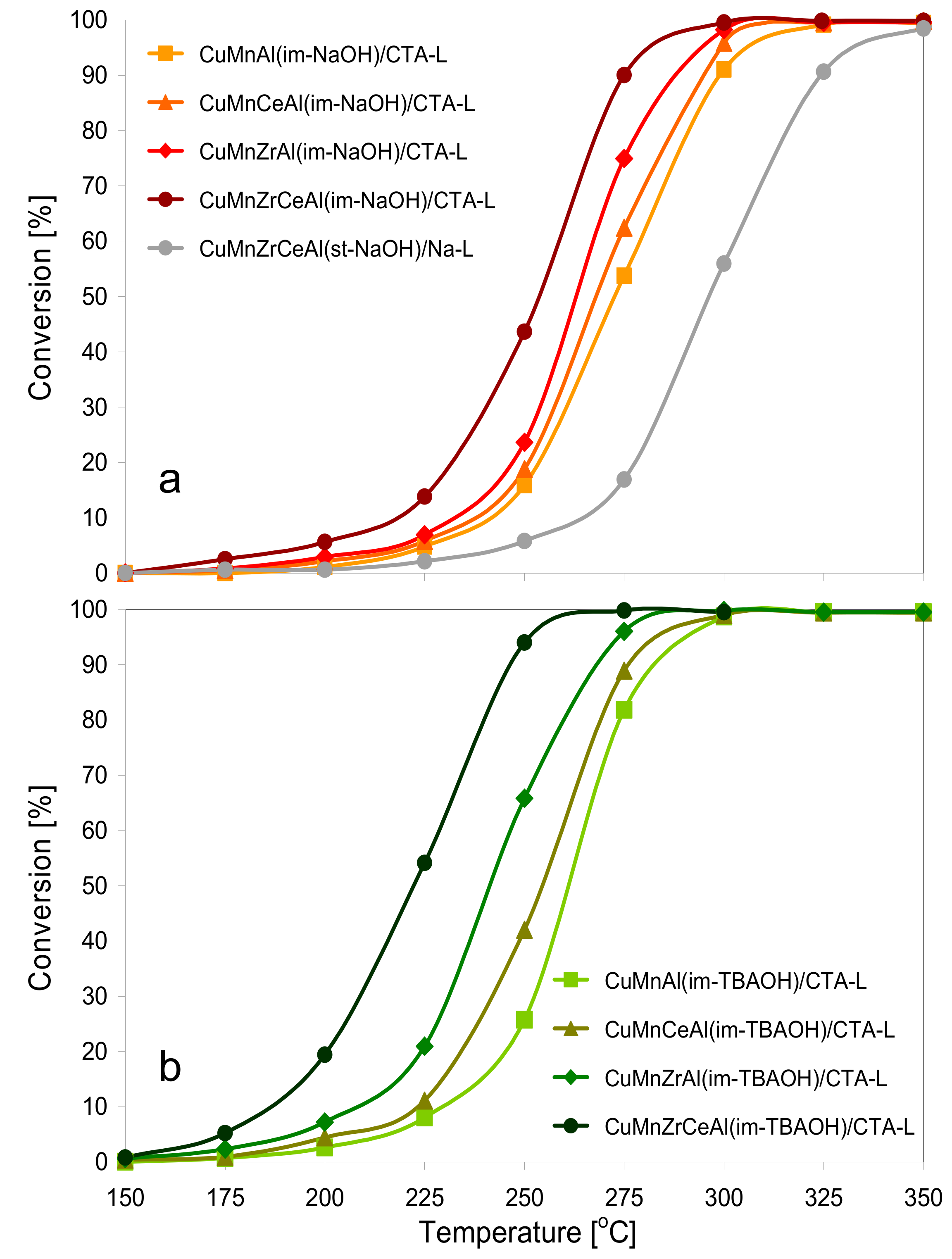
3.2. Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shanguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Ciambelli, P.; Perathoner, S.; Russo, P. Environmental catalysis: Trends and outlook. Catal. Today 2002, 75, 3–15. [Google Scholar] [CrossRef]
- Lamb, A.B.; Bray, W.C.; Frazer, J.C.W. The Removal of Carbon Monoxide from Air. J. Ind. Eng. Chem. 1920, 12, 213–221. [Google Scholar] [CrossRef]
- Merrill, D.R.; Scalione, C.C. The Catalytic Oxidation of Carbon Monoxide at Ordinary Temperatures. J. Am. Chem. Soc. 1921, 43, 1982–2002. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadús, L.E. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B Environ. 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Zimowska, M.; Michalik-Zym, A.; Janik, R.; Machej, T.; Gurgul, J.; Socha, R.P.; Podobiński, J.; Serwicka, E.M. Catalytic combustion of toluene over mixed Cu-Mn oxides. Catal. Today 2007, 119, 321–326. [Google Scholar] [CrossRef]
- Chen, H.; Tong, X.; Li, Y. Mesoporous Cu-Mn Hopcalite catalyst and its performance in low temperature ethylene combustion in a carbon dioxide stream. Appl. Catal. A Gen. 2009, 370, 59–65. [Google Scholar] [CrossRef]
- Vu, V.H.; Belkouch, J.; Ould-Dris, A.; Taouk, B. Removal of hazardous chlorinated VOCs over Mn–Cu mixed oxide based catalyst. J. Hazard. Mater. 2009, 169, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on γ-Al2O3. Appl. Catal. B Environ. 2011, 103, 275–286. [Google Scholar] [CrossRef]
- Behar, S.; Gonzalez, P.; Agulhon, P.; Quignard, F.; Świerczyński, D. New synthesis of nanosized Cu-Mn spinels as efficient oxidation catalysts. Catal. Today 2012, 189, 35–41. [Google Scholar] [CrossRef]
- Aguilera, D.A.; Perez, A.; Molina, R.; Moreno, S. Cu-Mn and Co-Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Machej, T.; Serwicka, E.M.; Zimowska, M.; Dula, R.; Michalik-Zym, A.; Napruszewska, B.; Rojek, W.; Socha, R. Cu/Mn-based mixed oxides derived from hydrotalcite-like precursors as catalysts for methane combustion. Appl. Catal. A Gen. 2014, 474, 87–94. [Google Scholar] [CrossRef]
- Behar, S.; Gómez-Mendoza, N.A.; Gómez-García, M.Á.; Świerczyński, D.; Quignard, F.; Tanchoux, N. Study and modelling of kinetics of the oxidation of VOC catalyzed by nanosized Cu-Mn spinels prepared via an alginate route. Appl. Catal. A Gen. 2015, 504, 203–210. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Shan, X.; Liu, G.; Chen, Y. Co-nanocasting synthesis of mesoporous Cu-Mn composite oxides and their promoted catalytic activities for gaseous benzene removal. Appl. Catal. B Environ. 2015, 162, 110–121. [Google Scholar] [CrossRef]
- Răciulete, M.; Layrac, G.; Papa, F.; Negrilă, C.; Tichit, D.; Marcu, I.C. Influence of Mn content on the catalytic properties of Cu-(Mn)-Zn-Mg-Al mixed oxides derived from LDH precursors in the total oxidation of methane. Catal. Today 2018, 306, 276–286. [Google Scholar] [CrossRef]
- Palacio, L.A.; Velásquez, J.; Echavarría, A.; Faro, A.; Ribeiro, F.R.; Ribeiro, M.F. Total oxidation of toluene over calcined trimetallic hydrotalcites type catalysts. J. Hazard. Mater. 2010, 177, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Giraudon, J.M.; Nuns, N.; Simon, P.; De Geyter, N.; Morent, R.; Lamonier, J.F. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation. Appl. Catal. B Environ. 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Napruszewska, B.D.; Michalik-Zym, A.; Dula, R.; Bielańska, E.; Rojek, W.; Machej, T.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. Composites derived from exfoliated Laponite and Mn-Al hydrotalcite prepared in inverse microemulsion: A new strategy for design of robust VOCs combustion catalysts. Appl. Catal. B Environ. 2017, 211, 46–56. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Rogowska, M.; Bielańska, E.; Rojek, W.; Gaweł, A.; Wójcik-Bania, M.; Bahranowski, K.; Serwicka, E.M. Novel Montmorillonite/TiO2/MnAl-Mixed Oxide Composites Prepared from Inverse Microemulsions as Combustion Catalysts. Materials 2017, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Napruszewska, B.D.; Michalik-Zym, A.; Dula, R.; Duraczyńska, D.; Rojek, W.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. VOCs combustion catalysts based on composites of exfoliated organo-Laponite and multimetallic (Mn, Al, Zr, Ce) hydrotalcites prepared by inverse microemulsion. Catal. Today 2018, in press. [Google Scholar] [CrossRef]
- Ying, J.Y. Design and synthesis of nanostructured catalysts. Chem. Eng. Sci. 2006, 61, 1540–1548. [Google Scholar] [CrossRef]
- Bellezza, F.; Cipiciani, A.; Costantino, U.; Nocchetti, M.; Posati, T. Hydrotalcite-like nanocrystals from water-in-oil microemulsions. Eur. J. Inorg. Chem. 2009, 18, 2603–2611. [Google Scholar] [CrossRef]
- Boutonnet, M.; Lögdberg, S.; Svensson, E. Recent developments in the application of nanoparticles prepared from w/o microemulsions in heterogeneous catalysis. Curr. Opin. Colloid Interface Sci. 2008, 13, 270–286. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1127. [Google Scholar] [CrossRef]
- Aisawa, S.; Hirahara, H.; Uchiyama, H.; Takahashi, S.; Narita, E. Synthesis and Thermal Decomposition of Mn-Al Layered Double Hydroxides. J. Solid State Chem. 2002, 167, 152–159. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimerk, A.V.; Olivier, J.P.; Rodriguez-Reinozo, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 879, 1051–1069. [Google Scholar] [CrossRef]
- Doornkamp, C.; Ponec, V. The universal character of the Mars and Van Krevelen mechanism. J. Mol. Catal. A Chem. 2000, 162, 19–32. [Google Scholar] [CrossRef]
- Buciuman, F.C.; Patcas, F.; Hahn, T. A spillover approach to oxidation catalysis over copper and manganese mixed oxides. Chem. Eng. Process. 1999, 38, 563–569. [Google Scholar] [CrossRef]
- Mirzaei, A.A.; Shaterian, H.R.; Habibi, M.; Hutchings, G.J.; Taylor, S.H. Characterisation of copper-manganese oxide catalysts: Effect of precipitate ageing upon the structure and morphology of precursors and catalysts. Appl. Catal. A Gen. 2003, 253, 499–508. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takeguchi, T.; Kikuchi, R.; Eguchi, K. Influence of preparation method and additive for Cu-Mn spinel oxide catalyst on water gas shift reaction of reformed fuels. Appl. Catal. A Gen. 2005, 279, 59–66. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Combined steam reforming of methanol over Cu-Mn spinel oxide catalysts. J. Catal. 2007, 251, 7–20. [Google Scholar] [CrossRef]
- Dua, Z.; Yuan, X.; Cao, L.; Zhang, C.; Wang, S. Water gas shift reaction over Cu-Mn mixed oxides catalysts: Effects of the third metal. Fuel Process. Technol. 2008, 89, 131–138. [Google Scholar] [CrossRef]
- Pokrovski, K.A.; Bell, A.T. An investigation of the factors influencing the activity of Cu/CexZr1−xO2 for methanol synthesis via CO hydrogenation. J. Catal. 2006, 241, 276–286. [Google Scholar] [CrossRef]
- Fokema, M.D.; Chiu, E.; Ying, J.Y. Synthesis and Characterization of Nanocrystalline Yttrium Oxide Prepared with Tetraalkylammonium Hydroxides. Langmuir 2000, 16, 3154–3159. [Google Scholar] [CrossRef]
- Paike, W.V.; Niphadkar, P.S.; Bokade, V.V.; Joshi, P.N. Synthesis of spinel CoFe2O4 via the co-precipitation method using tetraalkyl ammonium hydroxides as precipitating agents. J. Am. Ceram. Soc. 2007, 90, 3009–3012. [Google Scholar] [CrossRef]

| Sample | Si (at.%) | Mg (at.%) | Mn (at.%) | Cu (at.%) | Al (at.%) | Zr (at.%) | Ce (at.%) | Na (at.%) |
|---|---|---|---|---|---|---|---|---|
| CuMnAl(im-NaOH)/CTA-L | 46.4 | 31.8 | 10.9 | 5.5 | 5.4 | - | - | - |
| CuMnCeAl(im-NaOH)/CTA-L | 46.2 | 31.3 | 11.2 | 5.7 | 5.2 | - | 0.4 | - |
| CuMnZrAl(im-NaOH)/CTA-L | 46.8 | 31.6 | 10.7 | 5.5 | 3.3 | 2.1 | - | - |
| CuMnZrCeAl(im-NaOH)/CTA-L | 47.3 | 31.9 | 10.6 | 5.4 | 2.7 | 1.8 | 0.3 | - |
| CuMnAl(im-TBAOH)/CTA-L | 47.3 | 32.2 | 10.2 | 5.2 | 5.1 | - | - | - |
| CuMnCeAl(im-TBAOH)/CTA-L | 47.4 | 32.0 | 10.1 | 5.2 | 5.0 | - | 0.3 | - |
| CuMnZrAl(im-TBAOH)/CTA-L | 46.6 | 31.9 | 10.7 | 5.4 | 3.2 | 2.2 | - | - |
| CuMnZrCeAl(im-TBAOH)/CTA-L | 47.0 | 32.1 | 10.4 | 5.3 | 2.8 | 2.0 | 0.4 | - |
| CuMnZrCeAl(st)/Na-L | 44.5 | 30.2 | 11.1 | 5.6 | 3.0 | 2.1 | 0.4 | 3.1 |
| Sample | SBET (m2/g) | Vtot (cm3/g) | Smicro (m2/g) | Vmicro (cm3/g) | Dav (nm) | H/MnTPR | T50 (°C) | T90 (°C) |
|---|---|---|---|---|---|---|---|---|
| CuMnAl(im-NaOH)/CTA-L | 265 | 0.45 | 14 | 0.004 | 33.7 | 1.0 | 272 | 299 |
| CuMnCeAl(im-NaOH)/CTA-L | 253 | 0.41 | 21 | 0.008 | 32.3 | 1.0 | 268 | 295 |
| CuMnZrAl(im-NaOH)/CTA-L | 264 | 0.45 | 8 | 0.001 | 34.3 | 1.0 | 263 | 288 |
| CuMnZrCeAl(im-NaOH)/CTA-L | 259 | 0.41 | 10 | 0.002 | 32.0 | 0.9 | 254 | 275 |
| CuMnAl(im-TBAOH)/CTA-L | 273 | 0.46 | 13 | 0.004 | 33.7 | 0.9 | 261 | 282 |
| CuMnCeAl(im-TBAOH)/CTA-L | 254 | 0.39 | 15 | 0.005 | 30.6 | 1.1 | 255 | 275 |
| CuMnZrAl(im-TBAOH)/CTA-L | 262 | 0.45 | 16 | 0.005 | 34.4 | 0.9 | 241 | 268 |
| CuMnZrCeAl(im-TBAOH)/CTA-L | 257 | 0.45 | 14 | 0.004 | 36.3 | 1.0 | 222 | 246 |
| CuMnZrCeAl(st)/Na-L | 189 | 0.21 | 62 | 0.021 | 44.0 | 1.0 | 296 | 324 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napruszewska, B.D.; Michalik, A.; Walczyk, A.; Duraczyńska, D.; Dula, R.; Rojek, W.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. Composites of Laponite and Cu–Mn Hopcalite-Related Mixed Oxides Prepared from Inverse Microemulsions as Catalysts for Total Oxidation of Toluene. Materials 2018, 11, 1365. https://doi.org/10.3390/ma11081365
Napruszewska BD, Michalik A, Walczyk A, Duraczyńska D, Dula R, Rojek W, Lityńska-Dobrzyńska L, Bahranowski K, Serwicka EM. Composites of Laponite and Cu–Mn Hopcalite-Related Mixed Oxides Prepared from Inverse Microemulsions as Catalysts for Total Oxidation of Toluene. Materials. 2018; 11(8):1365. https://doi.org/10.3390/ma11081365
Chicago/Turabian StyleNapruszewska, Bogna D., Alicja Michalik, Anna Walczyk, Dorota Duraczyńska, Roman Dula, Wojciech Rojek, Lidia Lityńska-Dobrzyńska, Krzysztof Bahranowski, and Ewa M. Serwicka. 2018. "Composites of Laponite and Cu–Mn Hopcalite-Related Mixed Oxides Prepared from Inverse Microemulsions as Catalysts for Total Oxidation of Toluene" Materials 11, no. 8: 1365. https://doi.org/10.3390/ma11081365





