Anion Doping of Ferromagnetic Thin Films of La0.74Sr0.26MnO3−δ via Topochemical Fluorination
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
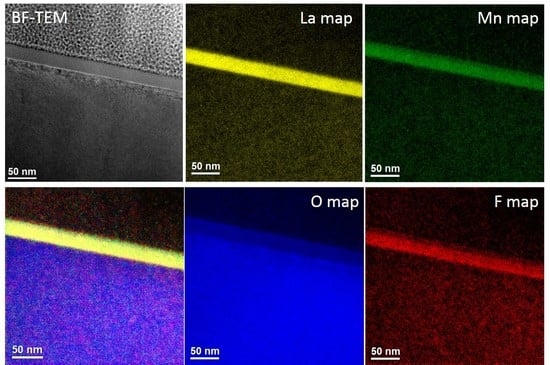
3.1. Morphology, Composition and Structure of the Films
- (a)
- Reductive fluorination: One fluoride ion replaces one oxygen ion, accompanied by a reduction of the transition metal oxidation state
- (b)
- Substitutive fluorination: Two Fluoride ions replace one oxygen ion and fill a vacancy under maintenance of the transition metal oxidation state
- (c)
- Oxidative fluorination: One Fluoride ion fills a vacancy, accompanied by an increase of the transition metal oxidation state
- (LSMO_F): La1−xSrxMn3+1−x+2yMn4+x−2yO3−y + 2y HF ➔ La1−xSrxMn3+1−x+2yMn4+x−2yO3−2yF2y + y H2O
- (LSMO_O): La1−xSrxMn3+1−x+2yMn4+x−2yO3−y + y/2 O2 ➔ La1−xSrxMn3+1−xMn4+xO3
- (LSMO_F+O): La1−xSrxMn3+1−x+2yMn4+x−2yO3−2yF2y + O2 ➔ no reaction
- (LSMO_O+F): La1−xSrxMn3+1−xMn4+xO3 + HF ➔ no reaction
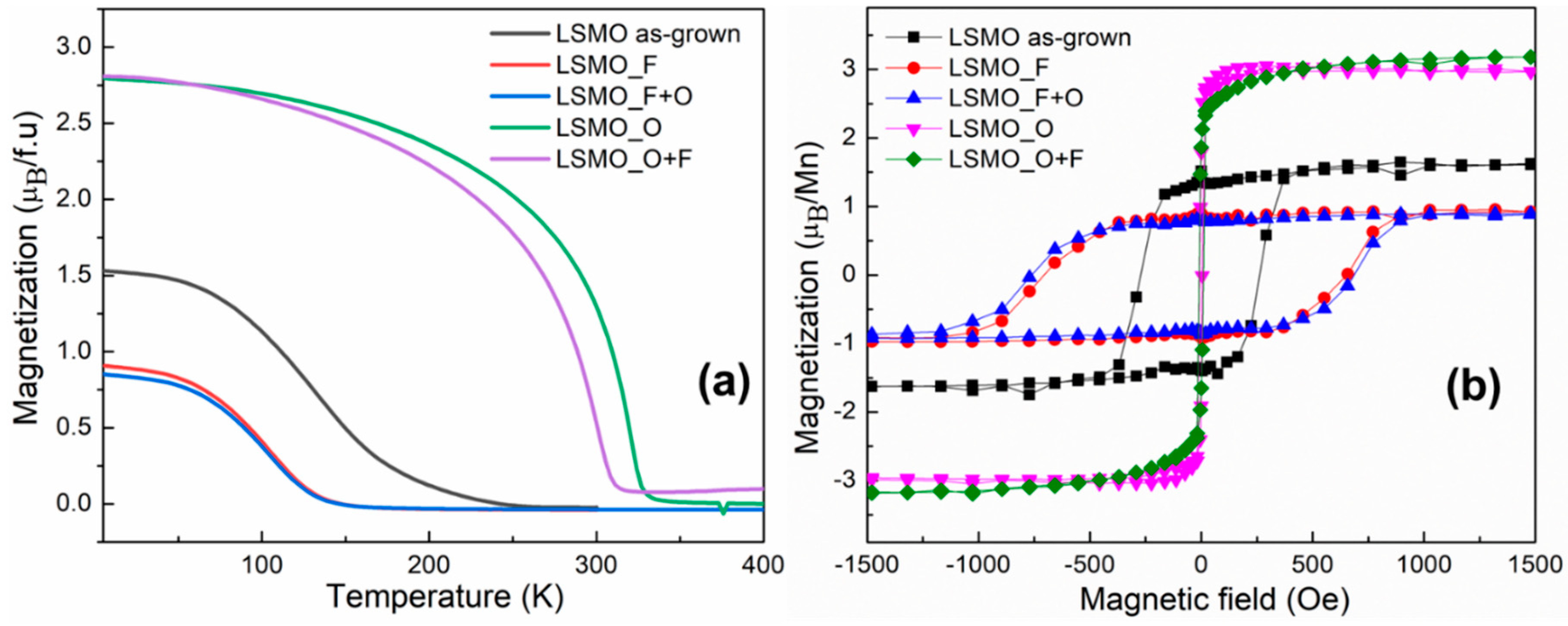
3.2. Magnetic Characterization
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gopalarao, T.R.; Dash, B.; Ravi, S. Magnetic and electrical transport properties of La0.7Sr0.3MnO3/LaFeO3 bilayer thin films. J. Magn. Magn. Mater. 2017, 441, 531–536. [Google Scholar] [CrossRef]
- Shinde, K.P.; Pawar, S.S.; Pawar, S.H. Influence of annealing temperature on morphological and magnetic properties of La0.9Sr0.1MnO3. Appl. Surf. Sci. 2011, 257, 9996–9999. [Google Scholar] [CrossRef]
- Bejar, M.; Dhahri, R.; El Halouani, F.; Dhahri, E. Magnetocaloric effect at room temperature in powder of La0.5(CaSr)0.5MnO3. J. Alloys Compd. 2006, 414, 31–35. [Google Scholar] [CrossRef]
- Wang, C.B.; Zhu, Y.X.; Li, L.; Shen, Q.; Zhang, L.M. Epitaxial growth and transport property of La0.9Sr0.1MnO3 thin films deposited on MgO, LaAlO3 and SrTiO3 substrates. J. Alloys Compd. 2017, 693, 832–836. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Wold, A.; Arnott, R.J.; Menyuk, N. Relationship between Crystal Symmetry and Magnetic properties of ionic compounds containing Mn3+. Phys. Rev. 1961, 124, 373–384. [Google Scholar] [CrossRef]
- Sdiri, N.; Bejar, M.; Dhahri, E. The Effect of the B-Site Size on the Structural, magnetic and electrical properties of La0.7Ca0.3MnO3−d compounds. J. Magn. Magn. Mater. 2007, 311, 512–516. [Google Scholar] [CrossRef]
- Ott, F.; Viret, M.; Borges, R.; Lyonnet, R.; Jacquet, E.; Fermon, C.; Contour, J.P. Interface magnetism of La0.7Sr0.3MnO3 thin films studied by neutron reflectometry. J. Magn. Magn. Mater. 2000, 211, 200–205. [Google Scholar] [CrossRef]
- Bhattacharya, A.; May, S.J.; Te Velthuis, S.G.E.; Warusawithana, M.; Zhai, X.; Jiang, B.; Zuo, J.M.; Fitzsimmons, M.R.; Bader, S.D.; Eckstein, J.N. Metal-insulator transition and its relation to magnetic structure in (LaMnO3)2n/(SrMnO3)n superlattices. Phys. Rev. Lett. 2008, 100, 257203. [Google Scholar] [CrossRef] [PubMed]
- Hemberger, J.; Krimmel, A.; Kurz, T.; Krug von Nidda, H.-A.; Ivanov, V.Y.; Mukhin, A.A.; Balbashov, A.M.; Loidl, A. Structural, magnetic, and electrical properties of single-crystalline La1−xSrxMnO3 (0.4 < x <0.8). Phys. Rev. B 2002, 66, 94410. [Google Scholar] [CrossRef]
- Španková, M.; Štrbík, V.; Dobročka, E.; Chromik, S.; Sojková, M.; Zheng, D.N.; Li, J. Characterization of epitaxial LSMO thin films with high curie temperature prepared on different substrates. Vacuum 2016, 126, 24–28. [Google Scholar] [CrossRef]
- Mishra, A.K.; Darbandi, A.J.; Leufke, P.M.; Kruk, R.; Hahn, H. Room temperature reversible tuning of magnetism of electrolyte-gated La0.75Sr0.25MnO3 nanoparticles. J. Appl. Phys. 2013, 113, 033913. [Google Scholar] [CrossRef]
- Leufke, P.M.; Kruk, R.; Brand, R.A.; Hahn, H. In situ magnetometry studies of magnetoelectric LSMO/PZT heterostructures. Phys. Rev. B 2013, 87, 94416. [Google Scholar] [CrossRef]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Wissel, K.; Heldt, J.; Groszewicz, P.B.; Dasgupta, S.; Breitzke, H.; Donzelli, M.; Waidha, A.I.; Fortes, A.D.; Rohrer, J.; Slater, P.R.; et al. Topochemical fluorination of La2NiO4+d: Unprecedented ordering of oxide and fluoride ions in La2NiO3F2. Inorg. Chem. 2018, 57, 6549–6560. [Google Scholar] [CrossRef] [PubMed]
- AI-Mamouri, M.; Edwards, P.P.; Greaves, C. Synthesis and super conducting properties of the strontium copper oxyfluoride Sr2CuO2F2+δ. Nature 1994, 369, 382–384. [Google Scholar] [CrossRef]
- McCabe, E.E.; Greaves, C. Fluorine insertion reactions into pre-formed metal oxides. J. Fluor. Chem. 2007, 128, 448–458. [Google Scholar] [CrossRef]
- Sanjaya Ranmohotti, K.G.; Josepha, E.; Choi, J.; Zhang, J.; Wiley, J.B. Topochemical manipulation of perovskites: Low-temperature reaction strategies for directing structure and properties. Adv. Mater. 2011, 23, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Clemens, O.; Slater, P.R. Topochemical modifications of mixed metal oxide compounds by low-temperature fluorination routes. Rev. Inorg. Chem. 2013, 33, 105–117. [Google Scholar] [CrossRef]
- Kawahara, K.; Chikamatsu, A.; Katayama, T.; Onozuka, T.; Ogawa, D.; Morikawa, K.; Ikenaga, E.; Hirose, Y.; Harayama, I.; Sekiba, D.; et al. Topotactic fluorination of perovskite strontium ruthenate thin films using polyvinylidene fluoride. CrystEngComm 2017, 19, 313–317. [Google Scholar] [CrossRef]
- Katayama, T.; Chikamatsu, A.; Hirose, Y.; Fukumura, T.; Hasegawa, T. Topotactic reductive fluorination of strontium cobalt oxide epitaxial thin films. J. Sol-Gel Sci. Technol. 2015, 73, 527–530. [Google Scholar] [CrossRef]
- Moon, E.J.; Xie, Y.; Laird, E.D.; Keavney, D.J.; Li, C.Y.; May, S.J. Fluorination of epitaxial oxides: Synthesis of perovskite oxyfluoride thin films. J. Am. Chem. Soc. 2014, 136, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Chikamatsu, A.; Hirose, Y.; Takagi, R.; Kamisaka, H.; Fukumura, T.; Hasegawa, T. Topotactic fluorination of strontium iron oxide thin films using polyvinylidene fluoride. J. Mater. Chem. C 2014, 2, 5350–5356. [Google Scholar] [CrossRef] [Green Version]
- Peña, O.; Moure, C.; Barahona, P.; Baibich, M.; Martinez, G. Intra- and inter-network interactions in magnetic oxides. Phys. B Condens. Matter 2006, 384, 57–61. [Google Scholar] [CrossRef]
- Leufke, P.M.; Kumar, A.; Beck, A.; Wang, D.; Kübel, C.; Kruk, R.; Hahn, H. Large-distance RF- and DC-sputtering of epitaxial La1−xSrxMnO3 thin films. Thin Solid Films 2012, 520, 5521–5527. [Google Scholar] [CrossRef]
- Li, R.K.; Greaves, C. Double-layered ruthenate Sr3Ru2O7F2 formed by fluorine insertion into Sr3Ru2O7. Phys. Rev. B 2000, 62, 3811–3815. [Google Scholar] [CrossRef]
- TOPAS 6, General Profile and Structure Analysis Software for Powder Diffractoin Data; User’s Manual; Bruker AXS: Karlsruhe, Germany, 2017.
- Cheary, R.W.; Coelho, A. Fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25 Pt 2, 109–121. [Google Scholar] [CrossRef]
- Pesquera, D.; Marti, X.; Holy, V.; Bachelet, R.; Herranz, G.; Fontcuberta, J. X-ray interference effects on the determination of structural data in ultrathin La2/3Sr1/3MnO3 epitaxial thin films. Appl. Phys. Lett. 2011, 99, 221901. [Google Scholar] [CrossRef]
- Molinari, A.; Leufke, P.M.; Reitz, C.; Dasgupta, S.; Witte, R.; Kruk, R.; Hahn, H. Hybrid supercapacitors for reversible control of magnetism. Nat. Commun. 2017, 8, 15339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, P.R. Poly(vinylidene fluoride) as a reagent for the synthesis of K2NiF4-related inorganic oxide fluorides. J. Fluor. Chem. 2002, 117, 43–45. [Google Scholar] [CrossRef]
- Negas, T.; Roth, R.S. The xystem SrMnO3−x. J. Solid State Chem. 1970, 1, 409–418. [Google Scholar] [CrossRef]
- Clemens, O.; Kuhn, M.; Haberkorn, R. Synthesis and characterization of the La1−xSrxFeO3−δ system and the fluorinated phases La1−xSrxFeO3−xFx. J. Solid State Chem. 2011, 184, 2870–2876. [Google Scholar] [CrossRef]
- Sullivan, E.; Greaves, C. Fluorine insertion reactions of the brownmillerite materials Sr2Fe2O5, Sr2CoFeO5, and Sr2Co2O5. Mater. Res. Bull. 2012, 47, 2541–2546. [Google Scholar] [CrossRef]
- Clemens, O.; Rongeat, C.; Reddy, M.A.; Giehr, A.; Fichtner, M.; Hahn, H. Electrochemical fluorination of perovskite type BaFeO2.5. Dalt. Trans. 2014, 43, 15771–15778. [Google Scholar] [CrossRef] [PubMed]
- Clemens, O.; Haberkorn, R.; Slater, P.R.; Beck, H.P. Synthesis and characterisation of the SrxBa1−xFeO3−y-system and of the fluorinated phases SrxBa1−xFeO2F. Solid State Sci. 2010, 12, 1455–1463. [Google Scholar] [CrossRef]
- Berry, F.J.; Ren, X.; Heap, R.; Slater, P.; Thomas, M.F. Fluorination of perovskite-related SrFeO3−δ. Solid State Commun. 2005, 134, 621–624. [Google Scholar] [CrossRef]
- Petrisor, T.; Gabor, M.S.; Boulle, A.; Bellouard, C.; Tiusan, C.; Pana, O.; Petrisor, T. Oxygen incorporation effects in annealed epitaxial La1−xSrxMnO3 thin Films. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Aikens, L.D.; Gillie, L.J.; Li, R.K.; Greaves, C. Staged Fluorine insertion into manganese oxides with Ruddlesden–Popper structures: LaSrMnO4F and La1.2Sr1.8Mn2O7F. J. Mater. Chem. 2002, 12, 264–267. [Google Scholar] [CrossRef]
- Aikens, L.D.; Li, R.K.; Greaves, C. The Synthesis and structure of a new oxide fluoride, LaSrMnO4F, with staged fluorine insertion. Chem. Commun. 2000, 21, 2129–2130. [Google Scholar] [CrossRef]
- Takeiri, F.; Yamamoto, T.; Hayashi, N.; Hosokawa, S.; Arai, K. AgFeOF2: A fluorine-rich perovskite oxyfluoride. Inorg. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Moreo, A.; Yunoki, S.; Dagotto, E. Solid state Ppysics—Phase separation scenario for manganese oxides and related materials. Science 1999, 283, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Brey, L. Electronic phase separation in manganite-insulator interfaces. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 104423. [Google Scholar] [CrossRef]
- Jacob, K.T.; Saji, V.S. Lanthanum Oxyfluoride: Structure, stability, and ionic conductivity. Int. J. Appl. Ceram. Technol. 2006, 321, 312–321. [Google Scholar] [CrossRef]
- Nowroozi, M.A.; Wissel, K.; Rohrer, J.; Munnangi, A.R.; Clemens, O. LaSrMnO4: Reversible electrochemical intercalation of fluoride ions in the context of fluoride ion batteries. Chem. Mater. 2017, 29, 3441–3453. [Google Scholar] [CrossRef]
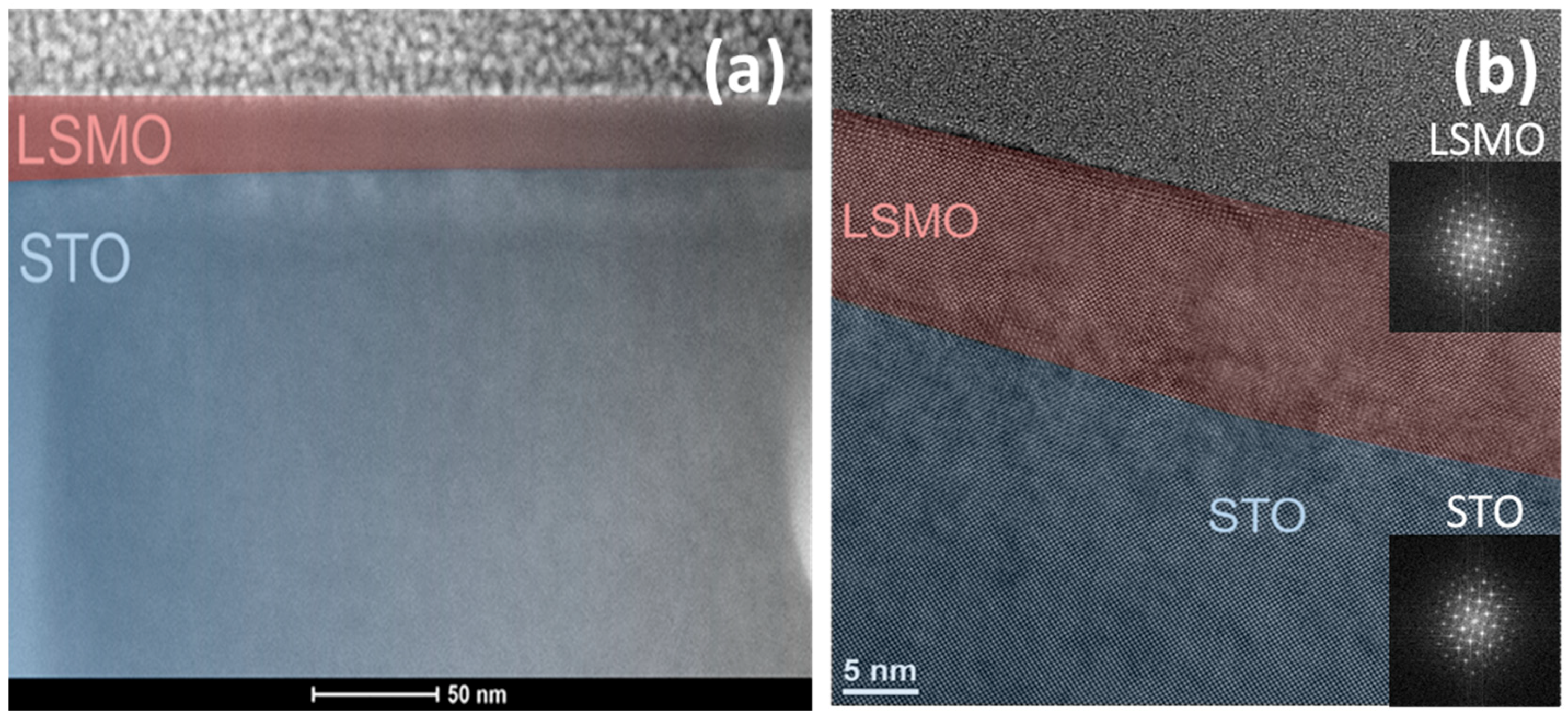
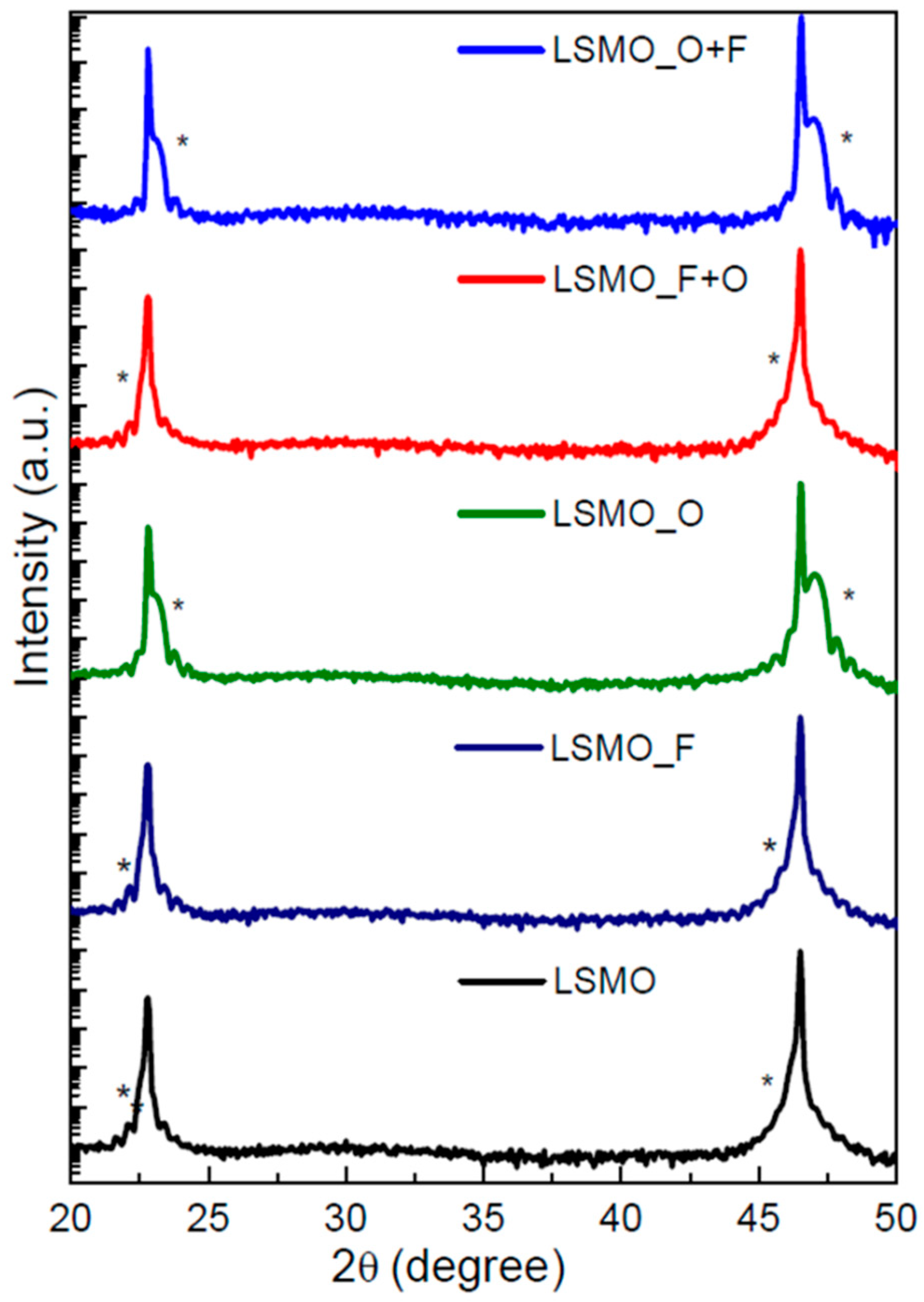
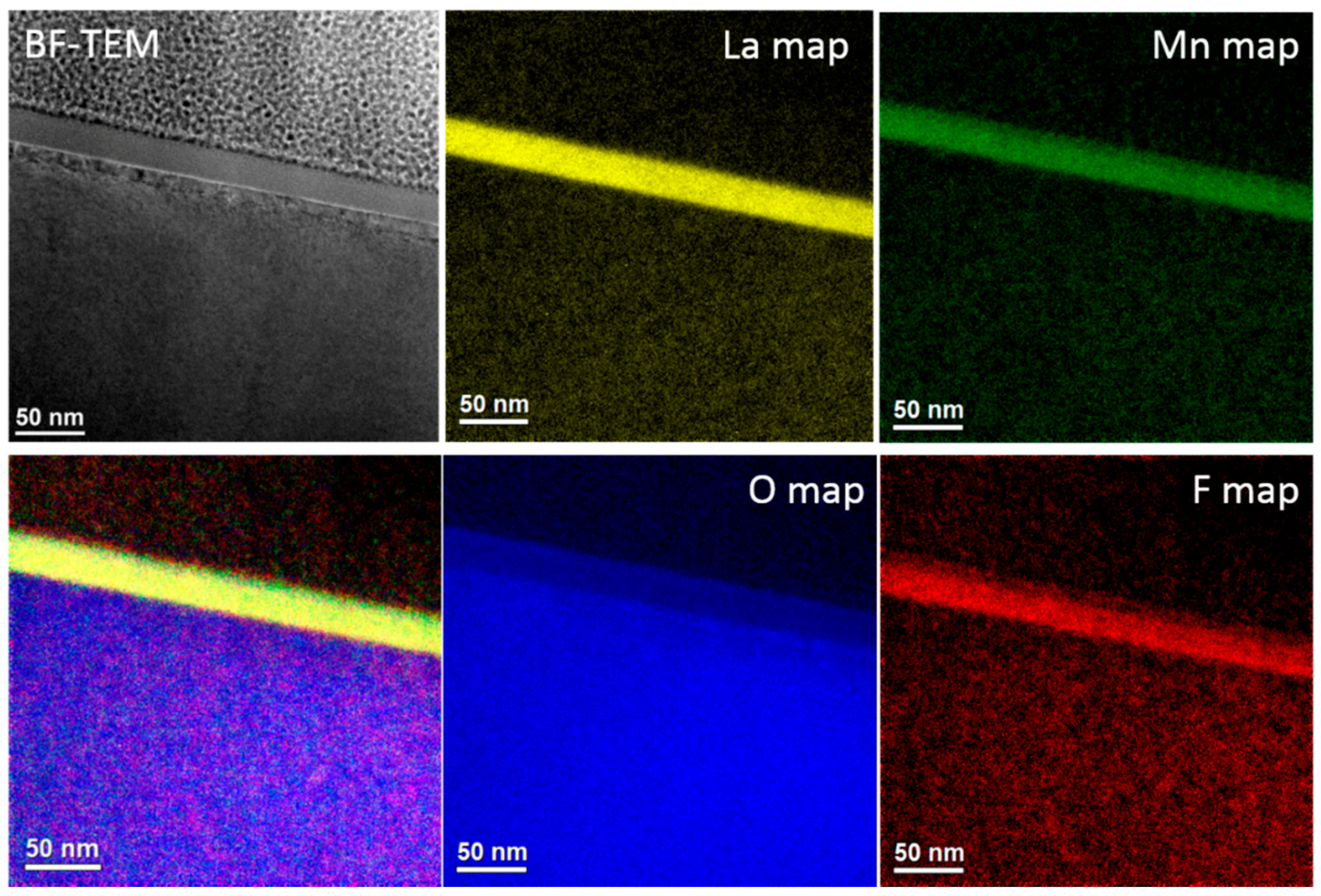
| Sample Label | Reaction Conditions |
|---|---|
| LSMO | As-grown LSMO film |
| LSMO_F | LSMO film fluorinated at 180 °C for 12 h in argon |
| LSMO_O | LSMO film oxidized at 900 °C for 1 h in air |
| LSMO_O+F | LSMO film oxidized at 900 °C in air for 1 h, followed by fluorination at 180 °C in argon carried out for 12 h |
| LSMO_F+O | LSMO film fluorinated at 180 °C for 12 h, followed by oxidation at 240 °C overnight in air (here reduced oxidation temperature is required to account for the metastable nature of the oxyfluoride compound) |
| Thin Film Label | Out-of-Plane Lattice Parameter of the Film (Å) |
|---|---|
| LSMO As-grown | 3.925 |
| LSMO_F | 3.920 |
| LSMO_F+O | 3.920 |
| LSMO_O | 3.860 |
| LSMO_O+F | 3.865 |
| STO substrate | 3.905 |
| Type of Film | Curie Temperature TC (K) | Saturation Magnetization MS at 5 K (μB per Mn ion) | Coercive Field HC (Oe) |
|---|---|---|---|
| LSMO as-grown | 135.1 | 1.5 | 271.5 |
| LSMO_O | 320.0 | 3.0 | 7.8 |
| LSMO_O+F | 301.1 | 3.2 | 9.2 |
| LSMO_F | 103.4 | 0.9 | 704.3 |
| LSMO_F+O | 103.2 | 0.8 | 760.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitha Sukkurji, P.; Molinari, A.; Reitz, C.; Witte, R.; Kübel, C.; Chakravadhanula, V.S.K.; Kruk, R.; Clemens, O. Anion Doping of Ferromagnetic Thin Films of La0.74Sr0.26MnO3−δ via Topochemical Fluorination. Materials 2018, 11, 1204. https://doi.org/10.3390/ma11071204
Anitha Sukkurji P, Molinari A, Reitz C, Witte R, Kübel C, Chakravadhanula VSK, Kruk R, Clemens O. Anion Doping of Ferromagnetic Thin Films of La0.74Sr0.26MnO3−δ via Topochemical Fluorination. Materials. 2018; 11(7):1204. https://doi.org/10.3390/ma11071204
Chicago/Turabian StyleAnitha Sukkurji, Parvathy, Alan Molinari, Christian Reitz, Ralf Witte, Christian Kübel, Venkata Sai Kiran Chakravadhanula, Robert Kruk, and Oliver Clemens. 2018. "Anion Doping of Ferromagnetic Thin Films of La0.74Sr0.26MnO3−δ via Topochemical Fluorination" Materials 11, no. 7: 1204. https://doi.org/10.3390/ma11071204