Advanced Chemical Looping Materials for CO2 Utilization: A Review
Abstract
:1. Introduction
2. Materials for Chemical Looping
2.1. CO2 Carrier
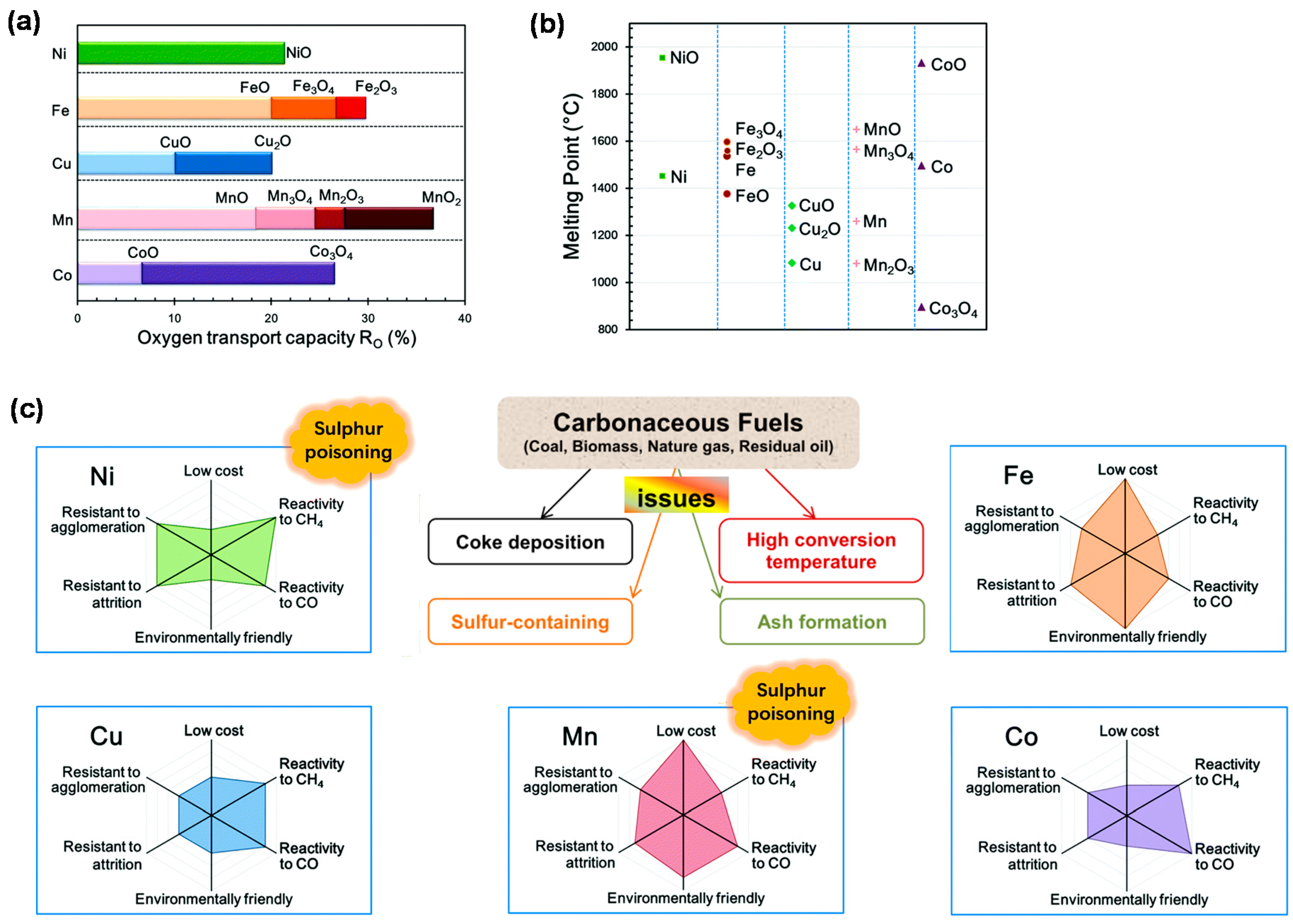
2.2. Oxygen Carrier
3. Improvements to Oxygen Carriers
3.1. Monometallic Materials
3.2. Mixed Oxide Materials
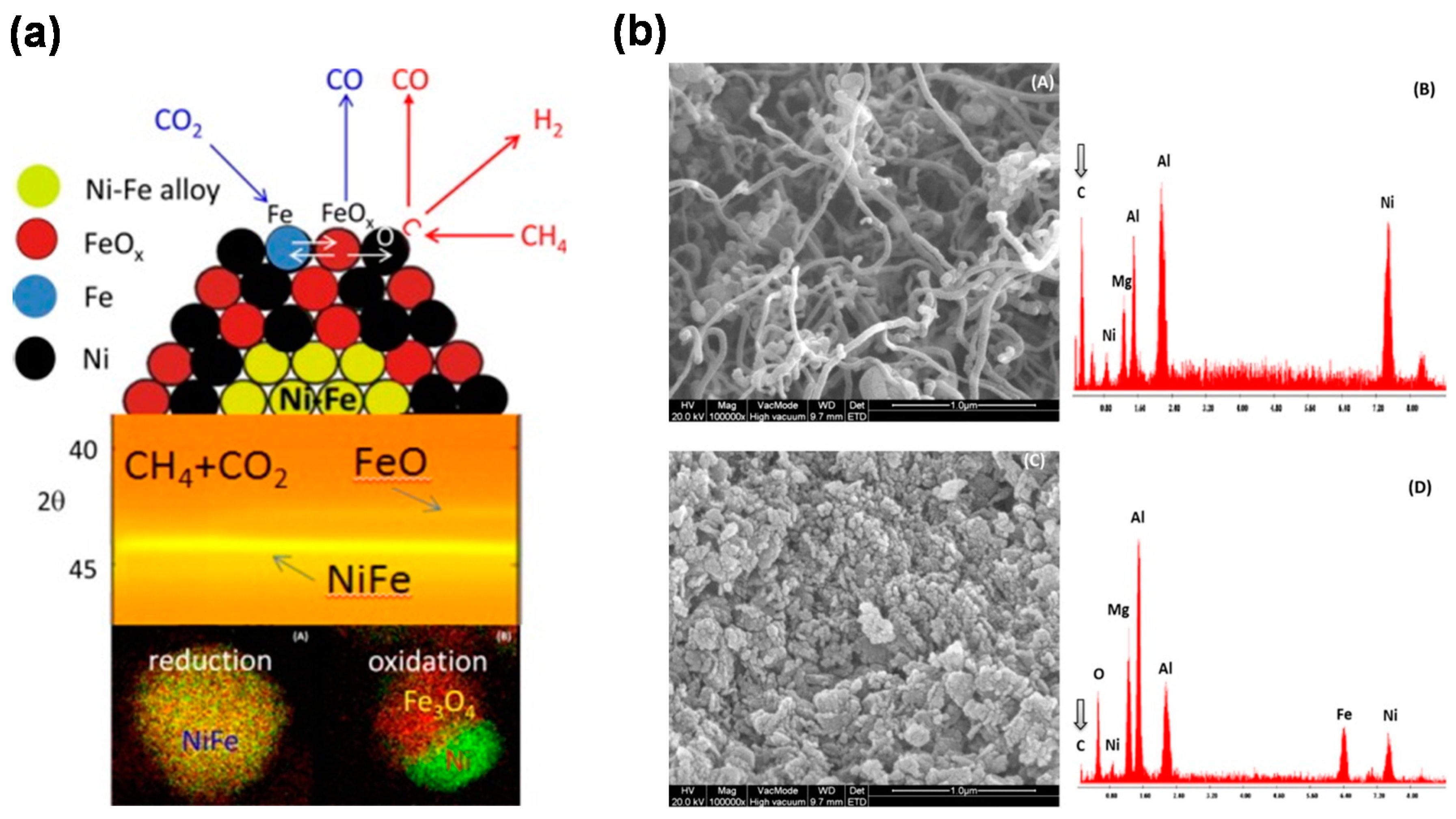
3.3. Structured Materials
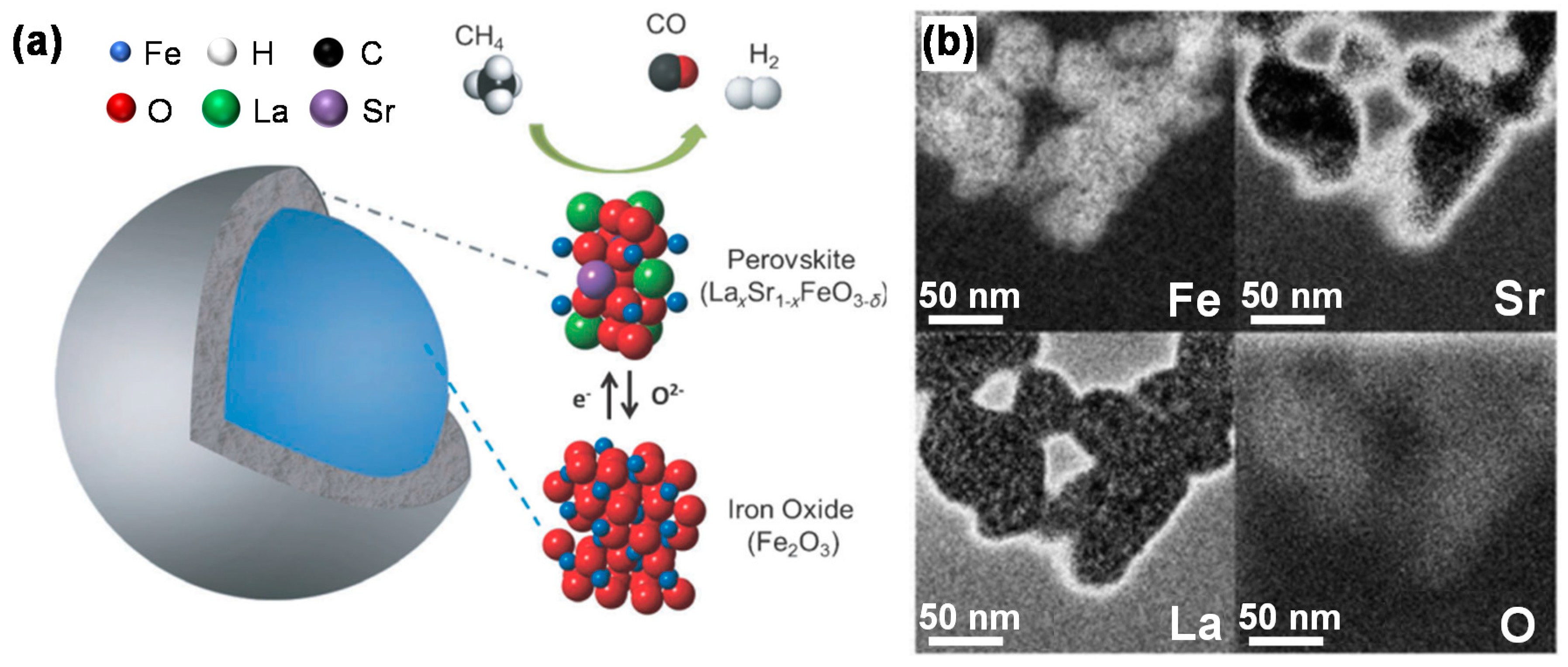
3.3.1. Perovskite Structure
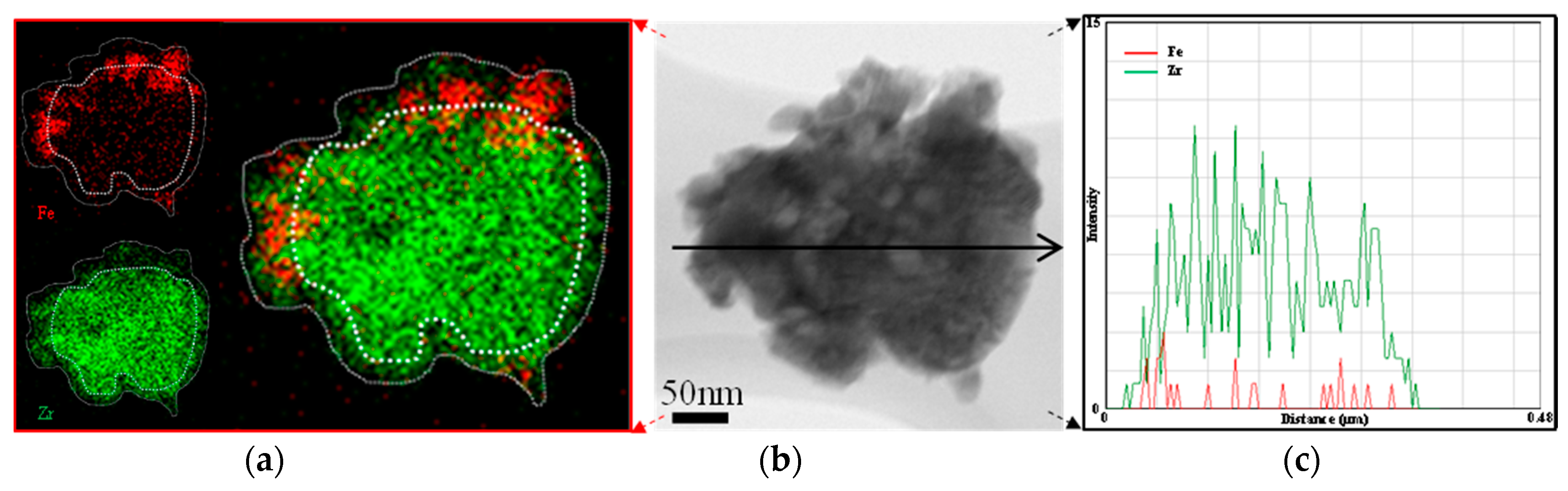
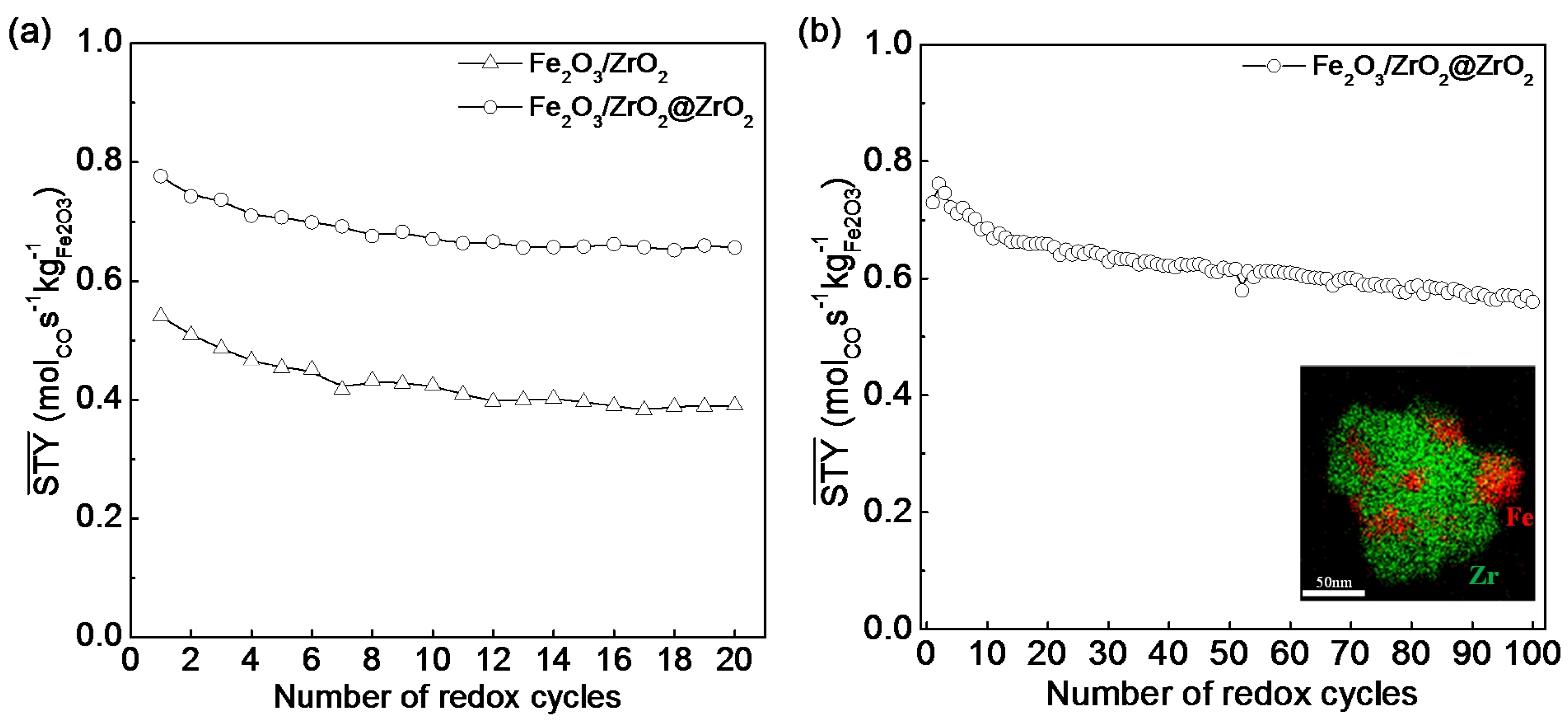
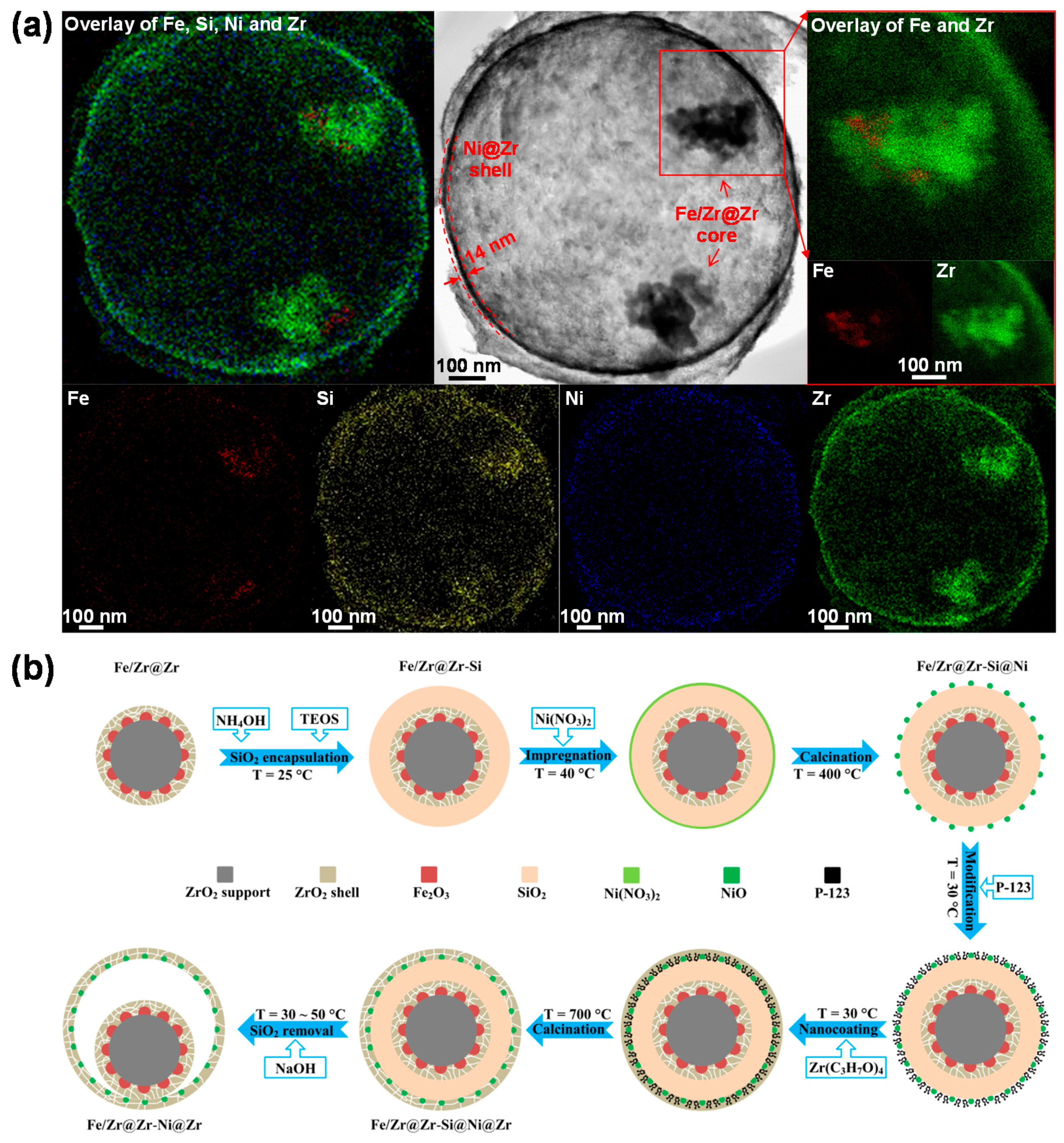
3.3.2. Core-Shell Structure
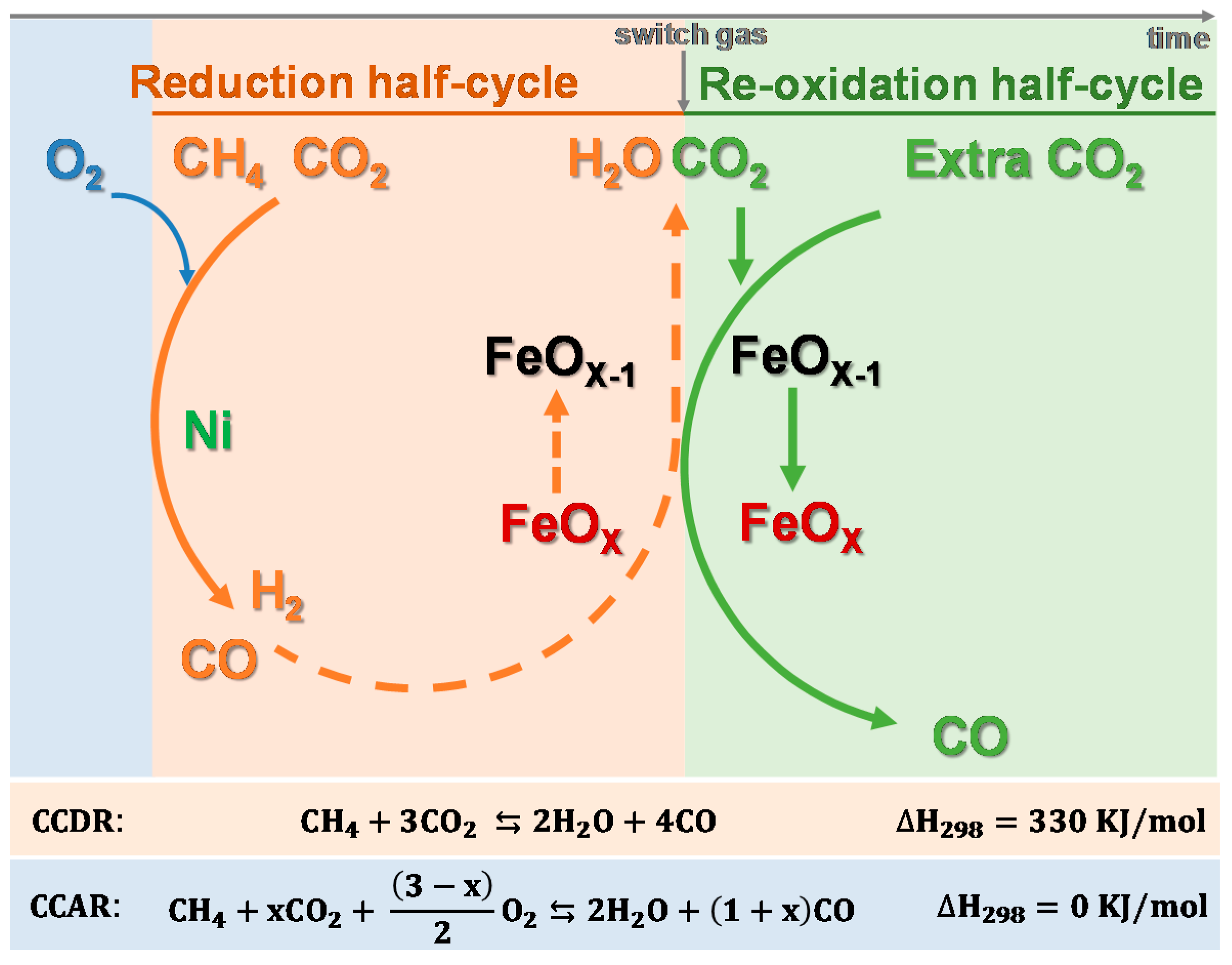
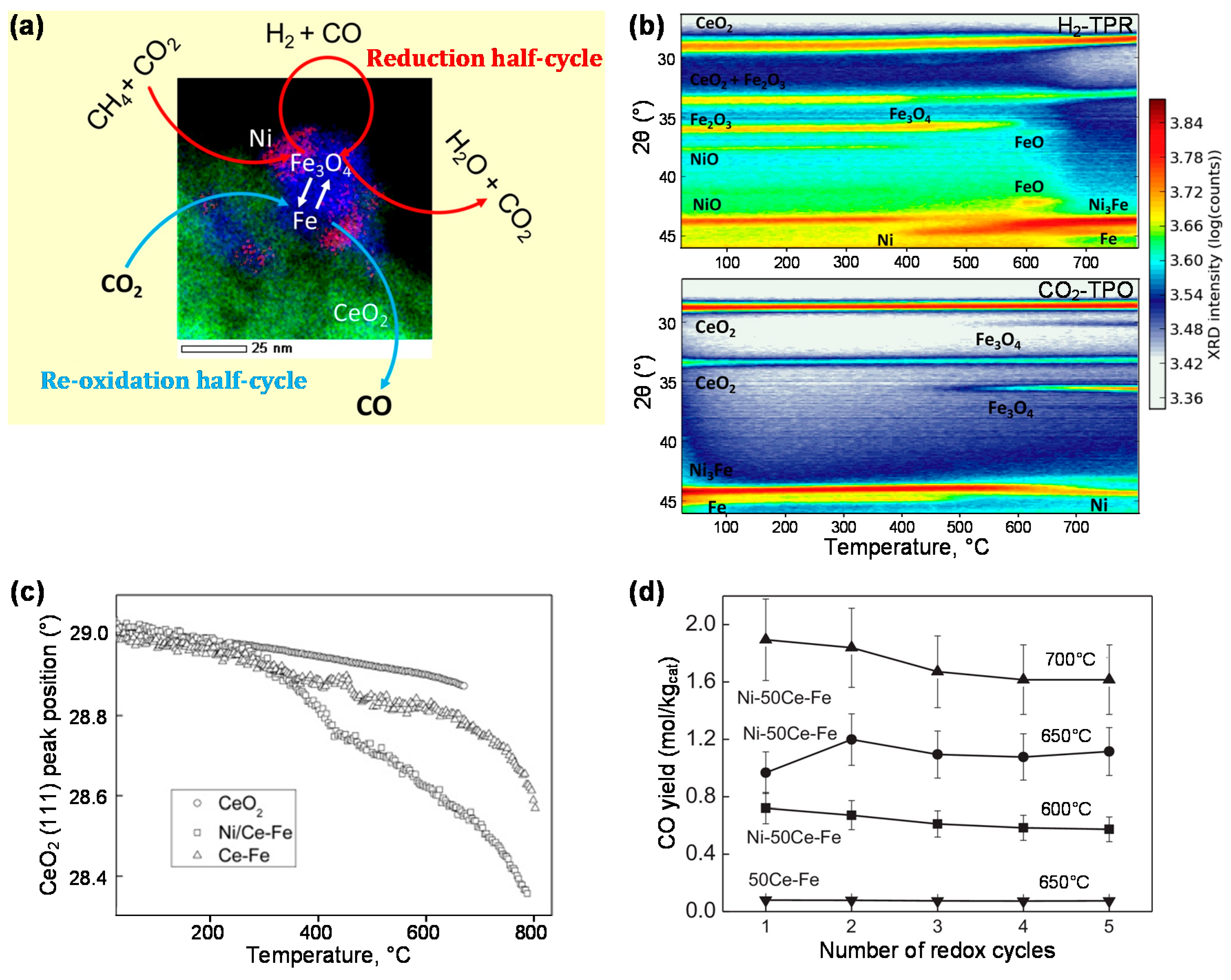
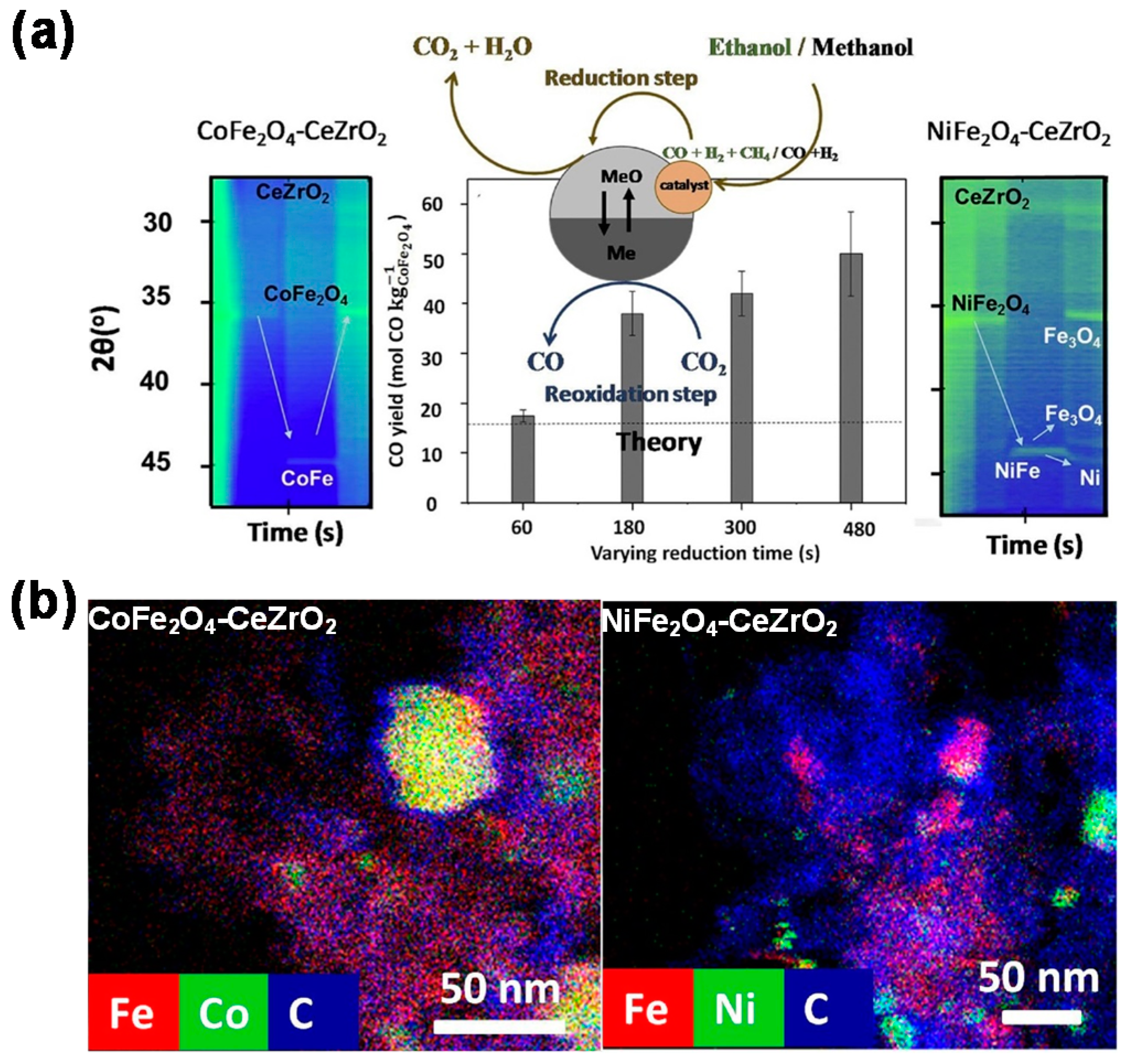
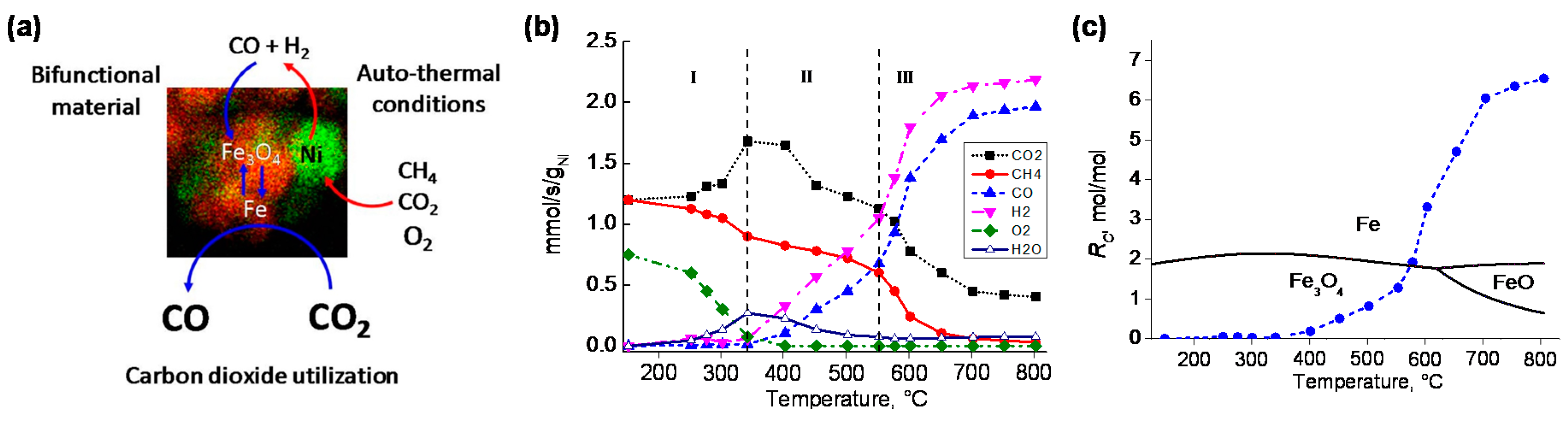
4. Bifunctional Materials for Catalyst-Assisted Chemical Looping
5. Conclusions
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Duan, Y.; Luebke, D.; Morreale, B. Advances in CO2 capture technology: A patent review. Appl. Energy 2013, 102, 1439–1447. [Google Scholar] [CrossRef]
- Das, A.; D’Alessandro, D.M.; Peterson, V.K. Carbon Dioxide Separation, Capture, and Storage in Porous Materials. In Neutron Applications in Materials for Energy. Neutron Scattering Applications and Techniques; Kearley, G., Peterson, V., Eds.; Springer: Cham, Switzerland, 2015; pp. 33–60. [Google Scholar]
- Guo, L.; Yang, J.; Hu, G.; Hu, X.; DaCosta, H.; Fan, M. CO2 removal from flue gas with amine-impregnated titanate nanotubes. Nano Energy 2016, 25, 1–8. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Rogelj, J.; Knutti, R. Geosciences after Paris. Nat. Geosci. 2016, 9, 187–189. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2015. Greenhouse Gas Emissions. 2017. Available online: www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2015 (accessed on 13 April 2017).
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef] [Green Version]
- Cebrucean, D.; Cebrucean, V.; Ionel, I. CO2 capture and storage from fossil fuel power plants. Energy Procedia 2014, 63, 18–26. [Google Scholar] [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.; Najera, M.; Solunke, R.; Veser, G. Chemical looping: To combustion and beyond. Catal. Today 2014, 228, 96–105. [Google Scholar] [CrossRef]
- Buelens, L.C.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Super-dry reforming of methane intensifies CO2 utilization via le chatelier’s principle. Science 2016, 354, 449–452. [Google Scholar] [CrossRef] [PubMed]
- D'Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Haszeldine, R.S. Carbon capture and storage: How green can black be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- De Diego, L.F.; Ortiz, M.; Adánez, J.; García-Labiano, F.; Abad, A.; Gayán, P. Synthesis gas generation by chemical-looping reforming in a batch fluidized bed reactor using Ni-based oxygen carriers. Chem. Eng. J. 2008, 144, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Buelens, L.; Theofanidis, S.-A.; Galvita, V.V.; Poelman, H.; Marin, G.B. CO2 conversion to CO by auto-thermal catalyst-assisted chemical looping. J. CO2 Util. 2016, 16, 8–16. [Google Scholar] [CrossRef]
- Fan, L.-S.; Zeng, L.; Luo, S. Chemical-looping technology platform. AIChE J. 2015, 61, 2–22. [Google Scholar] [CrossRef]
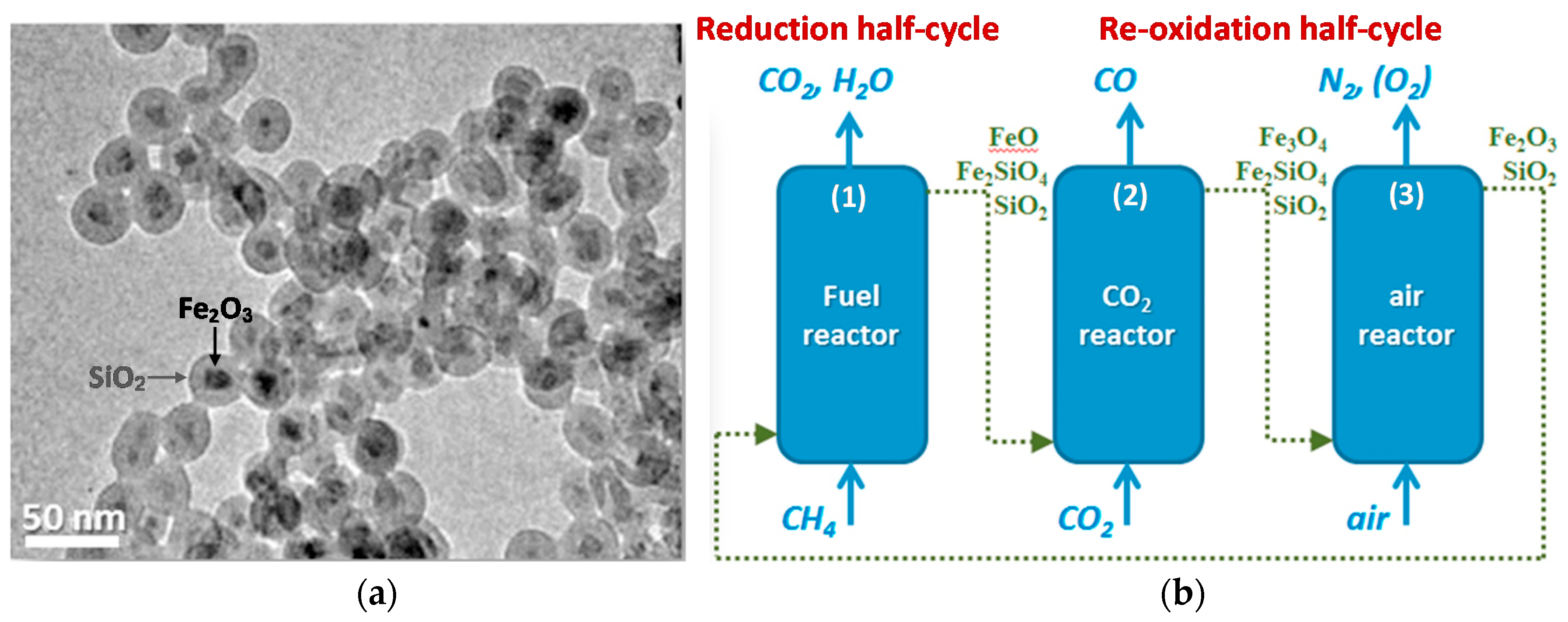
- Galvita, V.V.; Poelman, H.; Bliznuk, V.; Detavernier, C.; Marin, G.B. CeO2-modified Fe2O3 for CO2 utilization via chemical looping. Ind. Eng. Chem. Res. 2013, 52, 8416–8426. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping for CO2 conversion to CO. Appl. Catal. B 2015, 164, 184–191. [Google Scholar] [CrossRef]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Fan, L.-S. Chemical Looping Systems for Fossil Energy Conversions; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Nandy, A.; Loha, C.; Gu, S.; Sarkar, P.; Karmakar, M.K.; Chatterjee, P.K. Present status and overview of chemical looping combustion technology. Renew. Sustain. Energy Rev. 2016, 59, 597–619. [Google Scholar] [CrossRef]
- Yu, F.-C.; Phalak, N.; Sun, Z.; Fan, L.-S. Activation strategies for calcium-based sorbents for CO2 capture: A perspective. Ind. Eng. Chem. Res. 2011, 51, 2133–2142. [Google Scholar] [CrossRef]
- Dou, B.; Wang, C.; Song, Y.; Chen, H.; Jiang, B.; Yang, M.; Xu, Y. Solid sorbents for in-situ CO2 removal during sorption-enhanced steam reforming process: A review. Renew. Sustain. Energy Rev. 2016, 53, 536–546. [Google Scholar] [CrossRef]
- Fan, L.-S. Chemical Looping Partial Oxidation: Gasification, Reforming, and Chemical Syntheses; Cambridge University Press, Technology & Engineering: Cambridge, UK, 2017. [Google Scholar]
- Galvita, V.V.; Poelman, H.; Marin, G.B. Hydrogen production from methane and carbon dioxide by catalyst-assisted chemical looping. Top. Catal. 2011, 54, 907–913. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, J.; Li, K.; Tian, S.; Yan, F. Energy-efficient biogas reforming process to produce syngas: The enhanced methane conversion by O2. Appl. Energy 2017, 185, 687–697. [Google Scholar] [CrossRef]
- Christian Enger, B.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, S.-C. Characterization of catalytic partial oxidation of methane with carbon dioxide utilization and excess enthalpy recovery. Appl. Energy 2016, 162, 1141–1152. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, M.-R.; Lu, J.-J.; Chao, Y.; Leu, T.-S. Thermodynamic analysis of hydrogen production from methane via autothermal reforming and partial oxidation followed by water gas shift reaction. Int. J. Hydrog. Energy 2010, 35, 11787–11797. [Google Scholar] [CrossRef]
- Kohn, M.P.; Castaldi, M.J.; Farrauto, R.J. Auto-thermal and dry reforming of landfill gas over a Rh/γ-Al2O3 monolith catalyst. Appl. Catal. B 2010, 94, 125–133. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources; Weily-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Rao, C.N.R.; Dey, S. Solar thermochemical splitting of water to generate hydrogen. Proc. Natl. Acad. Sci. USA 2017, 114, 13385–13393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protasova, L.; Snijkers, F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel 2016, 181, 75–93. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. A core-shell structured Fe2O3/ZrO2@ZrO2 nanomaterial with enhanced redox activity and stability for CO2 conversion. J. CO2 Util. 2017, 17, 20–31. [Google Scholar] [CrossRef]
- González, B.; Blamey, J.; McBride-Wright, M.; Carter, N.; Dugwell, D.; Fennell, P.; Abanades, J.C. Calcium looping for CO2 capture: Sorbent enhancement through doping. Energy Procedia 2011, 4, 402–409. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Blamey, J.; Anthony, E.J.; Wang, J.; Fennell, P.S. The calcium looping cycle for large-scale CO2 capture. Prog. Energy Combust. Sci. 2010, 36, 260–279. [Google Scholar] [CrossRef]
- Dean, C.C.; Blamey, J.; Florin, N.H.; Al-Jeboori, M.J.; Fennell, P.S. The calcium looping cycle for CO2 capture from power generation, cement manufacture and hydrogen production. Chem. Eng. Res. Des. 2011, 89, 836–855. [Google Scholar] [CrossRef] [Green Version]
- Perejón, A.; Romeo, L.M.; Lara, Y.; Lisbona, P.; Martínez, A.; Valverde, J.M. The calcium-looping technology for CO2 capture: On the important roles of energy integration and sorbent behavior. Appl. Energy 2016, 162, 787–807. [Google Scholar] [CrossRef]
- Erans, M.; Manovic, V.; Anthony, E.J. Calcium looping sorbents for CO2 capture. Appl. Energy 2016, 180, 722–742. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Chen, L.; Qian, D.; Neathery, J.K.; Kozo, S.; Liu, K. Investigation of a canadian ilmenite as an oxygen carrier for chemical looping combustion. Energy Fuels 2013, 27, 5987–5995. [Google Scholar] [CrossRef]
- Keller, M.; Arjmand, M.; Leion, H.; Mattisson, T. Interaction of mineral matter of coal with oxygen carriers in chemical-looping combustion (CLC). Chem. Eng. Res. Des. 2014, 92, 1753–1770. [Google Scholar] [CrossRef]
- Lin, C.-L.; Kuo, J.-H.; Wey, M.-Y.; Chang, S.-H.; Wang, K.-S. Inhibition and promotion: The effect of earth alkali metals and operating temperature on particle agglomeration/defluidization during incineration in fluidized bed. Powder Technol. 2009, 189, 57–63. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Zhong, Z.; Zhou, Y.; Liu, W.; Niu, X.; Ge, H.; Jiang, S.; Wang, L. Interaction between biomass ash and iron ore oxygen carrier during chemical looping combustion. Chem. Eng. J. 2015, 277, 70–78. [Google Scholar]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Lyngfelt, A. Oxygen carriers for chemical looping combustion—4000 h of operational experience. Oil Gas Sci. Technol. 2011, 66, 161–172. [Google Scholar] [CrossRef]
- De Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Cabello, A.; Adánez, J.; Sprachmann, G. Performance of Cu- and Fe-based oxygen carriers in a 500 Wth CLC unit for sour gas combustion with high H2S content. Int. J. Greenh. Gas Control 2014, 28, 168–179. [Google Scholar] [CrossRef]
- Rydén, M.; Cleverstam, E.; Johansson, M.; Lyngfelt, A.; Mattisson, T. Fe2O3 on Ce-, Ca-, or Mg-stabilized ZrO2 as oxygen carrier for chemical-looping combustion using NiO as additive. AIChE J. 2010, 56, 2211–2220. [Google Scholar]
- De Diego, L.F.; García-Labiano, F.; Adánez, J.; Gayán, P.; Abad, A.; Corbella, B.M.; María Palacios, J. Development of Cu-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1749–1757. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Simonyi, T.; Poston, J.; Siriwardane, R. Effect of hydrogen sulfide on chemical looping combustion of coal-derived synthesis gas over bentonite-supported metal-oxide oxygen carriers. Ind. Eng. Chem. Res. 2009, 48, 8418–8430. [Google Scholar] [CrossRef]
- Wang, B.; Gao, C.; Wang, W.; Zhao, H.; Zheng, C. Sulfur evolution in chemical looping combustion of coal with MnFe2O4 oxygen carrier. J. Environ. Sci. 2014, 26, 1062–1070. [Google Scholar] [CrossRef]
- Adánez, J.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A. Selection of oxygen carriers for chemical-looping combusion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Zafar, Q.; Mattisson, T.; Gevert, B. Redox investigation of some oxides of transition-state metals Ni, Cu, Fe, and Mn supported on SiO2 and MgAl2O4. Energy Fuels 2006, 20, 34–44. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Mattisson, T.; Lyngfelt, A. Investigation of Mn3O4 with stabilized ZrO2 for chemical-looping combustion. Chem. Eng. Res. Des. 2006, 84, 807–818. [Google Scholar] [CrossRef]
- Jin, H.; Okamoto, T.; Ishida, M. Development of a novel chemical-looping combustion synthesis of a looping material with a double metal oxide of CoO-NiO. Energy Fuels 1998, 12, 1272–1277. [Google Scholar] [CrossRef]
- Mattisson, T.; Järdnäs, A.; Lyngfelt, A. Reactivity of some metal oxides supported on alumina with alternating methane and oxygen application for chemical-looping combustion. Energy Fuels 2003, 17, 643–651. [Google Scholar] [CrossRef]
- Sedor, K.E.; Hossain, M.M.; De Lasa, H.I. Reactivity and stability of Ni/Al2O3 oxygen carrier for chemical-looping combustion (CLC). Chem. Eng. Sci. 2008, 63, 2994–3007. [Google Scholar] [CrossRef]
- Dueso, C.; Abad, A.; García-Labiano, F.; De Diego, L.F.; Gayán, P.; Adánez, J.; Lyngfelt, A. Reactivity of a NiO/Al2O3 oxygen carrier prepared by impregnation for chemical-looping combustion. Fuel 2010, 89, 3399–3409. [Google Scholar] [CrossRef] [Green Version]
- Quddus, M.R.; Hossain, M.M.; de Lasa, H.I. Ni based oxygen carrier over γ-Al2O3 for chemical looping combustion: Effect of preparation method on metal support interaction. Catal. Today 2013, 210, 124–134. [Google Scholar] [CrossRef]
- Corbella, B.M.; De Diego, L.F.; García-Labiano, F.; Adánez, J.; Palacios, J.M. Performance in a fixed-bed reactor of titania-supported nickel oxide as oxygen carriers for the chemical-looping combustion of methane in multicycle tests. Ind. Eng. Chem. Res. 2006, 45, 157–165. [Google Scholar] [CrossRef]
- Antzara, A.; Heracleous, E.; Silvester, L.; Bukur, D.B.; Lemonidou, A.A. Activity study of NiO-based oxygen carriers in chemical looping steam methane reforming. Catal. Today 2016, 272, 32–41. [Google Scholar] [CrossRef]
- Silvester, L.; Antzara, A.; Boskovic, G.; Heracleous, E.; Lemonidou, A.A.; Bukur, D.B. NiO supported on Al2O3 and ZrO2 oxygen carriers for chemical looping steam methane reforming. Int. J. Hydrog. Energy 2015, 40, 7490–7501. [Google Scholar] [CrossRef]
- Rydén, M.; Johansson, M.; Lyngfelt, A.; Mattisson, T. NiO supported on Mg-ZrO2 as oxygen carrier for chemical-looping combustion and chemical-looping reforming. Energy Environ. Sci. 2009, 2, 970–981. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H.; Okamoto, T. A fundamental study of a new kind of medium material for chemical-looping combustion. Energy Fuels 1996, 10, 958–963. [Google Scholar] [CrossRef]
- Readman, J.E.; Olafsen, A.; Smith, J.B.; Blom, R. Chemical looping combustion using NiO/NiAl2O4: Mechanisms and kinetics of reduction−oxidation (red-ox) reactions from in situ powder X-ray diffraction and thermogravimetry experiments. Energy Fuels 2006, 20, 1382–1387. [Google Scholar] [CrossRef]
- Mattisson, T.; Jerndal, E.; Linderholm, C.; Lyngfelt, A. Reactivity of a spray-dried NiO/NiAl2O4 oxygen carrier for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 4636–4644. [Google Scholar] [CrossRef]
- Ryden, M.; Lyngfelt, A.; Mattisson, T. Synthesis gas generation by chemical-looping reforming in a continuously operating laboratory reactor. Fuel 2006, 85, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Gayán, P.; De Diego, L.F.; García-Labiano, F.; Adánez, J.; Abad, A.; Dueso, C. Effect of support on reactivity and selectivity of Ni-based oxygen carriers for chemical-looping combustion. Fuel 2008, 87, 2641–2650. [Google Scholar] [CrossRef] [Green Version]
- Cabello, A.; Gayán, P.; García-Labiano, F.; De Diego, L.F.; Abad, A.; Izquierdo, M.T.; Adánez, J. Relevance of the catalytic activity on the performance of a NiO/CaAl2O4 oxygen carrier in a CLC process. Appl. Catal. B 2014, 147, 980–987. [Google Scholar] [CrossRef]
- Bhavsar, S.; Veser, G. Reducible supports for Ni-based oxygen carriers in chemical looping combustion. Energy Fuels 2013, 27, 2073–2084. [Google Scholar] [CrossRef]
- He, F.; Wang, H.; Dai, Y. Application of Fe2O3/Al2O3 composite particles as oxygen carrier of chemical looping combustion. J. Nat. Gas Chem. 2007, 16, 155–161. [Google Scholar] [CrossRef]
- Wang, B.; Yan, R.; Lee, D.H.; Zheng, Y.; Zhao, H.; Zheng, C. Characterization and evaluation of Fe2O3/Al2O3 oxygen carrier prepared by sol–gel combustion synthesis. J. Anal. Appl. Pyrolysis 2011, 91, 105–113. [Google Scholar] [CrossRef]
- Mei, D.; Abad, A.; Zhao, H.; Adánez, J.; Zheng, C. On a highly reactive Fe2O3/Al2O3 oxygen carrier for in situ gasification chemical looping combustion. Energy Fuels 2014, 28, 7043–7052. [Google Scholar] [CrossRef]
- Cabello, A.; Dueso, C.; García-Labiano, F.; Gayán, P.; Abad, A.; De Diego, L.F.; Adánez, J. Performance of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier with CH4 and H2S in a 500 Wth CLC unit. Fuel 2014, 121, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Rihko-Struckmann, L.K.; Datta, P.; Wenzel, M.; Sundmacher, K.; Dharanipragada, N.V.R.A.; Poelman, H.; Galvita, V.V.; Marin, G.B. Hydrogen and carbon monoxide production by chemical looping over iron-aluminium oxides. Energy Technol. 2016, 4, 304–313. [Google Scholar] [CrossRef]
- Qin, W.; Chen, Q.; Wang, Y.; Dong, C.; Zhang, J.; Li, W.; Yang, Y. Theoretical study of oxidation–reduction reaction of Fe2O3 supported on MgO during chemical looping combustion. Appl. Surf. Sci. 2013, 266, 350–354. [Google Scholar] [CrossRef]
- Corbella, B.M.; Palacios, J.M. Titania-supported iron oxide as oxygen carrier for chemical-looping combustion of methane. Fuel 2007, 86, 113–122. [Google Scholar] [CrossRef]
- Ksepko, E.; Sciazko, M.; Babinski, P. Studies on the redox reaction kinetics of Fe2O3-CuO/Al2O3 and Fe2O3/TiO2 oxygen carriers. Appl. Energy 2014, 115, 374–383. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Ku, Y.; Tseng, Y.-H.; Lee, H.-Y.; Kuo, Y.-L. Fabrication of Fe2O3/TiO2 oxygen carriers for chemical looping combustion and hydrogen generation. Aerosol Air Qual. Res. 2016, 16, 2023–2032. [Google Scholar] [CrossRef]
- Zafar, Q.; Mattisson, T.; Gevert, B. Integrated hydrogen and power production with CO2 capture using chemical-looping reforming redox reactivity of particles of CuO, Mn2O3, NiO, and Fe2O3 using SiO2 as a support. Ind. Eng. Chem. Res. 2005, 44, 3485–3496. [Google Scholar] [CrossRef]
- Johansson, M.; Mattisson, T.; Lyngfelt, A. Investigation of Fe2O3 with MgAl2O4 for chemical-looping combustion. Ind. Eng. Chem. Res. 2004, 43, 6978–6987. [Google Scholar] [CrossRef]
- Dharanipragada, N.V.R.A.; Buelens, L.C.; Poelman, H.; De Grave, E.; Galvita, V.V.; Marin, G.B. Mg–Fe–Al–O for advanced CO2 to CO conversion: Carbon monoxide yield vs. oxygen storage capacity. J. Mater. Chem. A 2015, 3, 16251–16262. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M. Inhibiting Fe–Al spinel formation on a narrowed mesopore-sized MgAl2O4 support as a novel catalyst for H2 production in chemical looping technology. Catalysts 2018, 8, 27. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Driessche, I.; Van Der Voort, P.; Snijkers, F.; Verberckmoes, A. Processing and characterization of Fe-based oxygen carriers for chemical looping for hydrogen production. Int. J. Greenh. Gas Control 2018, 70, 12–21. [Google Scholar] [CrossRef]
- Ismail, M.; Liu, W.; Scott, S.A. The performance of Fe2O3-CaO oxygen carriers and the interaction of iron oxides with CaO during chemical looping combustion and H2 production. Energy Procedia 2014, 63, 87–97. [Google Scholar] [CrossRef]
- Ismail, M.; Liu, W.; Dunstan, M.T.; Scott, S.A. Development and performance of iron based oxygen carriers containing calcium ferrites for chemical looping combustion and production of hydrogen. Int. J. Hydrog. Energy 2016, 41, 4073–4084. [Google Scholar] [CrossRef]
- Liu, W.; Dennis, J.S.; Scott, S.A. The effect of addition of ZrO2 to Fe2O3 for hydrogen production by chemical looping. Ind. Eng. Chem. Res. 2012, 51, 16597–16609. [Google Scholar] [CrossRef]
- Tan, Q.; Qin, W.; Chen, Q.; Dong, C.; Li, W.; Yang, Y. Synergetic effect of ZrO2 on the oxidation–reduction reaction of Fe2O3 during chemical looping combustion. Appl. Surf. Sci. 2012, 258, 10022–10027. [Google Scholar] [CrossRef]
- Kang, K.-S.; Kim, C.-H.; Bae, K.-K.; Cho, W.-C.; Jeong, S.-U.; Lee, Y.-J.; Park, C.-S. Reduction and oxidation properties of Fe2O3/ZrO2 oxygen carrier for hydrogen production. Chem. Eng. Res. Des. 2014, 92, 2584–2597. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Wei, Y.; Wang, H.; Sun, L. Chemical-looping steam methane reforming over a CeO2-Fe2O3 oxygen carrier: Evolution of its structure and reducibility. Energy Fuels 2014, 28, 754–760. [Google Scholar] [CrossRef]
- Machida, M.; Kawada, T.; Fujii, H.; Hinokuma, S. The role of CeO2 as a gateway for oxygen storage over CeO2-grafted Fe2O3 composite materials. J. Phys. Chem. C 2015, 119, 24932–24941. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, M.; Cai, L.; Zhang, S.; Zeng, D.; Xiao, R. Performance of CeO2-modified iron-based oxygen carrier in the chemical looping hydrogen generation process. Energy Fuels 2015, 29, 7612–7621. [Google Scholar] [CrossRef]
- Dharanipragada, N.V.R.A.; Meledina, M.; Galvita, V.V.; Poelman, H.; Turner, S.; Van Tendeloo, G.; Detavernier, C.; Marin, G.B. Deactivation study of Fe2O3–CeO2 during redox cycles for CO production from CO2. Ind. Eng. Chem. Res. 2016, 55, 5911–5922. [Google Scholar] [CrossRef]
- Jin, H.; Ishida, M. Reactivity study on natural-gas-fueled chemical-looping combustion by a fixed-bed reactor. Ind. Eng. Chem. Res. 2002, 41, 4004–4007. [Google Scholar] [CrossRef]
- Hossain, M.M.; De Lasa, H.I. Reactivity and stability of Co-Ni/Al2O3 oxygen carrier in multicycle CLC. AIChE J. 2007, 53, 1817–1829. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Jiang, F.; Luo, M.; Li, H. Chemical looping combustion of coke oven gas by using Fe2O3/CuO with MgAl2O4 as oxygen carrier. Energy Environ. Sci. 2010, 3, 1353. [Google Scholar] [CrossRef]
- Mungse, P.; Saravanan, G.; Rayalu, S.; Labhsetwar, N. Mixed oxides of iron and manganese as potential low-cost oxygen carriers for chemical looping combustion. Energy Technol. 2015, 3, 856–865. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Investigation of different Mn–Fe oxides as oxygen carrier for chemical-looping with oxygen uncoupling (CLOU). Energy Fuels 2012, 27, 367–377. [Google Scholar] [CrossRef]
- Azimi, G.; Mattisson, T.; Leion, H.; Rydén, M.; Lyngfelt, A. Comprehensive study of Mn–Fe–Al oxygen-carriers for chemical-looping with oxygen uncoupling (CLOU). Int. J. Greenh. Gas Control 2015, 34, 12–24. [Google Scholar] [CrossRef]
- Frick, V.; Rydén, M.; Leion, H. Investigation of Cu–Fe and Mn–Ni oxides as oxygen carriers for chemical-looping combustion. Fuel Process. Technol. 2016, 150, 30–40. [Google Scholar] [CrossRef]
- Pans, M.A.; Gayán, P.; Abad, A.; García-Labiano, F.; De Diego, L.F.; Adánez, J. Use of chemically and physically mixed iron and nickel oxides as oxygen carriers for gas combustion in a CLC process. Fuel Process. Technol. 2013, 115, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Bhavsar, S.; Veser, G. Bimetallic Fe–Ni oxygen carriers for chemical looping combustion. Ind. Eng. Chem. Res. 2013, 52, 15342–15352. [Google Scholar] [CrossRef]
- Wei, G.; He, F.; Zhao, Z.; Huang, Z.; Zheng, A.; Zhao, K.; Li, H. Performance of Fe–Ni bimetallic oxygen carriers for chemical looping gasification of biomass in a 10 kwth interconnected circulating fluidized bed reactor. Int. J. Hydrog. Energy 2015, 40, 16021–16032. [Google Scholar] [CrossRef]
- Wei, G.; He, F.; Zhao, W.; Huang, Z.; Zhao, K.; Zhao, Z.; Zheng, A.; Wu, X.; Li, H. Experimental investigation of Fe–Ni–Al oxygen carrier derived from hydrotalcite-like precursors for the chemical looping gasification of biomass char. Energy Fuels 2017, 31, 5174–5182. [Google Scholar] [CrossRef]
- Lim, H.S.; Kang, D.; Lee, J.W. Phase transition of Fe2O3–NiO to NiFe2O4 in perovskite catalytic particles for enhanced methane chemical looping reforming-decomposition with CO2 conversion. Appl. Catal. B 2017, 202, 175–183. [Google Scholar] [CrossRef]
- Mihai, O.; Chen, D.; Holmen, A. Catalytic consequence of oxygen of lanthanum ferrite perovskite in chemical looping reforming of methane. Ind. Eng. Chem. Res. 2011, 50, 2613–2621. [Google Scholar] [CrossRef]
- Antigoni, E.; Vassilis, Z.; Lori, N. La1−xSrxFeO3−δ perovskites as redox materials for application in a membrane reactor for simultaneous production of pure hydrogen and synthesis gas. Fuel 2010, 89, 1265–1273. [Google Scholar]
- He, F.; Li, X.; Zhao, K.; Huang, Z.; Wei, G.; Li, H. The use of La1−xSrxFeO3 perovskite-type oxides as oxygen carriers in chemical-looping reforming of methane. Fuel 2013, 108, 465–473. [Google Scholar] [CrossRef]
- Lori, N.; Antigoni, E.; Vassilis, Z. La1−xSrxMyFe1−yO3−δ perovskites as oxygen-carrier materials for chemical-looping reforming. Int. J. Hydrog. Energy 2011, 36, 6657–6670. [Google Scholar]
- Li, Z.; Mo, L.; Kathiraser, Y.; Kawi, S. Yolk–satellite–shell structured Ni–yolk@Ni@SiO2 nanocomposite: Superb catalyst toward methane CO2 reforming reaction. ACS Catal. 2014, 4, 1526–1536. [Google Scholar] [CrossRef]
- Baktash, E.; Littlewood, P.; Schomäcker, R.; Thomas, A.; Stair, P.C. Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane. Appl. Catal. B 2015, 179, 122–127. [Google Scholar] [CrossRef]
- Park, J.N.; Zhang, P.; Hu, Y.S.; McFarland, E.W. Synthesis and characterization of sintering-resistant silica-encapsulated Fe3O4 magnetic nanoparticles active for oxidation and chemical looping combustion. Nanotechnology 2010, 21, 225708. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.; Najera, M.; Veser, G. Chemical looping dry reforming as novel, intensified process for CO2 activation. Chem. Eng. Technol. 2012, 35, 1281–1290. [Google Scholar] [CrossRef]
- Shafiefarhood, A.; Galinsky, N.; Huang, Y.; Chen, Y.; Li, F. Fe2O3@LaxSr1−xFeO3 core-shell redox catalyst for methane partial oxidation. ChemCatChem 2014, 6, 790–799. [Google Scholar] [CrossRef]
- Neal, L.; Shafiefarhood, A.; Li, F. Effect of core and shell compositions on MeOx@LaySr1−yFeO3 core–shell redox catalysts for chemical looping reforming of methane. Appl. Energy 2015, 157, 391–398. [Google Scholar] [CrossRef]
- Sarshar, Z.; Sun, Z.; Zhao, D.; Kaliaguine, S. Development of sinter-resistant core–shell LaMnxFe1–xO3@mSiO2 oxygen carriers for chemical looping combustion. Energy Fuels 2012, 26, 3091–3102. [Google Scholar] [CrossRef]
- Tian, X.; Wei, Y.; Zhao, H. Evaluation of a hierarchically-structured CuO@TiO2-Al2O3 oxygen carrier for chemical looping with oxygen uncoupling. Fuel 2017, 209, 402–410. [Google Scholar] [CrossRef]
- Dharanipragada, N.V.R.A.; Galvita, V.V.; Poelman, H.; Buelens, L.C.; Detavernier, C.; Marin, G.B. Bifunctional Co- and Ni-ferrites for catalyst-assisted chemical looping with alcohols. Appl. Catal. B 2018, 222, 59–72. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping auto-thermal dry reforming: Spatial structuring effects on process efficiency. Appl. Catal. B 2018, 231, 123–136. [Google Scholar] [CrossRef]
- Vladimir, G.V.; Hilde, P.; Esteban, F.; Mark, S.; Marin, G.B. Development and performance of iron based oxygen carriers for chemical looping, in nanotechnology in catalysis. In Applications in the Chemical Industry, Energy Development, and Environment Protection; Voorde, M.V.d., Sels, B., Eds.; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Zhu, T.; Flytzani-Stephanopoulos, M. Catalytic partial oxidation of methane to syngas over Ni-CeO2. Appl. Catal. A 2001, 208, 403–417. [Google Scholar] [CrossRef]
- Pengpanich, S.; Meeyoo, V.; Rirksomboon, T.; Bunyakiat, K. Catalytic oxidation of methane over CeO2-ZrO2 mixed oxide solid solution catalysts prepared via urea hydrolysis. Appl. Catal. A 2002, 234, 221–233. [Google Scholar] [CrossRef]
- Tobias, M.; Anders, L.; Paul, C. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 1953–1962. [Google Scholar]
- Rifflart, S.; Lambert, A.; Delebarre, A.; Salmi, J.; Durand, B.; Carpentier, S. Climate project-material development for chemical looping combustion. In Proceedings of the 1st International Conference on Chemical Looping, Lyon, France, 17–19 March 2010. [Google Scholar]
- Tian, Q.; Che, L.; Ding, B.; Wang, Q.; Su, Q. Performance of a Cu-Fe-based oxygen carrier combined with a Ni-based oxygen carrier in a chemical-looping combustion process based on fixed-bed reactors. Greenh. Gas Sci. Technol. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Johansson, M.; Mattisson, T.; Lyngfelt, A. Creating a synergy effect by using mixed oxides of iron- and nickel oxides in the combustion of methane in a chemical-looping combustion reactor. Energy Fuels 2006, 20, 2399–2407. [Google Scholar] [CrossRef]
- Pishahang, M.; Larring, Y.; Sunding, M.; Jacobs, M.; Snijkers, F. Performance of perovskite-type oxides as oxygen-carrier materials for chemical looping combustion in the presence of H2S. Energy Technol. 2016, 4, 1305–1316. [Google Scholar] [CrossRef]
- Dai, X.P.; Li, R.J.; Yu, C.C.; Hao, Z.P. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A = La, Nd, Eu) perovskite-type oxides as oxygen storage. J. Phys. Chem. B 2006, 110, 22525–22531. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Yu, C.C.; Ji, W.J.; Shen, S.K. Methane oxidation to synthesis gas using lattice oxygen. In Studies in Surface Science and Catalysis; Bao, X., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 147, pp. 199–204. [Google Scholar]
- Solunke, R.D.; Veser, G.t. Hydrogen production via chemical looping steam reforming in a periodically operated fixed-bed reactor. Ind. Eng. Chem. Res. 2010, 49, 11037–11044. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Park, J.C.; Bang, J.U.; Lee, J.; Ko, C.H.; Song, H. Ni@SiO2 yolk-shell nanoreactor catalysts: High temperature stability and recyclability. J. Mater. Chem. 2010, 20, 1239–1246. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Song, Y.; Zhao, J.; Ji, W.; Au, C.-T. Fine-tunable Ni@porous silica core–shell nanocatalysts: Synthesis, characterization, and catalytic properties in partial oxidation of methane to syngas. J. Catal. 2012, 288, 54–64. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, H.; Wei, Y.; Zheng, C. Self-assembly template combustion synthesis of a core–shell CuO@TiO2-Al2O3 hierarchical structure as an oxygen carrier for the chemical-looping processes. Combust. Flame 2015, 162, 3030–3045. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Huang, Z.; Wu, G.; Gong, J. A Ni@ZrO2 nanocomposite for ethanol steam reforming: Enhanced stability via strong metal-oxide interaction. Chem. Commun. (Camb.) 2013, 49, 4226–4228. [Google Scholar] [CrossRef] [PubMed]
- Ushakov, S.V.; Navrotsky, A.; Yang, Y.; Stemmer, S.; Kukli, K.; Ritala, M.; Leskelä, M.A.; Fejes, P.; Demkov, A.; Wang, C.; et al. Crystallization in hafnia- and zirconia-based systems. Phys. Status Solidi 2004, 241, 2268–2278. [Google Scholar] [CrossRef]
- Zhao, F.; Gao, Y.; Li, W.; Luo, H. Nanocoating Fe2O3 powders with a homogeneous ultrathin ZrO2 shell. J. Ceram. Soc. Jpn. 2008, 116, 1164–1166. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, F.; Liu, Y.; Luo, H. Synthesis and characterization of ZrO2 capsules and crystalline ZrO2 thin layers on Fe2O3 powders. CrystEngComm 2011, 13, 3511–3514. [Google Scholar] [CrossRef]
- Arnal, P.M.; Weidenthaler, C.; Schüth, F. Highly monodisperse zirconia-coated silica spheres and zirconia-silica hollow spheres with remarkable textural properties. Chem. Mater. 2006, 18, 2733–2739. [Google Scholar] [CrossRef]
- Feyen, M.; Weidenthaler, C.; Guttel, R.; Schlichte, K.; Holle, U.; Lu, A.H.; Schuth, F. High-temperature stable, iron-based core-shell catalysts for ammonia decomposition. Chemistry 2011, 17, 598–605. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst design with atomic layer deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Galinsky, N.L.; Shafiefarhood, A.; Chen, Y.; Neal, L.; Li, F. Effect of support on redox stability of iron oxide for chemical looping conversion of methane. Appl. Catal. B 2015, 164, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Evdou, A.; Zaspalis, V.; Nalbandian, L. Ferrites as redox catalysts for chemical looping processes. Fuel 2016, 165, 367–378. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Hu, M.; Tian, Y.; Jin, X.; Ma, S.; Xu, T.; Hu, Z.; Liu, S.; Guo, D.; et al. Chemical looping combustion of biomass using metal ferrites as oxygen carriers. Chem. Eng. J. 2017, 312, 252–262. [Google Scholar] [CrossRef]
- Srdic, V.; Mojic, B.; Nikolic, M.; Ognjanovic, S. Recent progress on synthesis of ceramics core/shell nanostructures. Process. Appl. Ceram. 2013, 7, 45–62. [Google Scholar] [CrossRef]
- Yoo, J.B.; Kim, H.S.; Kang, S.H.; Lee, B.; Hur, N.H. Hollow nickel-coated silica microspheres containing rhodium nanoparticles for highly selective production of hydrogen from hydrous hydrazine. J. Mater. Chem. A 2014, 2, 18929–18937. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Li, X.; Liu, X.; Yang, Q. Organosilane-assisted transformation from core–shell to yolk–shell nanocomposites. Chem. Mater. 2011, 23, 3676–3684. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]




| Oxygen Carrier(Active Metal) | Promotors/Support | Preparation | Structure | Process | Reactant Gas | Ref. | |
|---|---|---|---|---|---|---|---|
| Reducing | Oxidizing | ||||||
| Monometallic-Based Material | |||||||
| NiO | α-Al2O3 | incipient wetness impregnation | supported | CLC | CH4 or 30%CH4/N2 | air or 5%O2/N2 | [64,65] |
| La-Co/γ-Al2O3 | impregnation | supported | CLC | CH4 | air | [66] | |
| Al2O3, sepiolite, SiO2, TiO2, ZrO2 | mechanical mixing | mixed | CLC | 70%CH4/H2O | air | [58] | |
| SiO2, MgAl2O4 | dry impregnation | supported | CLC and CLR | 10%CH4 10%H2O 5%CO2 75%N2 | 5%O2/N2 | [59] | |
| TiO2 | incipient wetness impregnation | supported | CLC | 20%CH4/N2 | air | [67] | |
| ZrO2, TiO2, SiO2, Al2O3, NiAl2O4 | wet impregnation | supported | CLR | CH4/H2O (1/3) | air | [68] | |
| ZrO2, γ-Al2O3 | wet impregnation | supported | CLR | CH4/H2O (1/3) | air | [69] | |
| Mg-stabilized ZrO2 | freeze granulation | supported | CLC CLR | CH4/N2 CH4/H2O | 5%O2/N2 | [70] | |
| Yttria-stabilized ZrO2 | sol-gel and dissolution | mixed | CLC | H2 | air | [71] | |
| NiAl2O4 | sol-gel | spinel | CLC | 17%CH4/He | air | [72] | |
| NiAl2O4 | spray-drying | mixed | CLC | 50%CH4/H2O | 5%O2/N2 | [73] | |
| MgAl2O4 | freeze granulation | supported | CLR | Nature gas | air | [74] | |
| α-Al2O3, MgAl2O4, CaAl2O4 | dry impregnation | supported | CLC | 10%CH4 20%H2O 70%N2 | air | [75] | |
| CaAl2O4 | impregnation | supported | CLC | 15%CH4/N2 | 10%O2/N2 | [76] | |
| CeO2, La2O3 | incipient wetness impregnation | supported | CLC | 17%CH4/Ar | air | [77] | |
| Fe2O3 | γ-Al2O3 | impregnation | supported | CLC | CH4 | air | [78] |
| α-Al2O3 | sol-gel combustion | mixed | CLC | H2 | air | [79] | |
| Al2O3 | sol-gel | mixed | CLC | lignite | air | [80] | |
| γ-Al2O3 | impregnation | supported | CLC | H2S ppm/CH4 | air | [81] | |
| Al2O3 | co-precipitation | mixed | CL a | H2 | CO2 | [82] | |
| MgO | theoretical study | supported | CLC | CO | O2 | [83] | |
| TiO2 | incipient wetness impregnation | supported | CLC | CH4 | O2 | [84] | |
| TiO2 | solid-state mixing | mixed | CLC | 3%H2/Ar | air | [85] | |
| TiO2 | mechanical mixing | mixed | CLC | syngas | H2O | [86] | |
| SiO2 | dry impregnation | supported | CLR | 50%CH4/H2O | 5%O2/N2 | [87] | |
| MgAl2O4 | freeze granulation | supported | CLC | 50%CH4/H2O | air | [88] | |
| MgFeAlOx | co-precipitation | spinel | CL | 5%H2/He | CO2 | [89] | |
| MgAl2O4 | sequential wetness impregnation | supported | CL a | CO | H2O | [90] | |
| MgAl2O4 | spray-drying | mixed | CLR | CH4 | H2O | [91] | |
| CaO | wet impregnation | doped | CLC | 10%CO/N2 | 20%CO2/N2 | [92] | |
| CaO | mechanical mixing | mixed | CLC | 10%CO/N2 | 20%CO2/N2 | [93] | |
| ZrO2 | co-precipitation | mixed | CLC | 10%CO/N2 | 20%CO2/N2 | [94] | |
| ZrO2 | theoretical study | supported | CLC | CO | O2 | [95] | |
| ZrO2 | co-precipitation | mixed | CLC CLR | CO CH4 | O2 H2O | [96] | |
| Ce-, Ca-, Mg-stabilized ZrO2 | freeze granulation | supported | CLC | CH4/CO2/CO | O2 | [54] | |
| CeO2 | co-precipitation | mixed | CL a | 5%H2/He | CO2 | [22] | |
| CeO2 | co-precipitation | mixed | CLR | CH4 | H2O/N2 | [97] | |
| CeO2 | nanometric colloidal sol technique | Surfacegrafted | CL a | CO or H2/N2 | O2/N2 | [98] | |
| CeO2 | sol-gel | mixed | CLR | CO/H2/N2 | H2O/N2 | [99] | |
| CeO2 | co-precipitation | solid solution | CL a | H2 | CO2 | [100] | |
| Mixed oxide-based material | |||||||
| CoO-NiO | Yttria-stabilized ZrO2 | dissolution | supported | CLC | CH4/H2O (1/2) | air | [101] |
| Al2O3 | incipient wetness impregnation | supported | CLC | CH4 | air | [102] | |
| Fe2O3-CuO | MgAl2O4 | mechanical mixing | mixed | CLC | syngas or nature gas | air | [103] |
| FexMn(1-x)O | - | co-precipitation | mixed | CLC | 5%CH4/He | air | [104] |
| - | spray-drying | mixed | CLOU | CH4 or syngas | 5%O2/N2 | [105] | |
| Al2O3 | spray-drying | supported | CLOU | CH4 or syngas | 5%O2/N2 | [106] | |
| NixMn(1−x)O | - | spray-drying | mixed | CLOU | CH4 or syngas | 5%O2/N2 | [107] |
| Fe2O3-NiO | Al2O3 | sequential impregnation | supported | CLC | 25%CH4/N2 | 10%O2/N2 | [108] |
| CeO2, Al2O3 | incipient wetness impregnation | supported | CLC | 17%CH4/Ar | 20%O2/He | [109] | |
| Al2O3 | mechanical mixing and impregnation | supported | CLG b | biomass | air | [110] | |
| Al2O3 | co-precipitation | mixed | CLG b | biomass | air | [111] | |
| La0.8Sr0.2FeO3 | sol-gel | supported | CLR | 30%CH4/N2 | 30%CO2/N2 | [112] | |
| Perovskite-structured material | |||||||
| LaFeO3 | - | Solution combustion | perovskite | CLR | CH4/Ar | O2/Ar | [113] |
| La1−xSrxFeO3 | - | co-precipitation | perovskite | CLR | CH4 | H2O | [114] |
| - | solution combustion | perovskite | CLR | 40%CH4/N2 | air | [115] | |
| La1−xSrxMyFe1−yO3 | M = Ni, Co, Cr, Cu | citrate method | perovskite | CLR | CH4 | O2 or H2O | [116] |
| Core-shell structured material | |||||||
| NiO (core) | SiO2 (shell) | SiO2 coating | core-shell | DR | CO2:CH4:N2 = 1:1:1 | [117] | |
| Al2O3 (shell) | atomic layer deposition | core-shell | DR | CO2:CH4:He = 1:1:8 | [118] | ||
| Fe3O4 (core) | SiO2 (shell) | microemulsion-based synthesis | core-shell | CLC | 50%CH4/N2 | 50%O2/N2 | [119] |
| Fe2O3 (core) | SiO2 (shell) | reverse microemulsion | core-shell | CL a | H2 | CO2 | [120] |
| La1−xSrxFeO3 (shell) | sequential sol-gel | core-shell | CLR | CH4 | O2 | [121] | |
| MeOx (core) (Me = Mn, Co, Fe) | La1-xSrxFeO3 (shell) | modified Pechini method | core-shell | CLR | 10%CH4/He | 10%O2/He | [122] |
| LaMn0.7Fe0.3O3.15 (core) | SiO2 (shell) | surfactant-templating | core-shell | CLC | CH4 | air | [123] |
| CuO (shell) | TiO2-Al2O3 (core) | self-assembly template combustion | core-shell | CLC CLOU | CH4 N2 | air O2 | [124] |
| Fe2O3/ZrO2 (core) | ZrO2 (shell) | impregnation and nanocoating | core-shell | CL a | 5%H2/Ar | CO2 | [40] |
| Bifunctional material | |||||||
| Ni-Fe2O3 | CeO2 | co-precipitation and impregnation | supported | CCDR | CO2:CH4 = 1:1 | CO2 | [23] |
| NiFe2O4 or CoFe2O4 | CeZrO2 | co-precipitation | spinel | CCDR | methanol or ethanol-H2O | CO2 | [125] |
| Ni-Fe2O3 | MgAl2O4 | co-impregnation | supported | CCAR | CO2:CH4:O2= 1:1:0.5 | CO2 | [20] |
| Ni(shell)-Fe2O3(core) | ZrO2 | impregnation, nanocoating and SiO2-template assisted | core-shell | CCAR | CO2:CH4:O2= 1:1:0.2 | CO2 | [126] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Galvita, V.V.; Poelman, H.; Marin, G.B. Advanced Chemical Looping Materials for CO2 Utilization: A Review. Materials 2018, 11, 1187. https://doi.org/10.3390/ma11071187
Hu J, Galvita VV, Poelman H, Marin GB. Advanced Chemical Looping Materials for CO2 Utilization: A Review. Materials. 2018; 11(7):1187. https://doi.org/10.3390/ma11071187
Chicago/Turabian StyleHu, Jiawei, Vladimir V. Galvita, Hilde Poelman, and Guy B. Marin. 2018. "Advanced Chemical Looping Materials for CO2 Utilization: A Review" Materials 11, no. 7: 1187. https://doi.org/10.3390/ma11071187






