Research on the Thermal Behaviour of a Selectively Laser Melted Aluminium Alloy: Simulation and Experiment
Abstract
:1. Introduction
2. Model for SLM Process
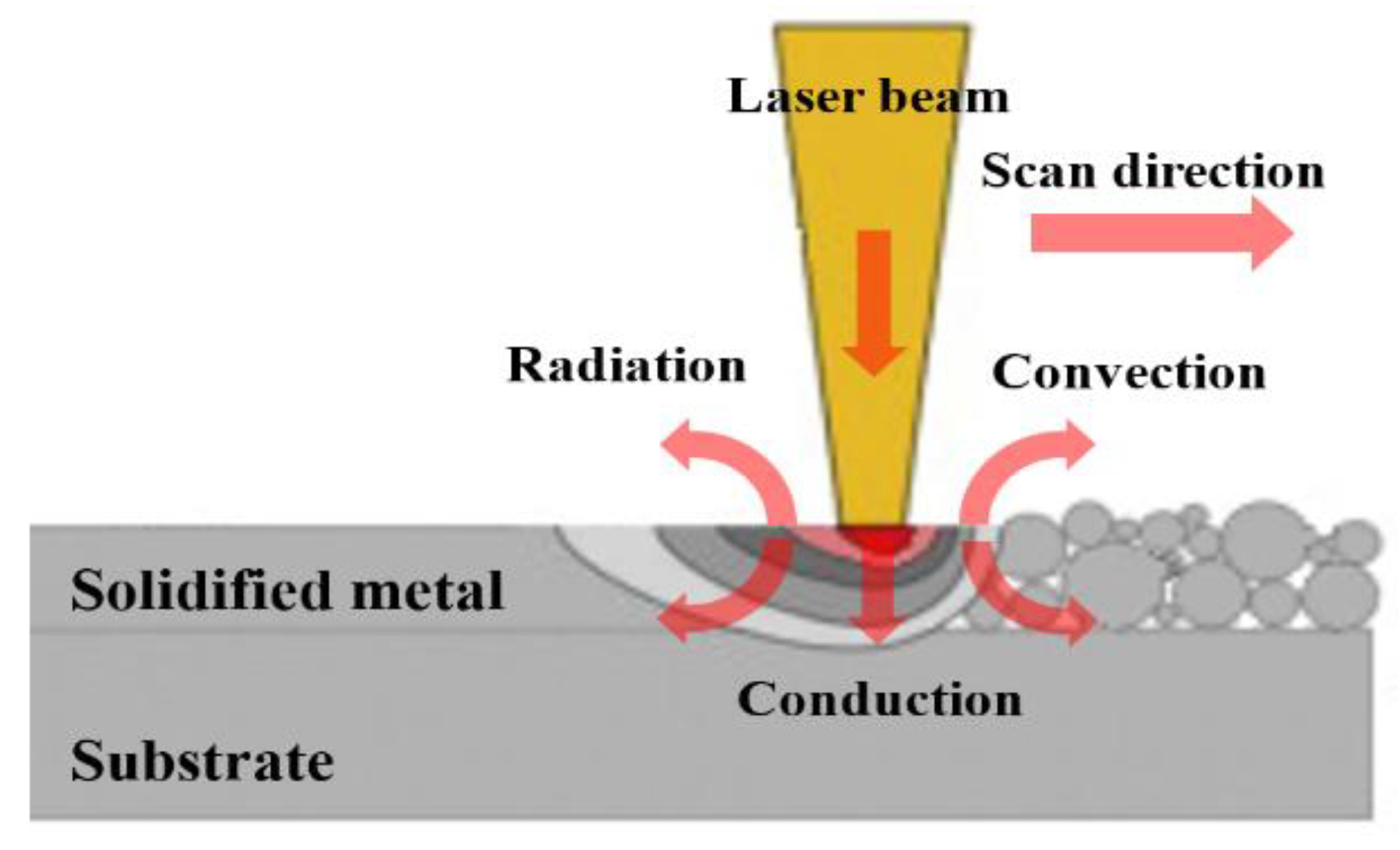
2.1. Mathematical Modelling of Heat Transfer
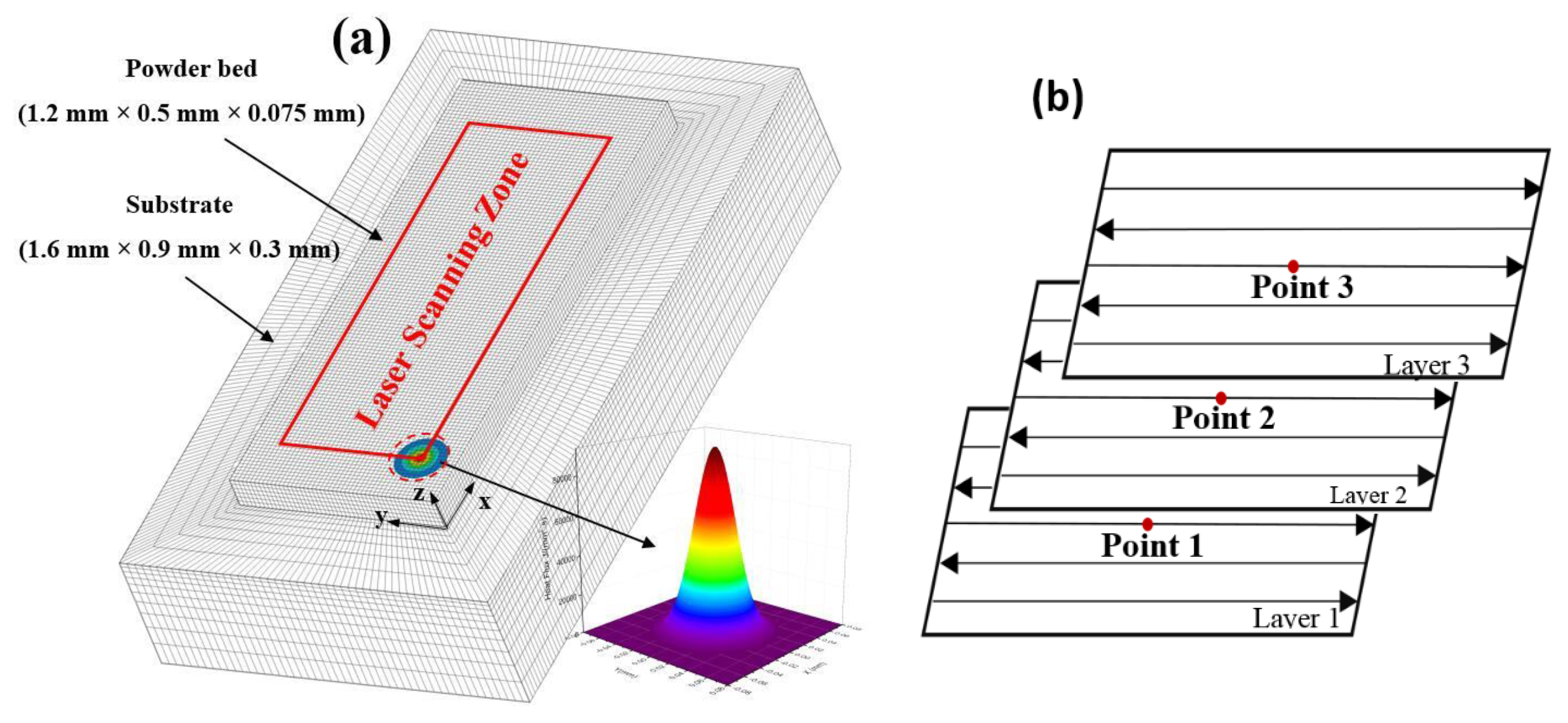
2.2. Numerical Model Establishment
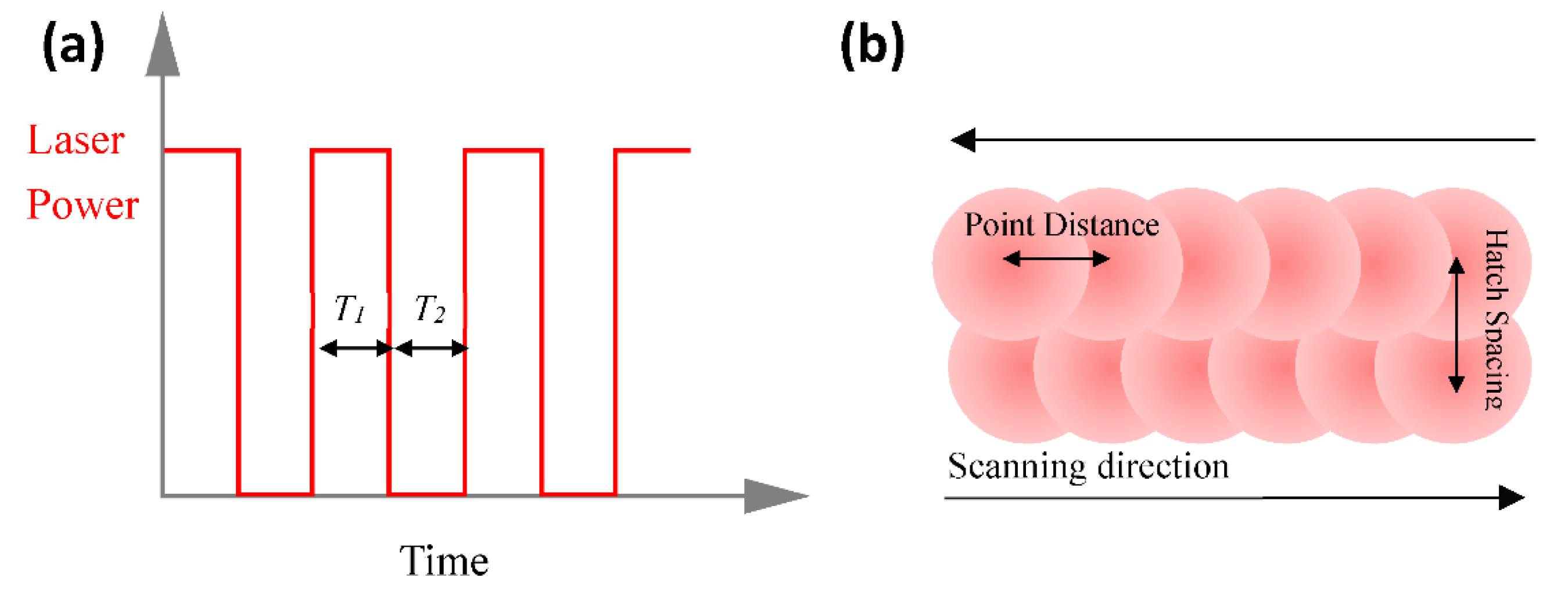
2.3. Modelling of Laser Energy
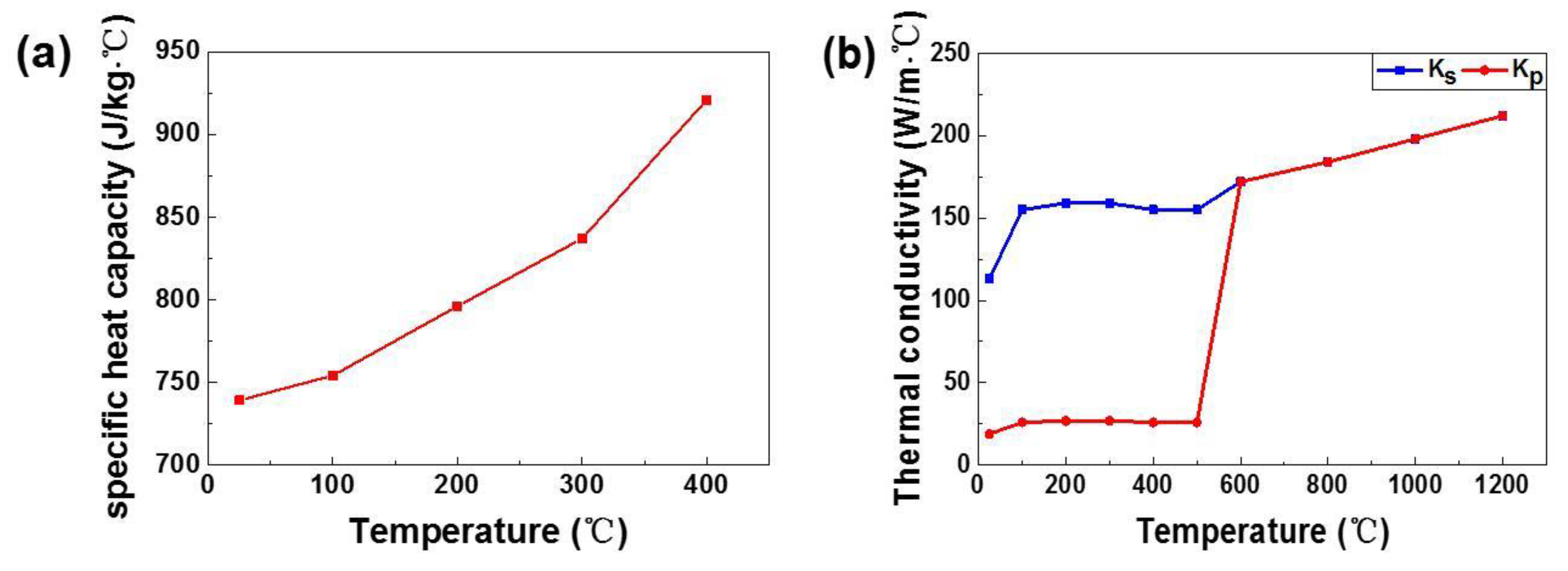
2.4. Thermal Physical Parameters
3. Experiments
4. Results and Discussion
4.1. Temperature Distribution
4.2. Thermal Behaviours
4.3. Melt Pool Configurations
4.4. Experimental Investigation
5. Conclusions
- (1)
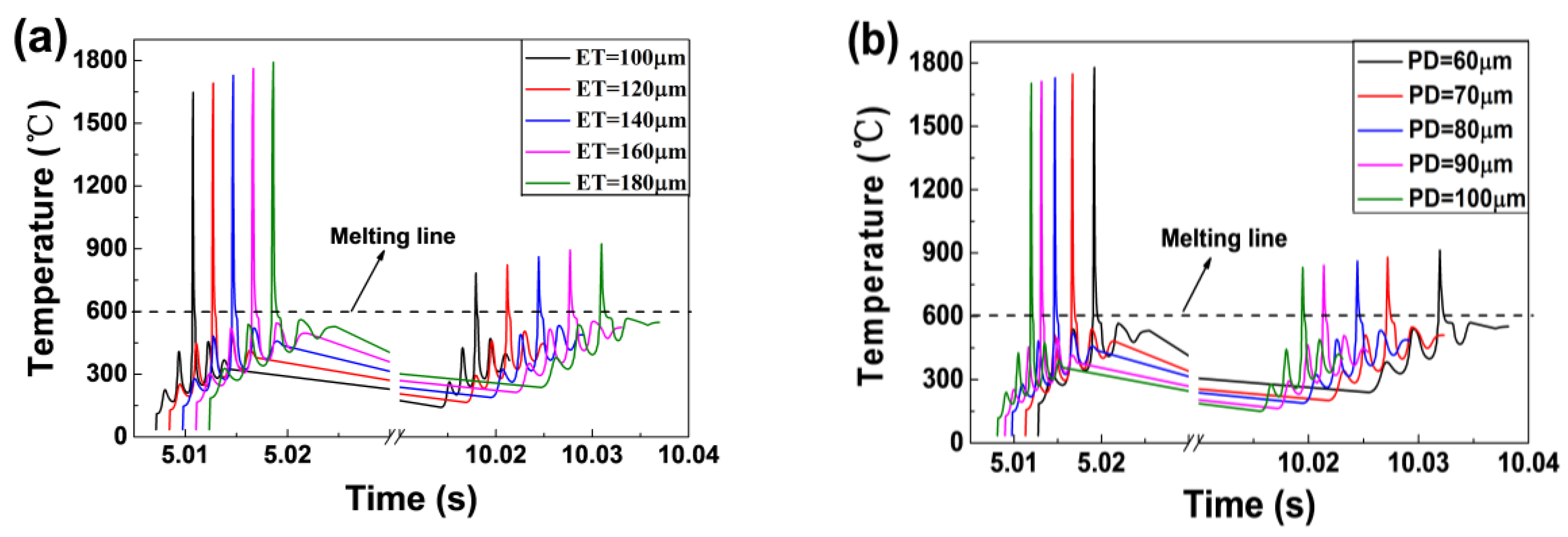
- As the laser moved from the centre of the first layer to the centre of the third layer, the maximum temperature of the melt pool decreased firstly and then increased slightly. The width and the depth of the melt pool increased continuously but in small amounts. This is mainly due to the heat accumulation phenomenon although the recoating phase.
- (2)
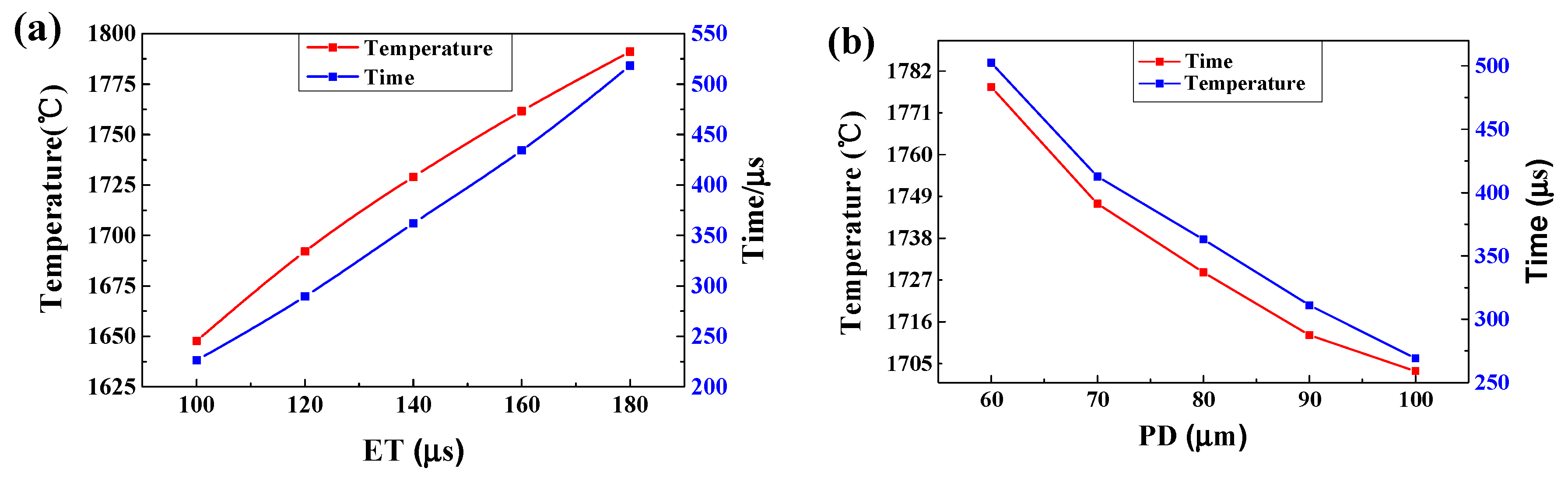
- The cooling rate of the melt pool decreased from 7.93 × 106 °C/s to 3.61 × 106 °C/s as the ET increased from 100 μs to 180 μs. However, the rate rose significantly from 3.25 × 106 °C/s to 7.48 × 106 °C/s as the PD increased from 60 μm to 100 μm. The cooling rate during the recoating phase elevated as the ET increased and the PD decreased. This was due to increasing heat accumulation in the underlying solid material.
- (3)
- The maximum temperature and the liquid lifetime rose as the ET increased and the PD decreased. However, the PD had more notable effects on the liquid lifetime.
- (4)
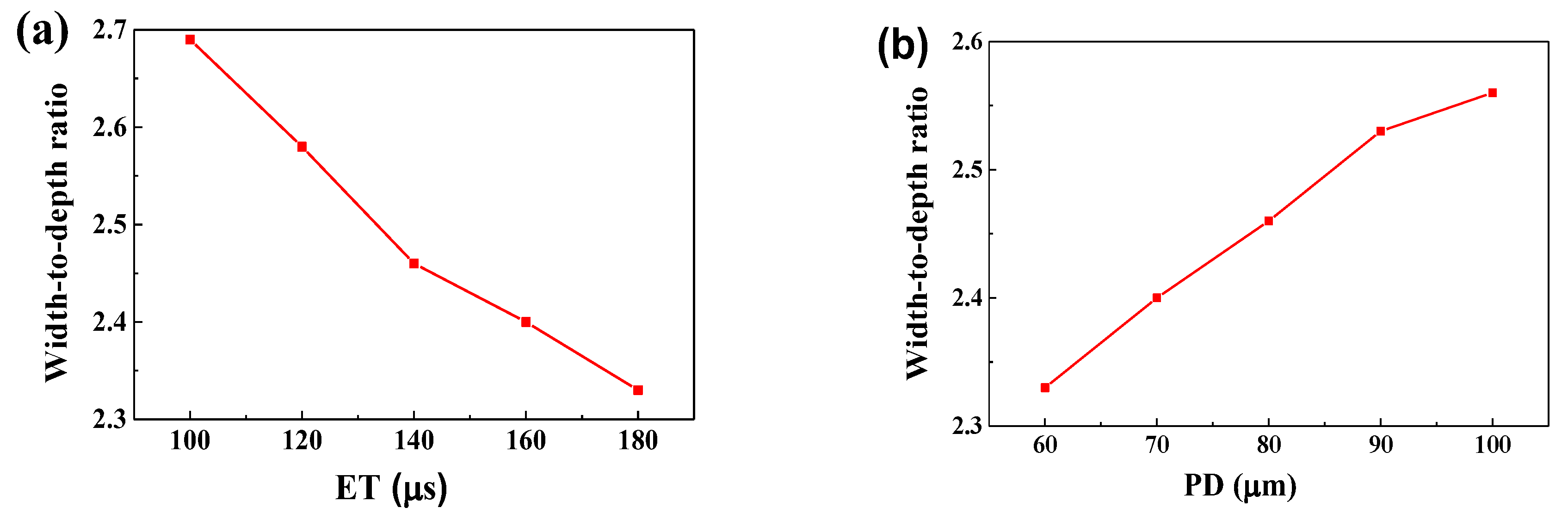
- The dimensions of the melt pool increased (the width from 107.2 μm to 134 μm and the depth from 39.8 μm to 57.5 μm) as the ET elevated from 100 μs to 180 μs, however, said dimensions decreased (the width from 131.9 μm to 114 μm and the depth from 56.5 μm to 44.5 μm) as the PD elevated from 60 μm to 100 μm. It can be seen that the depth and the width were more sensitive to the PD than to the ET. The proper melt pool width (119.8 μm) and depth (48.65 μm) were obtained for a successful SLM process with a combination of ET = 140 μs and PD = 80 μm.
- (5)
- The best forming quality—free of apparent pores, holes, cracks, and the balling phenomenon—was obtained at the optimised combination of ET = 140 μs and PD = 80 μm.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fousová, M.; Dvorský, D.; Michalcová, A.; Vojtěch, D. Changes in the microstructure and mechanical properties of additively manufactured AlSi10Mg alloy after exposure to elevated temperatures. Mater. Charact. 2018, 137, 119–126. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Wu, J. Experimental investigation on densification behavior and surface roughness of AlSi10Mg powders produced by selective laser melting. Opt. Laser Technol. 2017, 96, 88–96. [Google Scholar] [CrossRef]
- Almangour, B.; Grzesiak, D.; Cheng, J.; Ertas, Y. Thermal behavior of the molten pool, microstructural evolution, and tribological performance during selective laser melting of TiC/316L stainless steel nanocomposites: Experimental and simulation methods. J. Mater. Process. Technol. 2018, 257, 288–301. [Google Scholar] [CrossRef]
- Yu, G.; Gu, D.; Dai, D.; Xia, M.; Ma, C.; Shi, Q. On the role of processing parameters in thermal behavior, surface morphology and accuracy during laser 3D printing of aluminum alloy. J. Phys. D Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Ilin, A.; Logvinov, R.; Kulikov, A.; Prihodovsky, A.; Xu, H.; Ploshikhin, V.; Günther, B.; Bechmann, F. Computer Aided Optimisation of the Thermal Management During Laser Beam Melting Process. Phys. Procedia 2014, 56, 390–399. [Google Scholar] [CrossRef]
- Dai, D.; Gu, D. Tailoring surface quality through mass and momentum transfer modeling using a volume of fluid method in selective laser melting of TiC/AlSi10Mg powder. Int. J. Mach. Tools Manuf. 2015, 88, 95–107. [Google Scholar] [CrossRef]
- Ding, X.; Wang, L. Heat transfer and fluid flow of molten pool during selective laser melting of AlSi10Mg powder: Simulation and experiment. J. Manuf. Proc. 2017, 26, 280–289. [Google Scholar] [CrossRef]
- Li, Y.; Gu, D. Parametric analysis of thermal behavior during selective laser melting additive manufacturing of aluminum alloy powder. Mater. Des. 2014, 63, 856–867. [Google Scholar] [CrossRef]
- Loh, L.E.; Chua, C.K.; Yeong, W.-Y.; Song, J.; Mapar, M.; Sing, S.L.; Liu, Z.H.; Zhang, D.Q. Numerical investigation and an effective modelling on the Selective Laser Melting (SLM) process with aluminium alloy 6061. Int. J. Heat Mass Transf. 2015, 80, 288–300. [Google Scholar] [CrossRef]
- Han, Q.; Setchi, R.; Lacan, F.; Gu, D.; Evans, S.L. Selective laser melting of advanced Al-Al2O3 nanocomposites: Simulation, microstructure and mechanical properties. Mater. Sci. Eng. A 2017, 698, 162–173. [Google Scholar] [CrossRef]
- Cherry, J.A.; Davies, H.M.; Mehmood, S.; Lavery, N.P.; Brown, S.G.R.; Sienz, J. Investigation into the effect of process parameters on microstructural and physical properties of 316L stainless steel parts by selective laser melting. Int. J. Adv. Manuf. Technol. 2015, 76, 869–879. [Google Scholar] [CrossRef]
- Hussein, A.; Hao, L.; Yan, C.; Everson, R. Finite element simulation of the temperature and stress fields in single layers built without-support in selective laser melting. Mater. Des. 2013, 52, 638–647. [Google Scholar] [CrossRef]
- Pei, W.; Wei, Z.; Zhen, C.; Li, J.; Zhang, S.; Du, J. Numerical simulation and parametric analysis of selective laser melting process of AlSi10Mg powder. Appl. Phys. A 2017, 123, 540. [Google Scholar] [CrossRef]
- Ding, X.; Wang, L.; Wang, S. Comparison study of numerical analysis for heat transfer and fluid flow under two different laser scan pattern during selective laser melting. Optik Int. J. Light Electron Opt. 2016, 127, 10898–10907. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, D. Aluminum Alloys and Their Application; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Safdar, S.; Pinkerton, A.J.; Li, L.; Sheikh, M.A.; Withers, P.J. An anisotropic enhanced thermal conductivity approach for modelling laser melt pools for Ni-base super alloys. Appl. Math. Model. 2013, 37, 1187–1195. [Google Scholar] [CrossRef]
- Thijs, L.; Kempen, K.; Kruth, J.-P.; Van Humbeeck, J. Fine-structured aluminium products with controllable texture by selective laser melting of pre-alloyed AlSi10Mg powder. Acta Mater. 2013, 61, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Pistorius, P.C.; Narra, S.; Beuth, J.L. Rapid Solidification: Selective Laser Melting of AlSi10Mg. JOM 2016, 68, 960–969. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, Z.; Jiang, Y.; Wang, G.W.; Yang, Y.; Zhang, L.C. Gradient in microstructure and mechanical property of selective laser melted AlSi10Mg. J. Alloys Compd. 2017. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.Q.; Wang, W.; Attallah, M.M.; Loretto, M.H. Microstructure and strength of selectively laser melted AlSi10Mg. Acta Mater. 2016, 117, 311–320. [Google Scholar] [CrossRef]
- Zhao, Z.; Guan, R.; Zhang, J.; Zhao, Z.; Bai, P. Effects of Process Parameters of Semisolid Stirring on Microstructure of Mg-3Sn-1Mn-3SiC (wt %) Strip Processed by Rheo-rolling. Acta Metall. Sin. Engl. Lett. 2017, 30, 66–72. [Google Scholar] [CrossRef]
- Zhang, B.; Dembinski, L.; Coddet, C. The study of the laser parameters and environment variables effect on mechanical properties of high compact parts elaborated by selective laser melting 316L powder. Mater. Sci. Eng. A 2013, 584, 21–31. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Maskery, I.; Tuck, C.; Ashcroft, I.; Everitt, N.M. Improving the fatigue behaviour of a selectively laser melted aluminium alloy: Influence of heat treatment and surface quality. Mater. Des. 2016, 104, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Di, W.; Yang, Y.; Su, X.; Chen, Y. Study on energy input and its influences on single-track, multi-track, and multi-layer in SLM. Int. J. Adv. Manuf. Technol. 2012, 58, 1189–1199. [Google Scholar] [CrossRef]
- Cao, J.; Liu, F.; Lin, X.; Huang, C.; Chen, J.; Huang, W. Effect of overlap rate on recrystallization behaviors of Laser Solid Formed Inconel 718 superalloy. Opt. Laser Technol. 2013, 45, 228–235. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Lu, Z.; Huang, S. Numerical simulation of transient temperature field in selective laser melting. Chin. Mech. Eng. 2008, 19, 2492–2495. [Google Scholar] [CrossRef]
- Haboudou, A.; Peyre, P.; Vannes, A.B.; Peix, G. Reduction of porosity content generated during Nd:YAG laser welding of A356 and AA5083 aluminium alloys. Mater. Sci. Eng. A 2003, 363, 40–52. [Google Scholar] [CrossRef]
- Wu, H.; Ren, J.; Huang, Q.; Zai, X.; Liu, L.; Chen, C.; Liu, S.; Yang, X.; Li, R. Effect of laser parameters on microstructure, metallurgical defects and property of AlSi10Mg printed by selective laser melting. J. Micromech. Mol. Phys. 2018, 02, 1750017. [Google Scholar] [CrossRef]
- Weingarten, C.; Buchbinder, D.; Pirch, N.; Meiners, W.; Wissenbach, K.; Poprawe, R. Formation and reduction of hydrogen porosity during selective laser melting of AlSi10Mg. J. Mater. Process. Technol. 2015, 221, 112–120. [Google Scholar] [CrossRef]
- Han, X.; Zhu, H.; Nie, X.; Wang, G.; Zeng, X. Investigation on Selective Laser Melting AlSi10Mg Cellular Lattice Strut: Molten Pool Morphology, Surface Roughness and Dimensional Accuracy. Materials 2018, 11, 392. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Value |
|---|---|
| Laser power, P | 200 W |
| Hatching space, S | 80 μm |
| Spot diameter, D | 80 μm |
| Layer thickness | 25 μm |
| Laser absorptivity of the Al powder, A | 18% [13] |
| Spreading time | 5 s |
| Exposure Time, ET | 100, 120, 140, 160, 180 μs |
| Point Distance, PD | 60, 70, 80, 90, 100 μm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, B.-Q.; Bai, P.; Liu, B.; Wang, Y. Research on the Thermal Behaviour of a Selectively Laser Melted Aluminium Alloy: Simulation and Experiment. Materials 2018, 11, 1172. https://doi.org/10.3390/ma11071172
Li Z, Li B-Q, Bai P, Liu B, Wang Y. Research on the Thermal Behaviour of a Selectively Laser Melted Aluminium Alloy: Simulation and Experiment. Materials. 2018; 11(7):1172. https://doi.org/10.3390/ma11071172
Chicago/Turabian StyleLi, Zhonghua, Bao-Qiang Li, Peikang Bai, Bin Liu, and Yu Wang. 2018. "Research on the Thermal Behaviour of a Selectively Laser Melted Aluminium Alloy: Simulation and Experiment" Materials 11, no. 7: 1172. https://doi.org/10.3390/ma11071172





