The Effect of Radiative Cooling on Reducing the Temperature of Greenhouses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparing Plastic Films
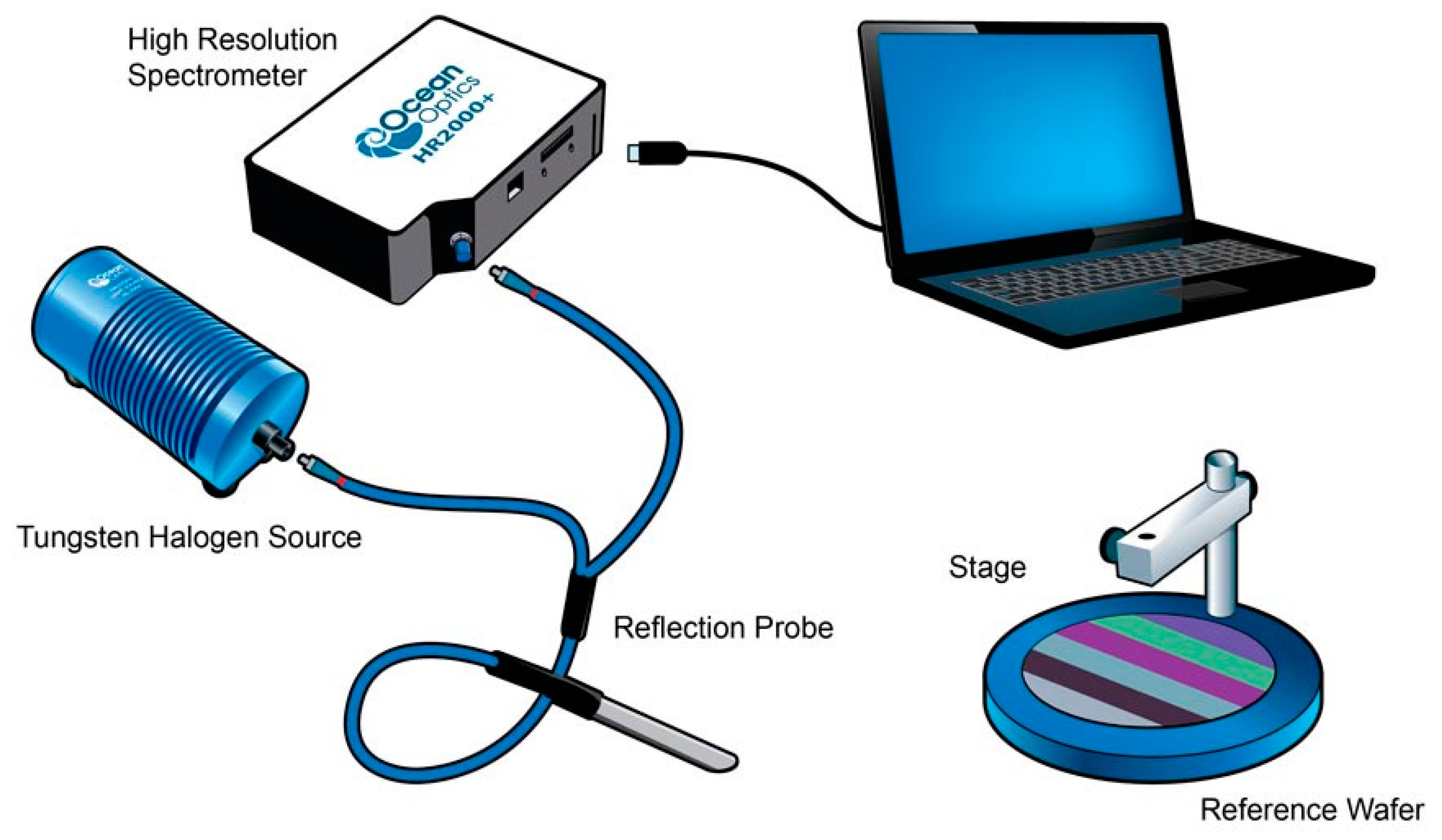
2.2. Characterization of Prepared Plastic Films
3. Results and Discussion
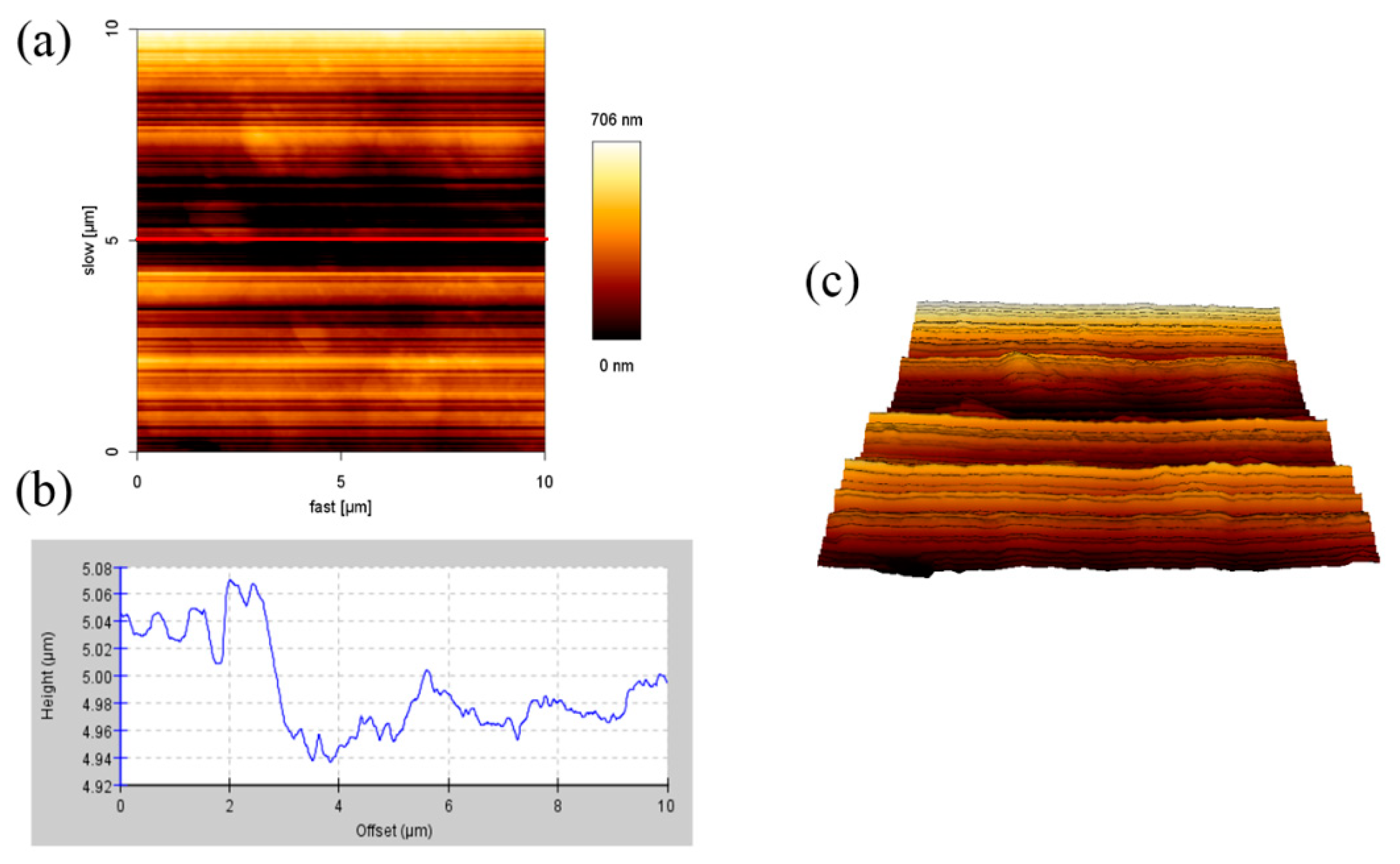
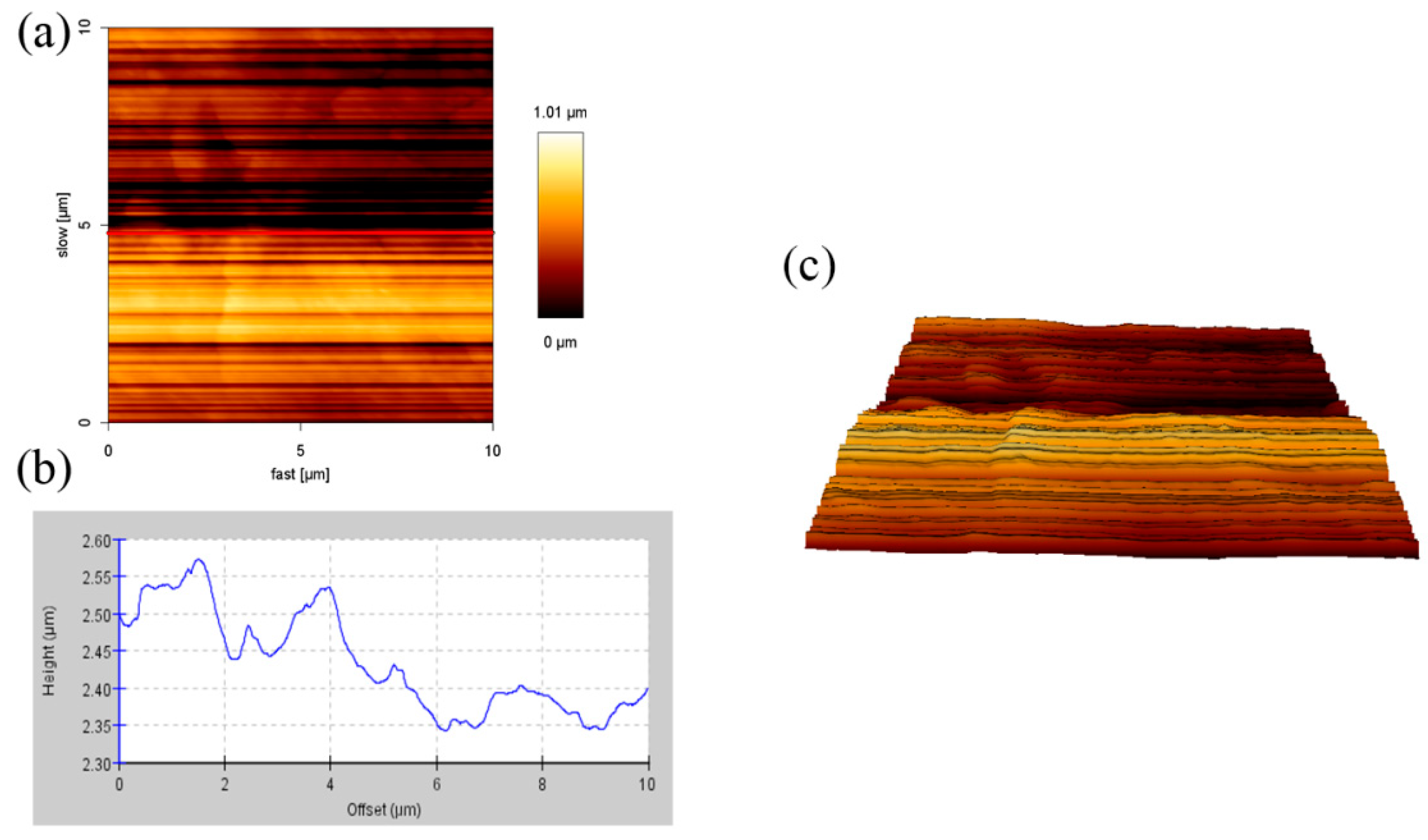
3.1. AFM Analysis of the Samples
3.2. Mechanical Properties of the Samples
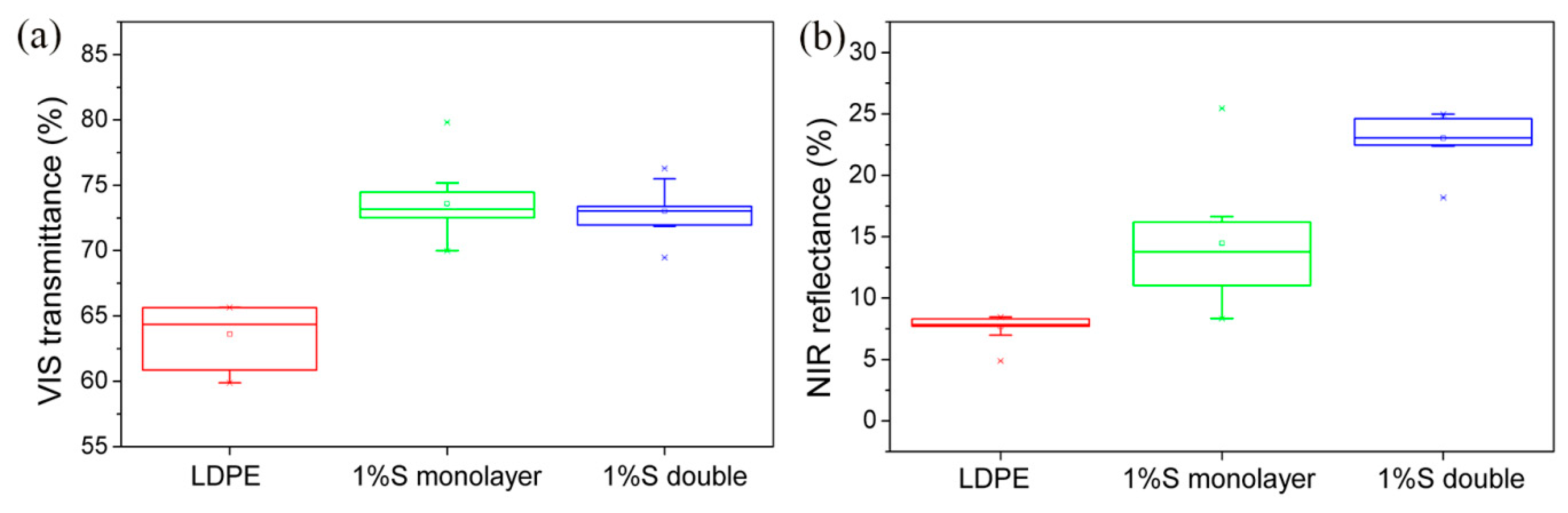
3.3. UV-VIS Spectra of the Samples
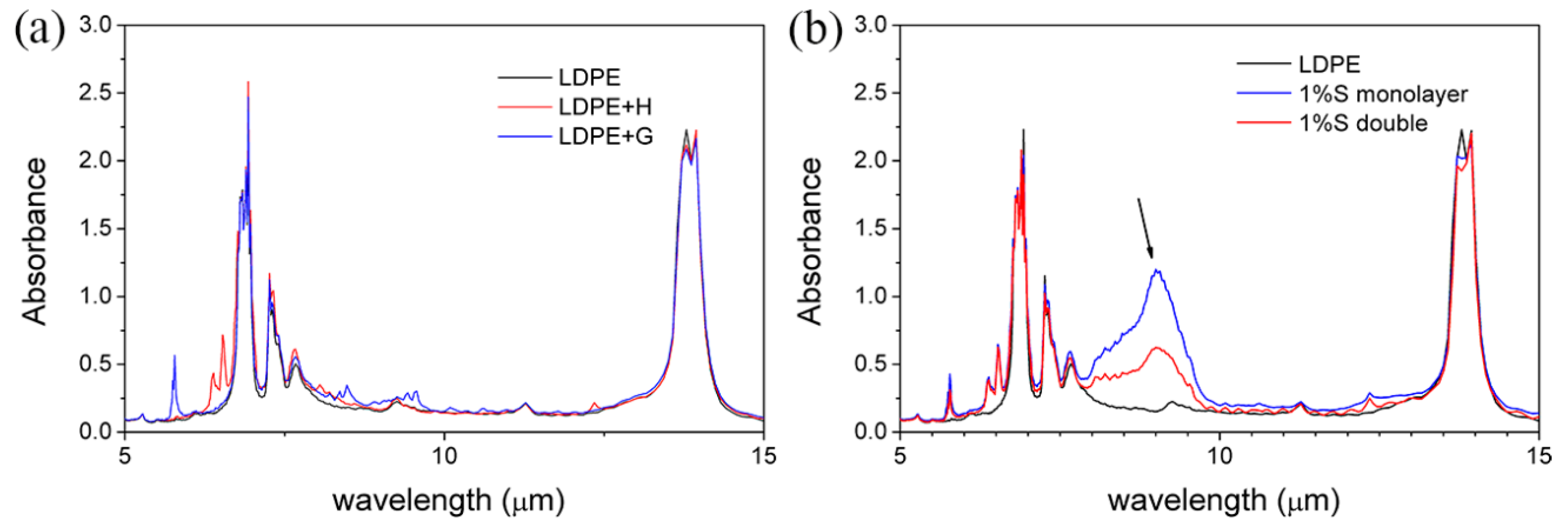
3.4. FT-IR Spectrum of the Samples
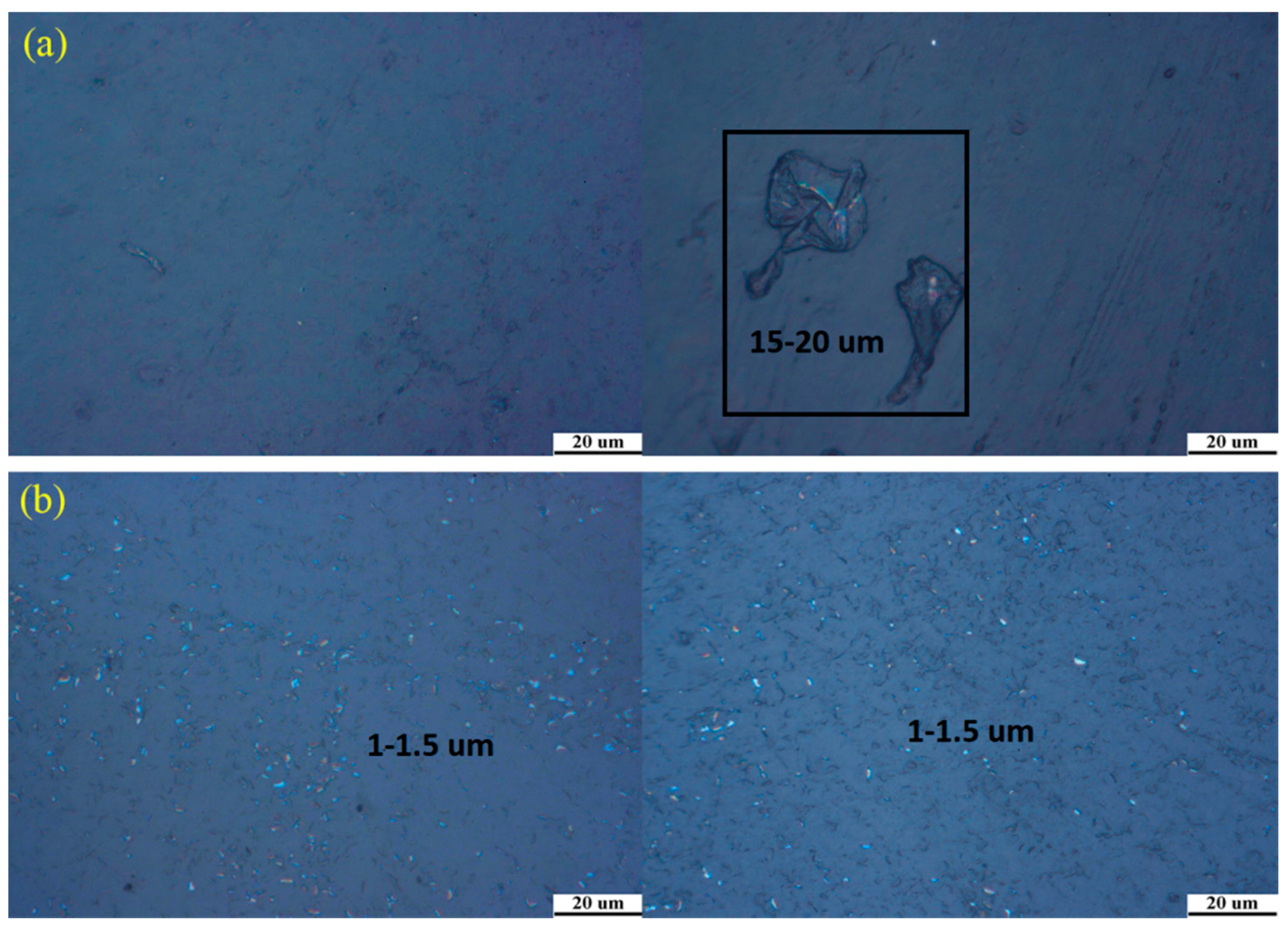
3.5. Inverted Metallurgical Microscope Images of the Samples
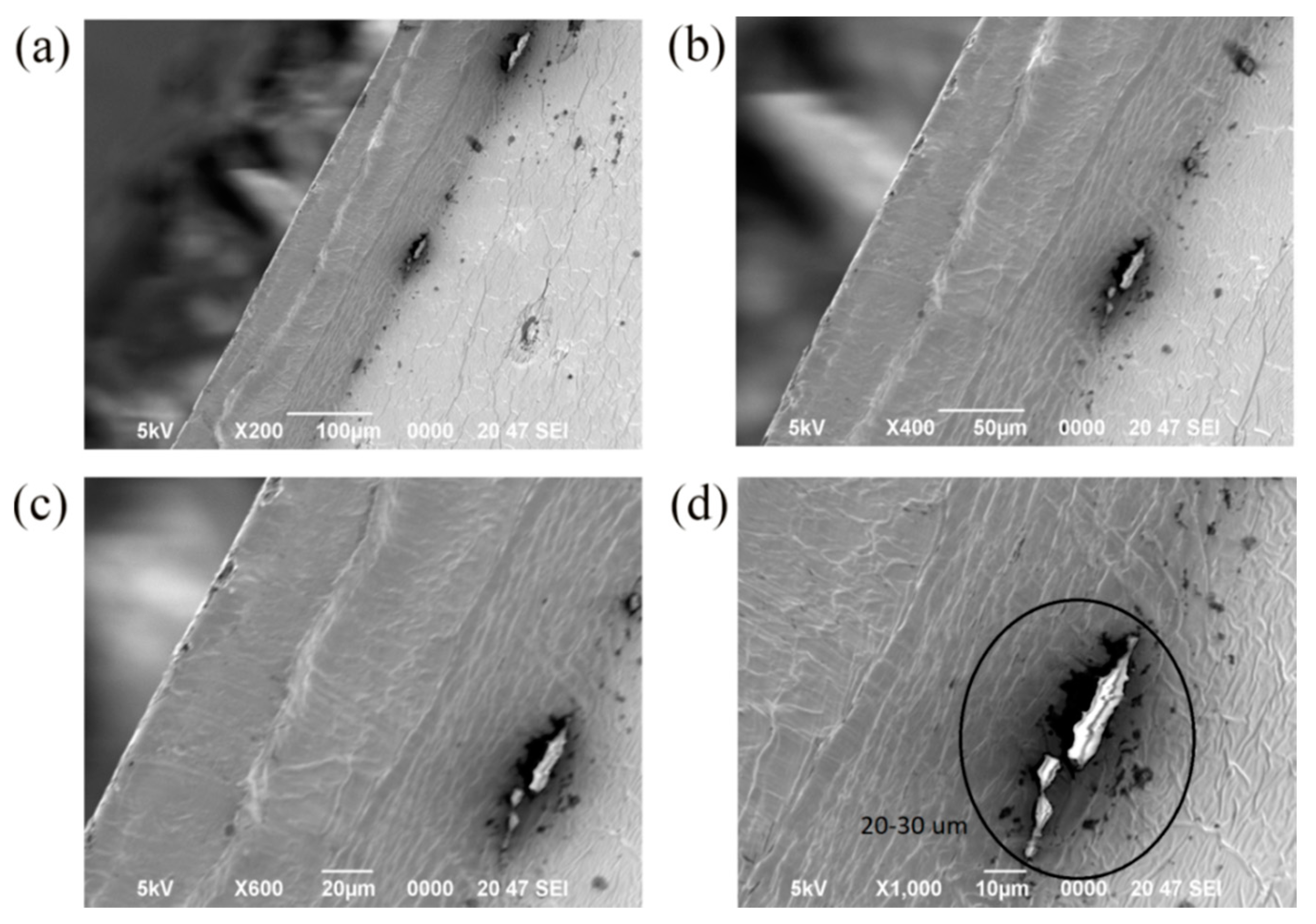
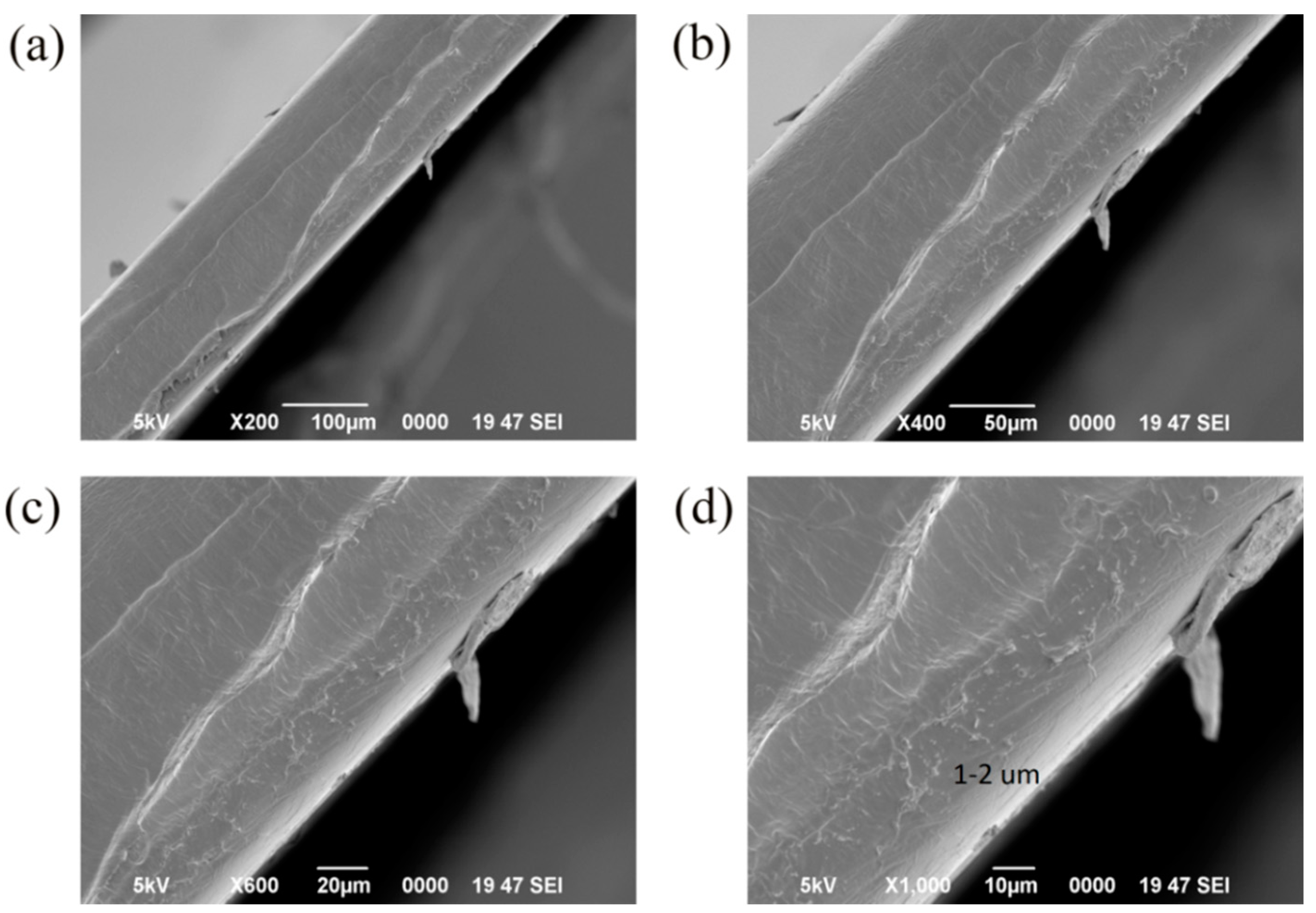
3.6. SEM Images of the Samples
3.7. Cooling Performance
4. Conclusions
- The VIS transmittance and the NIR reflectance of 1% SiO2 monolayer films and 1% SiO2 double layer films were better than that of LDPE.
- The images of inverted metallurgical microscope and SEM show that the silica particles in 1% SiO2 double layer films distributed more uniformly than that of monolayer films. It is suggested that the double layer film is the better choice.
- FT-IR spectra depict that strong absorption occurs in 9 µm for both 1% SiO2 monolayer films and 1% SiO2 double layer films.
- The mechanical properties of the raw LDPE used in this study are half those of the commercial agricultural PE films. However, with the addition of silica, the mechanical properties of the newly developed films increased as much as 60–70%.
- Under 35 °C ambient conditions, the inner temperature of the simulated greenhouse with the 1% SiO2 double layer films was 3 to 5 °C less than that of the simulated greenhouse with the commercial agricultural PE cladding. The temperature on the 1% SiO2 double layer films was reduced 2 to 4 °C in comparison with that of the commercial PE cladding. This is evidence that radiative cooling is helpful in the cooling performance of the greenhouse.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gulrez, S.K.; Abdel-Ghany, A.M.; Al-Helal, I.M.; Al-Zaharani, S.M.; Alsadon, A.A. Evaluation of PE films having NIR-reflective additives for greenhouse applications in arid regions. Adv. Mater. Sci. Eng. 2013, 2013, 575081. [Google Scholar] [CrossRef]
- Dehbi, A.; Youssef, B.; Chappey, C.; Mourad, A.-H.; Picuno, P.; Statuto, D. Multilayers Polyethylene Film for Crop Protection in Harsh Climatic Conditions. Adv. Mater. Sci. Eng. 2017, 2017, 4205862. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.; Al-Helal, I.; Picuno, P.; Shady, M. Modified plastic net-houses as alternative agricultural structures for saving energy and water in hot and sunny regions. Renew. Energy 2016, 93, 332–339. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Liu, L.; Yang, F.; Zhang, Y. Evaluation of cooling property of high density polyethylene (HDPE)/titanium dioxide (TiO2) composites after accelerated ultraviolet (UV) irradiation. Sol. Energy Mater. Sol. Cells 2015, 143, 120–127. [Google Scholar] [CrossRef]
- Haacke, G. Infrared Reflective Coating for Visible Light Transmitting Substrates. U.S. Patent No. 3,998,752, 21 December 1976. [Google Scholar]
- Landsberg, J.J.; White, B.; Thorpe, M. Computer analysis of the efficacy of evaporative cooling for glasshouses in high energy environments. J. Agric. Eng. Res. 1979, 24, 29–39. [Google Scholar] [CrossRef]
- Eriksson, T.; Granqvist, C. Infrared optical properties of silicon oxynitride films: Experimental data and theoretical interpretation. J. Appl. Phys. 1986, 60, 2081–2091. [Google Scholar] [CrossRef]
- Yilmazer, Ü.; Bakar, M.; Kioul, A. Development of thermal films for greenhouse applications using long infrared radiation absorbers. J. Plast. Film Sheeting 1991, 7, 43–55. [Google Scholar] [CrossRef]
- Giacomelli, G.A.; Roberts, W.J. Greenhouse covering systems. HortTechnology 1993, 3, 50–58. [Google Scholar]
- Abdel-Ghany, A.M.; Kozai, T.; Kubota, C.; Taha, I.S. Investigation of the spectral optical properties of the liquid radiation filters for using in the greenhouse applications. J. Agric. Meteorol. 2001, 57, 11–19. [Google Scholar] [CrossRef]
- Hassini, N.; Guenachi, K.; Hamou, A.; Saiter, J.; Marais, S.; Beucher, E. Polyethylene greenhouse cover aged under simulated sub-Saharan climatic conditions. Polym. Degrad. Stab. 2002, 75, 247–254. [Google Scholar] [CrossRef]
- Ye, Y.; King, R., III. Additives for Polyolefin Film Products: An Overview of Chemistry and Effects; Ciba Specialty Chemicals: Tarrytown, NY, USA, 2003. [Google Scholar]
- Caseri, W. Inorganic nanoparticles as optically effective additives for polymers. Chem. Eng. Commun. 2008, 196, 549–572. [Google Scholar] [CrossRef]
- Fernando, R.H. Nanocomposite and nanostructured coatings: Recent advancements. Nanotechnol. Appl. Coat. 2009, 2–21. [Google Scholar] [CrossRef]
- Schettini, E.; Vox, G. Greenhouse plastic films capable of modifying the spectral distribution of solar radiation. J. Agric. Eng. 2010, 41, 19–24. [Google Scholar] [CrossRef]
- Smith, G.B. Green nanotechnology. In Nanostructured Thin Films IV; International Society for Optics and Photonics: Bellingham, WA, USA, 2011; Volume 8104, p. 810402. [Google Scholar]
- Espejo, C.; Arribas, A.; Monzo, F.; Diez, P.P. Nanocomposite films with enhanced radiometric properties for greenhouse covering applications. J. Plast. Film Sheeting 2012, 28, 336–352. [Google Scholar]
- Fang, V.; Kennedy, J.V.; Futter, J.; Manning, J. A Review of Near Infrared Reflectance Properties of Metal Oxide Nanostructures; GNS Science: Lower Hutt, New Zealand, 2013. [Google Scholar]
- Čekon, M.; Kalousek, M.; Hraška, J.; Ingeli, R. Spectral optical properties and thermodynamic performance of reflective coatings in a mild climate zone. Energy Build. 2014, 77, 343–354. [Google Scholar]
- Wang, S.; Zhang, J. Effect of titanium dioxide (TiO2) on largely improving solar reflectance and cooling property of high density polyethylene (HDPE) by influencing its crystallization behavior. J. Alloys Compd. 2014, 617, 163–169. [Google Scholar] [CrossRef]
- Gentle, A.R.; Smith, G.B. A subambient open roof surface under the Mid-Summer sun. Adv. Sci. 2015, 2, 1500119. [Google Scholar] [CrossRef] [PubMed]
- Tawiah, B.; Narh, C.; Li, M.; Zhang, L.; Fu, S. Polymer-encapsulated colorful Al pigments with high NIR and UV reflectance and their application in textiles. Ind. Eng. Chem. Res. 2015, 54, 11858–11865. [Google Scholar] [CrossRef]
- Nawalany, G.; Bieda, W.; Radoń, J.; Herbut, P. Experimental study on development of thermal conditions in ground beneath a greenhouse. Energy Build. 2014, 69, 103–111. [Google Scholar] [CrossRef]
- Nawalany, G.; Radon, J.; Bieda, W.; Sokolowski, P. Influence of Selected Factors on Heat Exchange with the Ground in a Greenhouse. Trans. ASABE 2017, 60, 479–487. [Google Scholar]
- Rephaeli, E.; Raman, A.; Fan, S. Ultrabroadband photonic structures to achieve high-performance daytime radiative cooling. Nano Lett. 2013, 13, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.L.; Nagendra, S.S.; Maiya, M. Passive alternatives to mechanical air conditioning of building: A review. Build. Environ. 2013, 66, 54–64. [Google Scholar] [CrossRef]
- Zhu, L.; Raman, A.; Fan, S. Color-preserving daytime radiative cooling. Appl. Phys. Lett. 2013, 103, 223902. [Google Scholar] [CrossRef]
- Raman, A.P.; Anoma, M.A.; Zhu, L.; Rephaeli, E.; Fan, S. Passive radiative cooling below ambient air temperature under direct sunlight. Nature 2014, 515, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Jia, B.; Gu, M. A metamaterial emitter for highly efficient radiative cooling. Adv. Opt. Mater. 2015, 3, 1047–1051. [Google Scholar] [CrossRef]
- Zhu, L.; Raman, A.P.; Fan, S. Radiative cooling of solar absorbers using a visibly transparent photonic crystal thermal blackbody. Proc. Natl. Acad. Sci. USA 2015, 112, 12282–12287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.; Gu, M. Radiative cooling: Principles, progress, and potentials. Adv. Sci. 2016, 3, 1500360. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.-L.; Jurado, Z.; Chen, Z.; Fan, S.; Minnich, A.J. Daytime radiative cooling using near-black infrared emitters. ACS Photonics 2017, 4, 626–630. [Google Scholar] [CrossRef]
- Smith, G.; Gentle, A. Radiative cooling: Energy savings from the sky. Nat. Energy 2017, 2, 17142. [Google Scholar] [CrossRef]
- Zhai, Y.; Ma, Y.; David, S.N.; Zhao, D.; Lou, R.; Tan, G.; Yang, R.; Yin, X. Scalable-manufactured randomized glass-polymer hybrid metamaterial for daytime radiative cooling. Science 2017, 355, 1062–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granqvist, C.; Hjortsberg, A. Radiative cooling to low temperatures: General considerations and application to selectively emitting SiO films. J. Appl. Phys. 1981, 52, 4205–4220. [Google Scholar] [CrossRef]




| Samples | Tensile Strength (MPa) | Maximum Elongation (%) |
|---|---|---|
| PE | 21.32 | 1506.64 |
| LDPE | 10.39 | 997.43 |
| 1%S monolayer | 16.89 | 1244.02 |
| 1%S double | 16.62 | 1259.80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-H.; Ay, C.; Kan, J.-C.; Lee, M.-T. The Effect of Radiative Cooling on Reducing the Temperature of Greenhouses. Materials 2018, 11, 1166. https://doi.org/10.3390/ma11071166
Liu C-H, Ay C, Kan J-C, Lee M-T. The Effect of Radiative Cooling on Reducing the Temperature of Greenhouses. Materials. 2018; 11(7):1166. https://doi.org/10.3390/ma11071166
Chicago/Turabian StyleLiu, Chia-Hsin, Chyung Ay, Jo-Chuan Kan, and Maw-Tien Lee. 2018. "The Effect of Radiative Cooling on Reducing the Temperature of Greenhouses" Materials 11, no. 7: 1166. https://doi.org/10.3390/ma11071166




