Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges
Abstract
:1. Introduction
2. Tendon
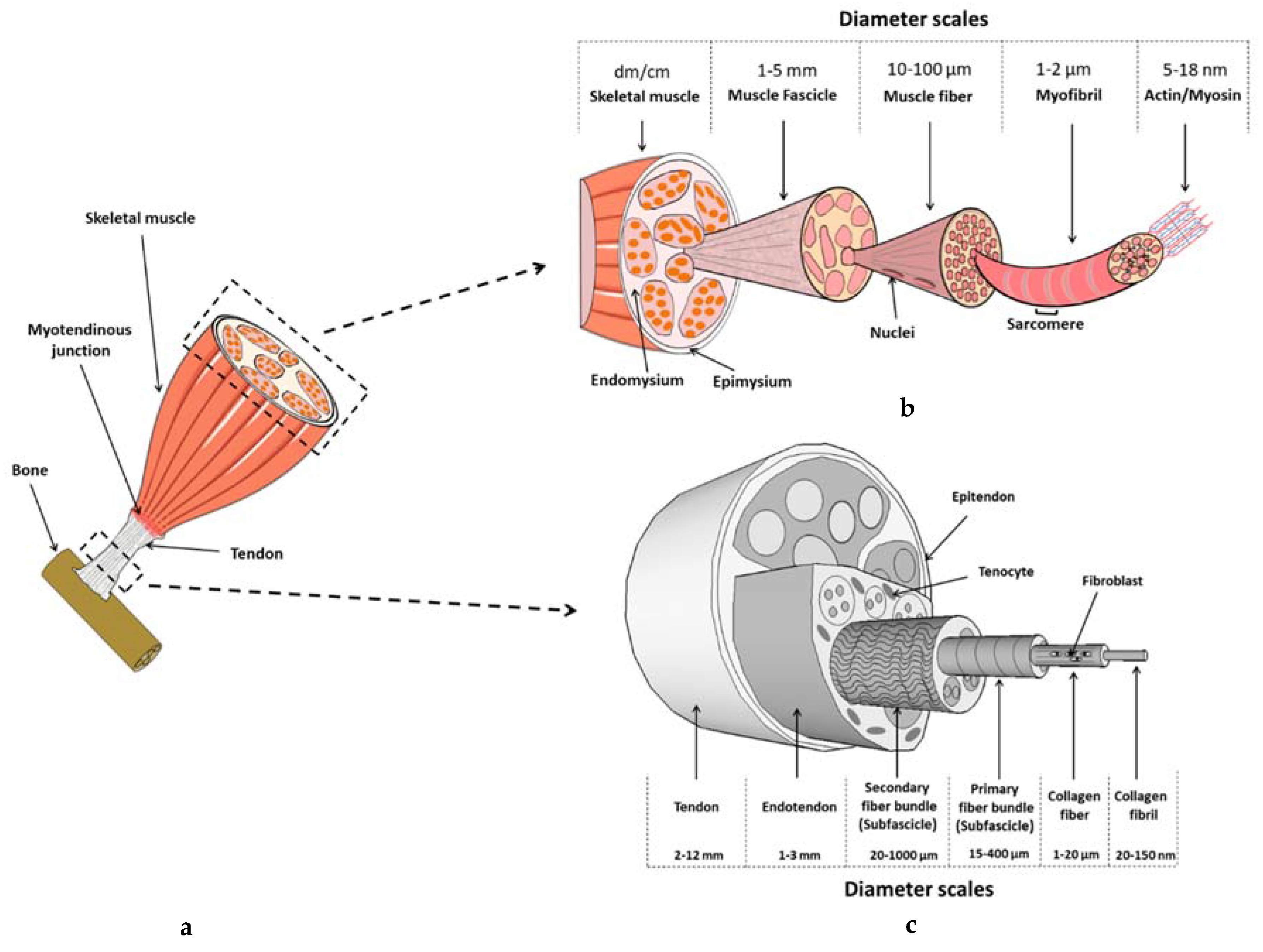
2.1. Tendon Composition and Structure
2.2. Tendon Injuries and Healing
2.3. Tendon Tissue Engineering
2.3.1. Cells
2.3.2. Modulation of the Environment
Biochemical Stimulation
Mechanical Stimulation
2.3.3. Materials
Biological Origin
Synthetic Material
Hybrid Material
2.4. From Biohybrid Tendon Design to Reconstructed Tissue’s Response
2.4.1. Macroporous Sponge
2.4.2. Collagen Extruded Fibers
2.4.3. Electrochemically-Aligned Collagen (ELAC) Fibers
2.4.4. Electrospun Scaffolds
Scaffold Structure and Mechanical Properties
Biological Response
Effect of Mechanical Stimulation
2.4.5. Knitted Scaffolds
3. Skeletal Muscle
3.1. Skeletal Muscle’s Composition and Structure
3.2. Muscle Injuries and Healing
3.2.1. Grafts
3.2.2. Cell Therapy
3.3. Skeletal Muscle Tissue Engineering
3.3.1. Cells
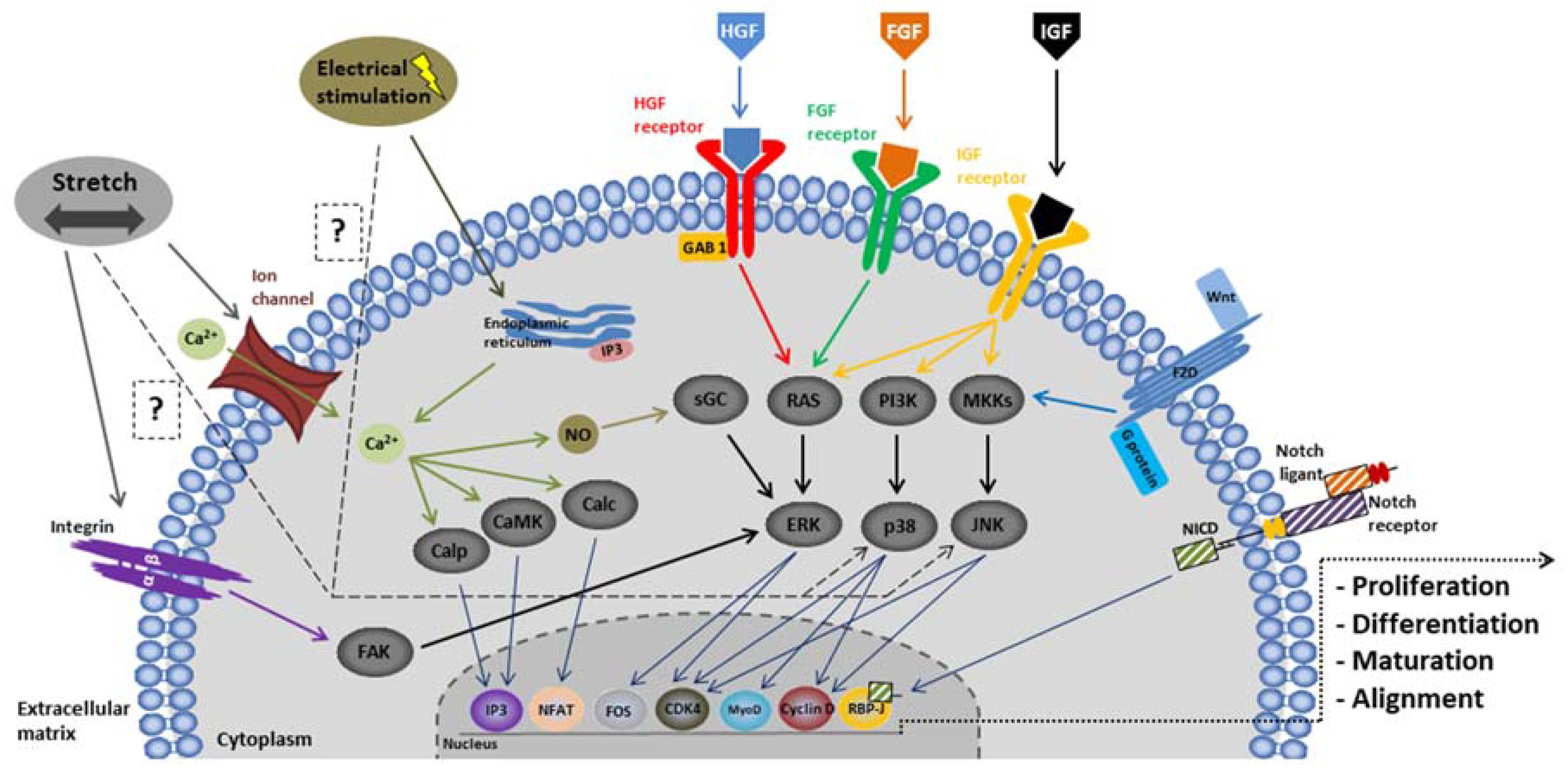
3.3.2. Modulation of the Environment
3.3.3. Materials
Biological Origin
Synthetic Materials
Hybrid Materials
3.4. From Biohybrid Muscle Design to Reconstructed Tissue’s Response
3.4.1. Films and Hydrogels
Effect of Scaffold Structure and Mechanical Properties on Biological Response
Effect of Electrical Stimulation on Biological Response
Effect of Mechanical Stimulation on Biological Response
3.4.2. Electrospun Scaffolds
Effect of Scaffold Structure and Mechanical Properties on Biological Response
Effect of Electrical Stimulation on Biological Response
Effect of Mechanical Stimulation on Biological Response
4. Reconstruction of the Myotendinous Junction
5. Conclusions and Perspectives—New Challenges
Funding
Conflicts of Interest
Abbreviations
| EDC | (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride |
| ASC | Adipose stem cell |
| ATMP | Advanced therapy medicinal products |
| bFGF | Basic fibroblast growth factor |
| BMSC | Bone marrow stem cell |
| BMP | Bone morphogenetic protein |
| JNK | c-Jun NH2-terminal kinases |
| camk | calmodulin-dependent protein kinases |
| calc | Calcineurin |
| Calp | Calpain |
| COL | Collagen |
| CTGF | Connective tissue growth factor |
| CTS | Cyclic tensile strain |
| DF | Dermal fibroblast |
| EM | Elastic modulus |
| ELAC | Electrochemically-aligned collagen |
| EDGE | Ethylene-glycol-diglycidyl-ether |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinases |
| Eya | Eye absent homolog |
| FAP | Fibro-adipogenic progenitors |
| FGF | Fibroblast growth factor |
| FAK | Focal adhesion Kinase |
| FIB | Focused ion beam |
| GelMA | Gelatin methacryloyl |
| GPa | GigaPascal |
| Tg | Glass transition |
| GO | Graphene oxydative |
| GDF | Growth and differentiation factor |
| HGH | Hepatocyte growth factor |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| LS | Linear stiffness |
| MKKs | McKusick-Kaufman syndrome |
| MSC | Mesenchymal stem cell |
| p38 | Mitogen-activated protein kinases |
| MWCNT | Multiwalled carbon nanotubes |
| MPCs | Muscle progenitor cells |
| MRF | Myogenic regulatory factor |
| MHC | Myosin heavy chain |
| MTJ | Myotendinous junction |
| NHS | N-Hydroxysuccinimide |
| OA | Oxidized alginate |
| PI3K | Phosphatidylinositol-3-kinase |
| PDGF | Platelet derived growth factor |
| PEEUR | Poly (esterurethane urea) |
| PCL | Poly (ε-caprolactone) |
| PEG | Poly ethylene glycol |
| PLGA | poly lactic-co-glycolic acid |
| PLLA | Poly-l-lactic acid |
| PAA | Poly(acrylic acid) |
| PCL | Poly(caprolactone) |
| Poly(l-lactide-co-d,l-lactide) | |
| PLA | Poly(l/d)lactide |
| PVA | Poly(vinyl alcohol) |
| PLGA | Poly[(lactic acid)-co-(glycolic acid)] |
| PANi | Polyaniline |
| PDMS | Polydimethylsiloxane |
| PGA | Poly(glycolic acid) |
| PU | Polyurethane |
| PVDF | Polyvinylidene fluoride |
| PG | Proteoglycan |
| Scs | Satellite cells |
| SEM | Scanning electron microscopy |
| SCX | Scleraxis |
| SDS | Sodium dodecyl sulfate |
| sGC | Soluble guanylyl cyclase |
| TNC | Tenascin-C |
| TDSC | Tendon derived stem cell |
| TSPC | Tendon stem/progenitor cells |
| TNMD | Tenomodulin |
| TE | Tissue engineering |
| TGF-β | Transforming growth factor-β |
| TEM | Transmission electron microscopy |
| TnBP | Tri(n-butyl)phosphate |
| UTS | Ultimate tensile strength |
| VEGF | Vascular endothelial growth factor |
| VMLs | Volumetric muscle losses |
| YM | Young modulus (E) |
References
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions-A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Font Tellado, S.; Balmayor, E.R.; Van Griensven, M. Strategies to engineer tendon/ligament-to-bone interface: Biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 2015, 94, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, D.T.; Garrett, W.E. Function and biomechanics of tendons. Scand. J. Med. Sci. Sports 2007, 7, 62–66. [Google Scholar] [CrossRef]
- Hart, D.A.; Kydd, A.; Reno, C. Gender and pregnancy affect neuropeptide responses of the rabbit Achilles tendon. Clin. Orthop. 1999, 365, 237–246. [Google Scholar] [CrossRef]
- Birch, H.L. Tendon matrix composition and turnover in relation to functional requirements. Int. J. Exp. Pathol. 1997, 88, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Freeman, J.W.; Seehra, G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Rigby, B.J.; Hirai, N.; Spikes, J.D.; Eyring, H. The Mechanical Properties of Rat Tail Tendon. J. Gen. Physiol. 1959, 43, 265–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Derwin, K.A.; Soslowsky, L.J.; Kimura, J.H.; Plaas, A.H. Proteoglycans and glycosaminoglycan fine structure in the mouse tail tendon fascicle. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2000, 19, 269–277. [Google Scholar] [CrossRef]
- Kuc, I.M.; Scott, P.G. Increased Diameters of Collagen Fibrils Precipitated in vitro in the Presence of Decorin from Various Connective Tissues. Connect. Tissue Res. 1997, 36, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, E.; Witsch-Prehm, P.; Harrach, B.; .Robenek, H.; Rauterberg, J.; Kresse, H. Interaction of biglycan with type I collagen. J. Biol. Chem. 1995, 270, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Ezura, Y.; Chakravarti, S.; Oldberg, A.; Chervoneva, I.; Birk, D.E. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000, 151, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Mccormick, R.J. Extracellular modifications to muscle collagen: Implications for meat quality. Poult. Sci. 1999, 78, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Vogel, K.G.; Koob, T.J. Structural specialization in tendons under compression. Int. Rev. Cytol. 1989, 115, 267–293. [Google Scholar] [PubMed]
- Martin, J.A.; Mehr, D.; Pardubsky, P.D.; Buckwalter, J.A. The role of tenascin-C in adaptation of tendons to compressive loading. Biorheology 2003, 40, 321–329. [Google Scholar] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. J. Bone Jt. Surg. Am. 1995, 87, 187–202. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.B.; Burr, D.B.; Sharkey, N.A.; Fyhrie, D.P. Mechanical Properties of Ligament and Tendon. In Skeletal Tissue Mechanics; Springer: New York, NY, USA, 2015; pp. 175–225. [Google Scholar]
- Silver, F.H.; Christiansen, D.L.; Snowhill, P.B.; Chen, Y. Role of storage on changes in the mechanical properties of tendon and self-assembled collagen fibers. Connect. Tissue Res. 2000, 41, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Robi, K.; Jakob, N.; Matevz, K.; Matjaz, V. The Physiology of Sports Injuries and Repair Processes. Curr. Issues Sports Exerc. Med. 2013. [Google Scholar] [CrossRef] [Green Version]
- Maganaris, C.N. Tensile properties of in vivo human tendinous tissue. J. Biomech. 2002, 35, 1019–1027. [Google Scholar] [CrossRef]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. Mechanical properties of the patellar tendon in adults and children. J. Biomech. 2010, 43, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon Healing: Repair and Regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Lamberti, A.; Maffulli, N.; Denaro, V. Tendon augmentation grafts: A systematic review. Br. Med. Bull. 2010, 94, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.L.; Hieu Nguyen, T.M.; Gao, L.; Ouyang, H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Bottagisio, M.; Lopa, S.; Granata, V.; Talò, G.; Bazzocchi, C.; Moretti, M.; Lovati, A.B. Different combinations of growth factors for the tenogenic differentiation of bone marrow mesenchymal stem cells in monolayer culture and in fibrin-based three-dimensional constructs. Differ. Res. Biol. Divers. 2017, 95, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.-W.; Sun, Y.-L.; Thoreson, A.R.; Steinmann, S.P.; Amadio, P.C.; An, K.-N.; Zhao, C. Effect of mechanical stimulation on bone marrow stromal cell-seeded tendon slice constructs: A potential engineered tendon patch for rotator cuff repair. Biomaterials 2015, 51, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younesi, M.; Islam, A.; Kishore, V.; Anderson, J.M.; Akkus, O. Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles. Adv. Funct. Mater. 2014, 24, 5762–5770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosworth, L.A.; Rathbone, S.R.; Bradley, R.S.; Cartmell, S.H. Dynamic loading of electrospun yarns guides mesenchymal stem cells towards a tendon lineage. J. Mech. Behav. Biomed. Mater. 2014, 39, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Ouyang, H.; Goh, J.C.-H.; Tay, T.E.; Toh, S.L. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006, 12, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, H.; Wang, Y.; Toh, S.L.; Goh, J.C.H. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials 2008, 29, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Juncosa-Melvin, N.; Shearn, J.T.; Boivin, G.P.; Gooch, C.; Galloway, M.T.; West, J.R.; Nirmalanandhan, V.S.; Bradica, G.; Butler, D.L. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006, 12, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Nirmalanandhan, V.S.; Rao, M.; Shearn, J.T.; Juncosa-Melvin, N.; Gooch, C.; Butler, D.L. Effect of scaffold material, construct length and mechanical stimulation on the in vitro stiffness of the engineered tendon construct. J. Biomech. 2008, 41, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Hu, X.; Zhang, X.; Zhu, J.; Zhang, J.; Fu, X.; Duan, X.; Ao, Y.; Zhou, C. Different tenogenic differentiation capacities of different mesenchymal stem cells in the presence of BMP-12. J. Transl. Med. 2015, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug. Deliv. Rev. 2017. [CrossRef] [PubMed]
- Reznikoff, C.A.; Brankow, D.W.; Heidelberger, C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973, 33, 3231–3238. [Google Scholar] [PubMed]
- Guerquin, M.-J.; Charvet, B.; Nourissat, G.; Havis, E.; Ronsin, O.; Bonnin, M.-A.; Ruggiu, M.; Olivera-Martinez, I.; Robert, N.; Lu, Y.; et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J. Clin. Investig. 2015, 123, 3564–3576. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, R.D.; Kluge, J.A.; Thayer, P.S.; Guelcher, S.A.; Dahlgren, L.A.; Kaplan, D.L.; Goldstein, A.S. Static and cyclic mechanical loading of mesenchymal stem cells on elastomeric, electrospun polyurethane meshes. J. Biomech. Eng. 2015, 137, 071010. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, R.D.; Dahlgren, L.A.; Goldstein, A.S. Electrospun fibre diameter, not alignment, affects mesenchymal stem cell differentiation into the tendon/ligament lineage. J. Tissue Eng. Regen. Med. 2014, 8, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Baudequin, T.; Gaut, L.; Mueller, M.; Huepkes, A.; Glasmacher, B.; Duprez, D.; Bedoui, F.; Legallais, C. The Osteogenic and Tenogenic Differentiation Potential of C3H10T1/2 (Mesenchymal Stem Cell Model) Cultured on PCL/PLA Electrospun Scaffolds in the Absence of Specific Differentiation Medium. Materils 2017, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.-F.; Lui, P.P.Y.; Li, G.; Fu, S.C.; Lee, Y.W.; Chan, K.M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 2010, 16, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.Y.; Wong, O.T.; Lee, Y.W. Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytotherapy 2016, 18, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Thaker, H.; Sharma, A.K. Engaging stem cells for customized tendon regeneration. Stem Cells Int. 2012, 2012, 309187. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Harley, B.A.C. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enea, D.; Henson, F.; Kew, S.; Wardale, J.; Getgood, A.; Brooks, R.; Rushton, N. Extruded collagen fibres for tissue engineering applications: Effect of crosslinking method on mechanical and biological properties. J. Mater. Sci. Mater. Med. 2011, 22, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Enea, D.; Gwynne, J.; Kew, S.; Arumugam, M.; Shepherd, J.; Brooks, R.; Ghose, S.; Best, S.; Cameron, R.; Rushton, N. Collagen fibre implant for tendon and ligament biological augmentation. In vivo study in an ovine model. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2013, 21, 1783–1793. [Google Scholar] [CrossRef]
- Surrao, D.C.; Fan, J.C.Y.; Waldman, S.D.; Amsden, B.G. A crimp-like microarchitecture improves tissue production in fibrous ligament scaffolds in response to mechanical stimuli. Acta Biomater. 2012, 8, 3704–3713. [Google Scholar] [CrossRef] [PubMed]
- Grier, W.K.; Iyoha, E.M.; Harley, B.A.C. The influence of pore size and stiffness on tenocyte bioactivity and transcriptomic stability in collagen-GAG scaffolds. J. Mech. Behav. Biomed. Mater. 2017, 65, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M. Stem Cell Applications in Tendon Disorders: A Clinical Perspective. Stem Cells Int. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, L.; Yan, X.; Cui, J.; Liu, W.; Rao, Y.; Sun, M.; Wei, Q.; Chen, F. Directing the Differentiation ofParthenogenetic Stem Cells into Tenocytes for Tissue-Engineered Tendon Regeneration. Stem Cells Transl. Med. 2017, 6, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Engineering tendon and ligament tissues: Present developments towards successful clinical products. J. Tissue Eng. Regen. Med. 2013, 7, 673–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Liu, H.; Stile, F.; Lei, M.-P.; Pang, Y.; Oswald, T.M.; Beck, J.; Dorsett-Martin, W.; Lineaweaver, W.C. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast. Reconstr. Surg. 2003, 112, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, S.O.; Lundborg, G.; Lohmander, L.S. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1991, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Evrova, O.; Buschmann, J. In vitro and in vivo effects of PDGF-BB delivery strategies on tendon healing: A review. Eur. Cells Mater. 2017, 34, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Najafbeygi, A.; Fatemi, M.J.; Lebaschi, A.H.; Mousavi, S.J.; Husseini, S.A.; Niazi, M. Effect of Basic Fibroblast Growth Factor on Achilles Tendon Healing in Rabbit. World J. Plast. Surg. 2017, 6, 26–32. [Google Scholar] [PubMed]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. 2008, 33, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Skutek, M.; van Griensven, M.; Zeichen, J.; Brauer, N.; Bosch, U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur. J. Appl. Physiol. 2001, 86, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.; Langberg, H.; Olesen, J.L.; Kjaer, M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J. Appl. Physiol. 2003, 95, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Kjaer, M. In vivo investigation of tendon responses to mechanical loading. J. Musculoskelet. Neuronal Interact. 2011, 11, 115–123. [Google Scholar] [PubMed]
- Loiselle, A.E.; Yukata, K.; Geary, M.B.; Kondabolu, S.; Shi, S.; Jonason, J.H.; Awad, H.A.; O’Keefe, R.J. Development of antisense oligonucleotide (ASO) technology against Tgf-β signaling to prevent scarring during flexor tendon repair. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 859–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juneja, S.C.; Schwarz, E.M.; O’Keefe, R.J.; Awad, H.A. Cellular and molecular factors in flexor tendon repair and adhesions: A histological and gene expression analysis. Connect. Tissue Res. 2013, 54, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Tu, Y.; Burns, M.; Silva, M.J.; Manske, P. BMP-12 gene transfer augmentation of lacerated tendon repair. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2001, 19, 1199–1202. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.P.; Fu, S.C.; Qin, L.; Rolf, C.; Chan, K.M. Supplementation-time Dependence of Growth Factors in Promoting Tendon Healing. Clin. Orthop. 2006, 448, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Musson, D.S.; Tay, M.L.; Chhana, A.; Pool, B.; Coleman, B.; Naot, D.; Cornish, J. Lactoferrin and parathyroid hormone are not harmful to primary tenocytesin vitro, but PDGF may be. Muscles Ligaments Tendons J. 2017, 7, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Schmidt, T.; Uckert, M.; Scheffler, S.; Kalus, U.; Rojewski, M.; Schrezenmeier, H.; Pruss, A.; Wildemann, B. Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Martin, J.I.; Maffulli, N. Advances with platelet rich plasma therapies for tendon regeneration. Expert Opin. Biol. Ther. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Berardi, A.C.; Muscari, C.; Campardelli, R.; Bonafè, F.; Guarnieri, C.; Reverchon, E.; Giordano, E.; Maffulli, N.; Della Porta, G. An Engineered Multiphase Three-Dimensional Microenvironment to Ensure the Controlled Delivery of Cyclic Strain and Human Growth Differentiation Factor 5 for the Tenogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells. Tissue Eng. Part A 2017, 23, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Vuornos, K.; Björninen, M.; Talvitie, E.; Paakinaho, K.; Kellomäki, M.; Huhtala, H.; Miettinen, S.; Seppänen-Kaijansinkko, R.; Haimi, S. Human Adipose Stem Cells Differentiated on Braided Polylactide Scaffolds Is a Potential Approach for Tendon Tissue Engineering. Tissue Eng. Part A 2016, 22, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Bhole, A.P.; Flynn, B.P.; Liles, M.; Saeidi, N.; Dimarzio, C.A.; Ruberti, J.W. Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: Implications for load-bearing matrix growth and stability. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 3339–3362. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, Y.; Grood, E.S.; Sakurai, A.; Herman, J.H. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1996, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Flynn, B.P.; Bhole, A.P.; Saeidi, N.; Liles, M.; Dimarzio, C.A.; Ruberti, J.W. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS ONE 2010, 5, e12337. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Zeichen, J.; van Griensven, M.; Bosch, U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am. J. Sports Med. 2000, 28, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Crawford, R.C.; Wang, J.H.-C. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J. Biomech. 2004, 37, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Gaut, L.; Duprez, D. Tendon development and diseases. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 5–23. [Google Scholar] [CrossRef]
- Doroski, D.M.; Levenston, M.E.; Temenoff, J.S. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng. Part A 2010, 16, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Manske, P.R.; Pruitt, D.L.; Larson, B.J. Effect of cyclic tension on lacerated flexor tendons in vitro. J. Hand Surg. 1995, 20, 467–473. [Google Scholar] [CrossRef]
- Govoni, M.; Muscari, C.; Lovecchio, J.; Guarnieri, C.; Giordano, E. Mechanical Actuation Systems for the Phenotype Commitment of Stem Cell-Based Tendon and Ligament Tissue Substitutes. Stem Cell Rev. 2016, 12, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Kinneberg, K.R.C.; Nirmalanandhan, V.S.; Juncosa-Melvin, N.; Powell, H.M.; Boyce, S.T.; Shearn, J.T.; Butler, D.L. Chondroitin-6-sulfate incorporation and mechanical stimulation increase MSC-collagen sponge construct stiffness. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Lamberti, A.; Petrillo, S.; Maffulli, N.; Denaro, V. Scaffolds in Tendon Tissue Engineering. Stem Cells Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.A.; Dürselen, L.; Heisterbach, P.; Evans, C.; Majewski, M. Effect of a Simple Collagen Type I Sponge for Achilles Tendon Repair in a Rat Model. Am. J. Sports Med. 2016, 44, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Lay, A.N.; Dickerson, D.A.; Nauman, E.A.; Livesay, G.A.; Dee, K.C. Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 2003, 24, 3805–3813. [Google Scholar] [CrossRef]
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 2008, 29, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Minoura, N.; Aiba, S.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 1995, 29, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Marturano, J.E.; Tuan, R.S. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Li, Y.; Chen, G.; He, J.; Han, Y.; Wang, X.; Kaplan, D.L. Silk-based biomaterials in biomedical textiles and fiber-based implants. Adv. Healthc. Mater. 2015, 4, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.; Naghashzargar, E.; Semnani, D. Silk Fibroin Nano-Coated Textured Silk Yarn by Electrospinning Method for Tendon and Ligament Scaffold Application. Nano Hybrids 2014, 7, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Yin, Z.; Shen, W.L.; Chen, X.; Heng, B.C.; Zou, X.H.; Ouyang, H.W. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 2010, 31, 9438–9451. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ran, J.; Chen, W.; Hu, Y.; Zhu, T.; Chen, X.; Yin, Z.; Heng, B.C.; Feng, G.; Le, H.; Tang, C.; Huang, J.; Chen, Y.; Zhou, Y.; Dominique, P.; Shen, W.; Ouyang, H.-W. Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair. Acta Biomater. 2017, 51, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, Y.; Liu, W.; Zhang, P.; Jiang, J.; Chen, S. Electrospun silk fibroin mat enhances tendon-bone healing in a rabbit extra-articular model. Biotechnol. Lett. 2016, 38, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Malcarney, H.L.; Bonar, F.; Murrell, G.A.C. Early Inflammatory Reaction after Rotator Cuff Repair with a Porcine Small Intestine Submucosal Implant: A Report of 4 Cases. Am. J. Sports Med. 2005, 33, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.K.; Cox, S.C. Biomaterial scaffolds for tissue engineering. Front. Biosci. Elite Ed. 2013, 5, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.J.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today Kidlington Engl. 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Moffat, K.L.; Kwei, A.S.-P.; Spalazzi, J.P.; Doty, S.B.; Levine, W.N.; Lu, H.H. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. Part A 2009, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Erisken, C.; Zhang, X.; Moffat, K.L.; Levine, W.N.; Lu, H.H. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng. Part A 2013, 19, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, J.; Wang, H.; Li, H.; Di, N.; Song, L.; Li, S.; Li, D.; Xiang, Y.; Liu, W.; Mo, X.; Zhou, Q. Fabrication of electrospun poly(L-lactide-co-ε-caprolactone)/collagen nanoyarn network as a novel, three-dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Eng. Part C Methods 2013, 19, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Horan, R.L.; Collette, A.L.; Lee, C.; Antle, K.; Chen, J.; Altman, G.H. Yarn design for functional tissue engineering. J. Biomech. 2006, 39, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, C.; Hu, X.; Sun, H.; You, C.; Gao, C.; Haiyang, Y. Applications of knitted mesh fabrication techniques to scaffolds for tissue engineering and regenerative medicine. J. Mech. Behav. Biomed. Mater. 2011, 4, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, Y.-J.; Moon, S.W.; Lee, B.H.; Shim, J.-H.; Cho, D.-W.; Wang, J.H. Three-Dimensional Bio-Printed Scaffold Sleeves With Mesenchymal Stem Cells for Enhancement of Tendon-to-Bone Healing in Anterior Cruciate Ligament Reconstruction Using Soft-Tissue Tendon Graft. Arthroscopy 2018, 34, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Mkhabela, V.J.; Ray, S.S. Poly(epsilon-caprolactone) nanocomposite scaffolds for tissue engineering: A brief overview. J. Nanosci. Nanotechnol. 2014, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Yang, J.-C.; Gupta, A.S.; Lopina, S.T. Synthesis and characterization of L-tyrosine based polyurethanes for biomaterial applications. J. Biomed. Mater. Res. A 2009, 90, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Juncosa-Melvin, N.; Boivin, G.P.; Galloway, M.T.; Shearn, J.T.; Gooch, C.; Awad, H. Functionaltissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.P.; Christiansen, D.L.; Hahn, R.A.; Shieh, S.J.; Goldstein, J.D.; Silver, F.H. Mechanical properties of collagen fibres: A comparison of reconstituted and rat tail tendon fibres. Biomaterials 1989, 10, 38–42. [Google Scholar] [CrossRef]
- Kew, S.J.; Gwynne, J.H.; Enea, D.; Abu-Rub, M.; Pandit, A.; Zeugolis, D.; Brooks, R.A.; Rushton, N.; Best, S.M.; Cameron, R.E. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011, 7, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Trelstad, R.L. Type I collagen in solution. Structure and properties of fibril fragments. J. Biol. Chem. 1980, 255, 9427–9433. [Google Scholar] [PubMed]
- Zeugolis, D.I.; Paul, G.R.; Attenburrow, G. Cross-linking of extruded collagen fibers—A biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. A 2009, 89, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Shepherd, J.H.; Shepherd, D.V.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M.; Brooks, R.A.; Wardale, J.; Rushton, N. Effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide concentrations on the mechanical and biological characteristics of cross-linked collagen fibres for tendon repair. Regen. Biomater. 2015, 2, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurkan, U.A.; Cheng, X.; Kishore, V.; Uquillas, J.A.; Akkus, O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J. Biomed. Mater. Res. A 2010, 94, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Paderi, J.E.; Akkus, A.; Smith, K.M.; Balachandran, D.; Beaudoin, S.; Panitch, A.; Akkus, O. Incorporation of a decorin biomimetic enhances the mechanical properties of electrochemically aligned collagen threads. Acta Biomater. 2011, 7, 2428–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uquillas, J.A.; Kishore, V.; Akkus, O. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads. Biomed. Mater. 2011, 6, 035008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uquillas, J.A.; Akkus, O. Modeling the electromobility of type-I collagen molecules in the electrochemical fabrication of dense and aligned tissue constructs. Ann. Biomed. Eng. 2012, 40, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Alfredo Uquillas, J.; Kishore, V.; Akkus, O. Genipin crosslinking elevates the strength of electrochemically aligned collagen to the level of tendons. J. Mech. Behav. Biomed. Mater. 2012, 15, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. A 2017, 105, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, C. One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; SpringerBriefs in Materials; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-36426-6. [Google Scholar]
- Teh, T.K.H.; Toh, S.-L.; Goh, J.C.H. Aligned fibrous scaffolds for enhanced mechanoresponse and tenogenesis of mesenchymal stem cells. Tissue Eng. Part A 2013, 19, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chen, S.-H.; Kuo, C.-Y.; Li, M.-L.; Chen, J.-P. Response of Dermal Fibroblasts to Biochemical and Physical Cues in Aligned Polycaprolactone/Silk Fibroin Nanofiber Scaffolds for Application in Tendon Tissue Engineering. Nanomaterials 2017, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.G.; Handorf, A.M.; Allee, T.J.; Li, W.-J. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng. Part A 2013, 19, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lipner, J.; Moran, C.H.; Feng, L.; Li, X.; Thomopoulos, S.; Xia, Y. Generation of electrospun nanofibers with controllable degrees of crimping through a simple, plasticizer-based treatment. Adv. Mater. 2015, 27, 2583–2588. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef]
- Voorneveld, J.; Oosthuysen, A.; Franz, T.; Zilla, P.; Bezuidenhout, D. Dual electrospinning with sacrificial fibers for engineered porosity and enhancement of tissue ingrowth. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.S.; Colello, R.J.; Bowman, J.R.; Sell, S.A.; Lee, K.D.; Bigbee, J.W.; Bowlin, G.L.; Chow, W.N.; Mathern, B.E.; Simpson, D.G. Two pole air gap electrospinning: Fabrication of highly aligned, three-dimensional scaffolds for nerve reconstruction. Acta Biomater. 2011, 7, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Neupane, M.P.; Pant, B.; Panthi, G.; Oh, H.-J.; Lee, M.H.; Kim, H.Y. Fabrication of highly porous poly (ε-caprolactone) fibers for novel tissue scaffold via water-bath electrospinning. Colloids Surf. B Biointerfaces 2011, 88, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, S.; Zhou, Q.; Mo, X.; Song, L.; Hou, T.; Wu, J.; Li, S.; Li, Y.; Li, P.; Gan, Y.; Xu, J. The effect of mechanical stimulation on the maturation of TDSCs-poly(L-lactide-co-e-caprolactone)/collagen scaffold constructs for tendon tissue engineering. Biomaterials 2014, 35, 2760–2772. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.M.; Erisken, C.; Iskratsch, T.; Sheetz, M.; Levine, W.N.; Lu, H.H. Polymer fiber-based models of connective tissue repair and healing. Biomaterials 2017, 112, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosworth, L.A.; Turner, L.-A.; Cartmell, S.H. State of the art composites comprising electrospun fibres coupled with hydrogels: A review. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Aibibu, D.; Hild, M.; Wöltje, M.; Cherif, C. Textile cell-free scaffolds for in situ tissue engineering applications. J. Mater. Sci. Mater. Med. 2016, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qi, Y.-Y.; Wang, L.-L.; Yin, Z.; Yin, G.-L.; Zou, X.-H.; Ouyang, H.-W. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials 2008, 29, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Jt. Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.; Gomes, E.R. Nuclear positioning in skeletal muscle. Semin. Cell Dev. Biol. 2017. [CrossRef] [PubMed]
- Greising, S.M.; Gransee, H.M.; Mantilla, C.B.; Sieck, G.C. Systems biology of skeletal muscle: Fiber type as an organizing principle. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef]
- Collinsworth, A.M.; Zhang, S.; Kraus, W.E.; Truskey, G.A. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am. J. Physiol. Cell Physiol. 2002, 283, C1219–C1227. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 2074–2079. [Google Scholar] [CrossRef]
- Relaix, F.; Zammit, P.S. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development 2012, 139, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Gillespie, M.A.; Rudnicki, M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2008, 2, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.N.; Low, M.; Eisner, C.; Rossi, F.M. Isolation, Culture, and Differentiation of Fibro/Adipogenic Progenitors (FAPs) from Skeletal Muscle. Methods Mol. Biol. 2017, 1668, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Counsel, P.; Breidahl, W. Muscle injuries of the lower leg. Semin. Musculoskelet. Radiol. 2010, 14, 162–175. [Google Scholar] [CrossRef] [PubMed]
- 2016 Plastic Surgery Statistics. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics?sub=2016+Plastic+Surgery+Statistics (accessed on 22 May 2018).
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Äärimaa, V.; Kääriäinen, M.; Vaittinen, S.; Tanner, J.; Järvinen, T.; Best, T.; Kalimo, H. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromuscular Disord. 2004, 14, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, M.; Järvinen, T.; Järvinen, M.; Rantanen, J.; Kalimo, H. Relation between myofibers and connective tissue during muscle injury repair. Scand. J. Med. Sci. Sports 2000, 10, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Kasukonis, B.; Kim, J.; Brown, L.; Jones, J.; Ahmadi, S.; Washington, T.; Wolchok, J. Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng. Part A 2016, 22, 1151–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariscalco, M.W.; Magnussen, R.A.; Mehta, D.; Hewett, T.E.; Flanigan, D.C.; Kaeding, C.C. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am. J. Sports Med. 2014, 42, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.M.; Oishi, S.N.; Ezaki, M. Brachioradialis flap for coverage after elbow flexion contracture release. Tech. Hand Up. Extrem. Surg. 2010, 14, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Vang, P. Advantages and Disadvantages between Allograft Versus Autograft in Anterior Cruciate Ligament Replacement. Available online: http://hdl.handle.net/10057/957 (accessed on 21 May 2006).
- Casaroli-Marano, R.P.; Tabera, J.; Vilarrodona, A.; Trias, E. Regulatory issues in cell-based therapy for clinical purposes. Dev. Ophthalmol. 2014, 53, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Shadrin, I.Y.; Khodabukus, A.; Bursac, N. Striated Muscle Function, Regeneration, and Repair. Cell. Mol. Life Sci. 2016, 73, 4175–4202. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J.E.; Turner, N.J.; Gilbert, T.W.; Badylak, S.F. Functional Skeletal Muscle Formation with a Biologic Scaffold. Biomaterials 2010, 31, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.M.; Tyraskis, A.; Maghsoudlou, P.; Urbani, L.; Totonelli, G.; Birchall, M.A.; De Coppi, P. Skeletal muscle tissue engineering: Which cell to use? Tissue Eng. Part B Rev. 2013, 19, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Sato, F.; Uemura, R.; Nakajima, A. Effect of Pulsatile Electric Field on Cultured Muscle Cells In Vitro. J. Syst. Cybern. Inform. 2012, 10, 1–6. [Google Scholar]
- Pennisi, C.P.; Olesen, C.G.; de Zee, M.; Rasmussen, J.; Zachar, V. Uniaxial Cyclic Strain Drives Assembly and Differentiation of Skeletal Myocytes. Tissue Eng. Part A 2011, 17, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Boonen, K.J.M.; Langelaan, M.L.P.; Polak, R.B.; van der Schaft, D.W.J.; Baaijens, F.P.T.; Post, M.J. Effects of a combined mechanical stimulation protocol: Value for skeletal muscle tissue engineering. J. Biomech. 2010, 43, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Satoh, S.; Oka, T.; Matsuda, T. Tissue engineering of skeletal muscle. Highly dense, highly oriented hybrid muscular tissues biomimicking native tissues. ASAIO J. 1997, 43, M749–M753. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Mashayekhan, S.; Fadaoddini, S.; Haghirsharifzamini, Y. Design, fabrication and characterization of oxidized alginate-gelatin hydrogels for muscle tissue engineering applications. J. Biomater. Appl. 2016, 31, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.; Sim, S.; Zhu, X.; Takayama, S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 2006, 27, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Rivera, J.A.; Marchwiany, D.; Solovyeva, V.; Bashir, R. Graphene-based patterning and differentiation of C2C12 myoblasts. Adv. Healthc. Mater. 2014, 3, 995–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altomare, L.; Gadegaard, N.; Visai, L.; Tanzi, M.C.; Farè, S. Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater. 2010, 6, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Charest, J.L.; García, A.J.; King, W.P. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials 2007, 28, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Fornetti, E.; Barbetta, A.; Trombetta, M.; Cannata, S.M.; Gargioli, C.; Rainer, A. Engineering Muscle Networks in 3D Gelatin Methacryloyl Hydrogels: Influence of Mechanical Stiffness and Geometrical Confinement. Front. Bioeng. Biotechnol. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, V.; Ahadian, S.; Ostrovidov, S.; Camci-Unal, G.; Chen, S.; Kaji, H.; Ramalingam, M.; Khademhosseini, A. Engineered Contractile Skeletal Muscle Tissue on a Microgrooved Methacrylated Gelatin Substrate. Tissue Eng. Part A 2012, 18, 2453–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, I.-C.; Liu, J.B.; Bursac, N.; Leong, K.W. Effect of Electromechanical Stimulation on the Maturation of Myotubes on Aligned Electrospun Fibers. Cell. Mol. Bioeng. 2008, 1, 133–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candiani, G.; Riboldi, S.A.; Sadr, N.; Lorenzoni, S.; Neuenschwander, P.; Montevecchi, F.M.; Mantero, S. Cyclic mechanical stimulation favors myosin heavy chain accumulation in engineered skeletal muscle constructs. J. Appl. Biomater. Biomech. 2010, 8, 68–75. [Google Scholar] [PubMed]
- Aviss, K.J.; Gough, J.E.; Downes, S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cells Mater. 2010, 19, 193–204. [Google Scholar] [CrossRef]
- Martins, P.M.; Ribeiro, S.; Ribeiro, C.; Sencadas, V.; Gomes, A.C.; Gama, F.M.; Lanceros-Méndez, S. Effect of poling state and morphology of piezoelectric poly(vinylidene fluoride) membranes for skeletal muscle tissue engineering. RSC Adv. 2013, 3, 17938. [Google Scholar] [CrossRef] [Green Version]
- Takeda, N.; Tamura, K.; Mineguchi, R.; Ishikawa, Y.; Haraguchi, Y.; Shimizu, T.; Hara, Y. In situ cross-linked electrospun fiber scaffold of collagen for fabricating cell-dense muscle tissue. J. Artif. Organs 2016, 19, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Guex, A.G.; Birrer, D.L.; Fortunato, G.; Tevaearai, H.T.; Giraud, M.-N. Anisotropically oriented electrospun matrices with an imprinted periodic micropattern: A new scaffold for engineered muscle constructs. Biomed. Mater. 2013, 8, 021001. [Google Scholar] [CrossRef] [PubMed]
- Abarzúa-Illanes, P.N.; Padilla, C.; Ramos, A.; Isaacs, M.; Ramos-Grez, J.; Olguín, H.C.; Valenzuela, L.M. Improving myoblast differentiation on electrospun poly(ε-caprolactone) scaffolds. J. Biomed. Mater. Res. A 2017, 105, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Jeong, S.; Shin, H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials 2009, 30, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Sirivisoot, S.; Harrison, B.S. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int. J. Nanomedicine 2011, 6, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M.M.; Ribeiro, S.; Ribeiro, C.; Francesko, A.; Maceiras, A.; Vilas, J.L.; Lanceros-Méndez, S. Relation between fiber orientation and mechanical properties of nano-engineered poly(vinylidene fluoride) electrospun composite fiber mats. Compos. Part B Eng. 2018, 139, 146–154. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Shi, X.; Zhang, L.; Liang, X.; Kim, S.B.; Fujie, T.; Ramalingam, M.; Chen, M.; Nakajima, K.; Al-Hazmi, F.; et al. Myotube formation on gelatin nanofibers—Multi-walled carbon nanotubes hybrid scaffolds. Biomaterials 2014, 35, 6268–6277. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, Y.-J.; Hyun, J.K.; Jung, T.-G.; Hong, S.W.; Han, D.-W. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Guo, B.; Ma, P.X. Nanofiber Yarn/Hydrogel Core-Shell Scaffolds Mimicking Native Skeletal Muscle Tissue for Guiding 3D Myoblast Alignment, Elongation, and Differentiation. ACS Nano 2015, 9, 9167–9179. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Lee, H.J.; Koh, W.-G. Study of myoblast differentiation using multi-dimensional scaffolds consisting of nano and micropatterns. Biomater. Res. 2017, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- McKeon-Fischer, K.D.; Flagg, D.H.; Freeman, J.W. Poly(acrylic acid)/poly(vinyl alcohol) compositions coaxially electrospun with poly(ε-caprolactone) and multi-walled carbon nanotubes to create nanoactuating scaffolds. Polymer 2011, 52, 4736–4743. [Google Scholar] [CrossRef]
- Serena, E.; Flaibani, M.; Carnio, S.; Boldrin, L.; Vitiello, L.; De Coppi, P.; Elvassore, N. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol. Res. 2008, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Langelaan, M.L.P.; Boonen, K.J.M.; Rosaria-Chak, K.Y.; van der Schaft, D.W.J.; Post, M.J.; Baaijens, F.P.T. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med. 2011, 5, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, B.; Shah, V.; Soram, M.; Viswanathan, C.; Ghosh, D. In vitro and in vivo evaluation of L-lactide/ε-caprolactone copolymer scaffold to support myoblast growth and differentiation. Biotechnol. Prog. 2013, 29, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Stern-Straeter, J.; Bach, A.D.; Stangenberg, L.; Foerster, V.T.; Horch, R.E.; Stark, G.B.; Beier, J.P. Impact of electrical stimulation on three-dimensional myoblast cultures—A real-time RT-PCR study. J. Cell. Mol. Med. 2005, 9, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Smiley, B.L.; Mills, J.; Vandenburgh, H.H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol. Cell Physiol. 2002, 283, C1557–C1565. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Juhas, M.; Pfeiler, T.W.; Bursac, N. Local tissue geometry determines contractile force generation of engineered muscle networks. Tissue Eng. Part A 2012, 18, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Kheradmandi, M.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Ganji, F. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res. A 2016, 104, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Shadrach, J.L.; Wagers, A.J. Stem cells for skeletal muscle repair. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2297–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.; Zhou, G.-Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng. Part B Rev. 2009, 15, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, S.F. Myogenesis: The Development of Muscle. In Developmental Biology, 6th ed.; 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10006/ (accessed on 22 May 2018).
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Ciccarelli, C.; De Cesaris, P.; Scoglio, A.; Bouché, M.; Molinaro, M.; Aquino, A.; Zani, B.M. PKCalpha-mediated ERK, JNK and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J. Cell Sci. 2002, 115, 3587–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michailovici, I.; Harrington, H.A.; Azogui, H.H.; Yahalom-Ronen, Y.; Plotnikov, A.; Ching, S.; Stumpf, M.P.H.; Klein, O.D.; Seger, R.; Tzahor, E. Nuclear to cytoplasmic shuttling of ERK promotes differentiation of muscle stem/progenitor cells. Development 2014, 141, 2611–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Xu, Q.; Xiao, F.; Jiang, Y.; Wu, Z. Involvement of the p38 Mitogen-activated Protein Kinase α, β, and γ Isoforms in Myogenic Differentiation. Mol. Biol. Cell 2008, 19, 1519–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, H.; Shimizu, K.; Yamamoto, Y.; Ito, A.; Kamihira, M.; Nagamori, E. Fabrication of scaffold-free contractile skeletal muscle tissue using magnetite-incorporated myogenic C2C12 cells. J. Tissue Eng. Regen. Med. 2010, 4, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Takayama, T.; Suzuki, N.; Shimada, K.; Otsuka, K.; Ito, K. Effects of low-intensity pulsed ultrasound on the differentiation of C2C12 cells. Life Sci. 2006, 79, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Fujie, T.; Vazão, H.; Ciofani, G.; Marotta, R.; Brescia, R.; Filippeschi, C.; Corradini, I.; Matteoli, M.; Mattoli, V.; et al. Boron Nitride Nanotube-Mediated Stimulation of Cell Co-Culture on Micro-Engineered Hydrogels. PLoS ONE 2013, 8, e71707. [Google Scholar] [CrossRef] [PubMed]
- Salgarella, A.R.; Cafarelli, A.; Ricotti, L.; Capineri, L.; Dario, P.; Menciassi, A. Optimal Ultrasound Exposure Conditions for Maximizing C2C12 Muscle Cell Proliferation and Differentiation. Ultrasound Med. Biol. 2017, 43, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Campion, D.R.; Richardson, R.L.; Kraeling, R.R.; Reagan, J.O. Regulation of skeletal muscle development by the central nervous system in the fetal pig. Growth 1978, 42, 189–204. [Google Scholar] [PubMed]
- Khodabukus, A.; Baar, K. Defined electrical stimulation emphasizing excitability for the development and testing of engineered skeletal muscle. Tissue Eng. Part C Methods 2012, 18, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hattori-Aramaki, N.; Sunohara, A.; Okabe, K.; Sakamoto, Y.; Ochiai, H.; Hayashi, R.; Kishi, K. Alignment of Skeletal Muscle Cells Cultured in Collagen Gel by Mechanical and Electrical Stimulation. Available online: https://www.hindawi.com/journals/ijte/2014/621529/ (accessed on 15 January 2018).
- Bajaj, P.; Reddy, B.; Millet, L.; Wei, C.; Zorlutuna, P.; Bao, G.; Bashir, R. Patterning the differentiation of C2C12 skeletal myoblasts. Integr. Biol. Quant. Biosci. Nano Macro 2011, 3, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Buvinic, S.; Almarza, G.; Bustamante, M.; Casas, M.; López, J.; Riquelme, M.; Sáez, J.C.; Huidobro-Toro, J.P.; Jaimovich, E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 2009, 284, 34490–34505. [Google Scholar] [CrossRef] [PubMed]
- Eltit, J.M.; García, A.A.; Hidalgo, J.; Liberona, J.L.; Chiong, M.; Lavandero, S.; Maldonado, E.; Jaimovich, E. Membrane Electrical Activity Elicits Inositol 1,4,5-Trisphosphate-dependent Slow Ca2+ Signals through a Gβγ/Phosphatidylinositol 3-Kinase γ Pathway in Skeletal Myotubes. J. Biol. Chem. 2006, 281, 12143–12154. [Google Scholar] [CrossRef] [PubMed]
- Rahnert, J.A.; Burkholder, T.J. ERK phosphorylation correlates with intensity of electrical stimulation in mouse tibialis anterior. FASEB J. 2011, 25, 1051. [Google Scholar]
- Zöllner, A.M.; Abilez, O.J.; Böl, M.; Kuhl, E. Stretching skeletal muscle: Chronic muscle lengthening through sarcomerogenesis. PLoS ONE 2012, 7, e45661. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.W.; Wolfram, T.; Goldyn, A.M.; Bruellhoff, K.; Rioja, B.A.; Möller, M.; Spatz, J.P.; Saif, T.A.; Groll, J.; Kemkemer, R. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials 2010, 31, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.G.; Christ, G.; Stitzel, J.D.; Atala, A.; Yoo, J.J. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng. Part A 2008, 14, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Truskey, G.A.; Kraus, W.E. Effect of cyclic stretch on β1D-integrin expression and activation of FAK and RhoA. Am. J. Physiol. Cell Physiol. 2007, 292, C2057–C2069. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Tabata, K.; Suzuki, T.; Do, M.-K.Q.; Mizunoya, W.; Nakamura, M.; Nishimura, S.; Tabata, S.; Ikeuchi, Y.; Sunagawa, K.; et al. Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. Am. J. Physiol. Cell Physiol. 2012, 302, C1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, R.; Hattori, A.; Ikeuchi, Y.; Anderson, J.E.; Allen, R.E. Release of Hepatocyte Growth Factor from Mechanically Stretched Skeletal Muscle Satellite Cells and Role of pH and Nitric Oxide. Mol. Biol. Cell 2002, 13, 2909–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, R.M.; Roth, J.A.; Cheng, H.-L.; Rice, D.C.; Khoury, J.; Bauer, S.B.; Peters, C.A.; Freeman, M.R. Signaling Through PI3K/Akt Mediates Stretch and PDGF-BB-Dependent DNA Synthesis in Bladder Smooth Muscle Cells. J. Urol. 2003, 169, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Hanke, N.; Kubis, H.-P.; Scheibe, R.J.; Berthold-Losleben, M.; Hüsing, O.; Meissner, J.D.; Gros, G. Passive mechanical forces upregulate the fast myosin heavy chain IId/x via integrin and p38 MAP kinase activation in a primary muscle cell culture. Am. J. Physiol. Cell Physiol. 2010, 298, C910–920. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, A.; Adriani, G.; Rasponi, M.; Zervantonakis, I.K.; Fiore, G.B.; Kamm, R.D. Controlled electromechanical cell stimulation on-a-chip. Sci. Rep. 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, V.; Zhen, Y.; Zakrzewska, M.; Haugsten, E.M.; Wälchli, S.; Nilsen, T.; Olsnes, S.; Wiedlocha, A. Phosphorylation of Fibroblast Growth Factor (FGF) Receptor 1 at Ser777 by p38 Mitogen-Activated Protein Kinase Regulates Translocation of Exogenous FGF1 to the Cytosol and Nucleus. Mol. Cell. Biol. 2008, 28, 4129–4141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, J.; Yamazaki, Y.; Guang, L.; Kaziro, Y.; Koide, H. Involvement of Ras and Ral in Chemotactic Migration of Skeletal Myoblasts. Mol. Cell. Biol. 2000, 20, 4658–4665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, N.; Kahamba, T.; Woudberg, N.; Goetsch, K.; Niesler, C. Dose-dependent modulation of myogenesis by HGF: Implications for c-Met expression and downstream signalling pathways. Growth Factors 2015, 33, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Fernández-Verdejo, R.; Jaimovich, E.; Buvinic, S. Electrical stimulation induces IL-6 in skeletal muscle through extracellular ATP by activating Ca2+ signals and an IL-6 autocrine loop. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E869–E882. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, A.; Gnocchi, V.F.; Zammit, P.S. Control of Myf5 activation in adult skeletal myonuclei requires ERK signalling. Cell Signal. 2007, 19, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Müller, M.; Jaimovich, E.; Pérez, F.; Buchuk, D.; Quest, A.F.G.; Carrasco, M.A. Depolarization of Skeletal Muscle Cells induces Phosphorylation of cAMP Response Element Binding Protein via Calcium and Protein Kinase Cα. J. Biol. Chem. 2004, 279, 39122–39131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dargelos, E.; Dedieu, S.; Moyen, C.; Poussard, S.; Veschambre, P.; Brustis, J.-J.; Cottin, P. Characterization of the calcium-dependent proteolytic system in a mouse muscle cell line. Mol. Cell. Biochem. 2002, 231, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Friday, B.B.; Horsley, V.; Pavlath, G.K. Calcineurin Activity Is Required for the Initiation of Skeletal Muscle Differentiation. J. Cell Biol. 2000, 149, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, S.Y.; Taylor, P.M. Integrin and cytoskeletal involvement in signalling cell volume changes to glutamine transport in rat skeletal muscle. J. Physiol. 1998, 512, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Kin, S.; Hagiwara, A.; Nakase, Y.; Kuriu, Y.; Nakashima, S.; Yoshikawa, T.; Sakakura, C.; Otsuji, E.; Nakamura, T.; Yamagishi, H. Regeneration of skeletal muscle using in situ tissue engineering on an acellular collagen sponge scaffold in a rabbit model. ASAIO J. 2007, 53, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Lehto, M.; Kvist, M.; Vieno, T.; Józsa, L. Macromolecular composition of the sarcolemma and endomysium in the rat. Acta Anat. 1988, 133, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Chen, C.; Xu, X.; Annabi, N.; Zadeh, H.H.; Wu, B.M.; Khademhosseini, A.; Shi, S.; Moshaverinia, A. Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors. Ann. Biomed. Eng. 2016, 44, 1908–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.; Huang, Y.-C.; Birla, R.K.; Takayama, S. Microfeature guided skeletal muscle tissue engineering for highly organized 3-dimensional free-standing constructs. Biomaterials 2009, 30, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Ruini, F.; Ramella, M.; Boccafoschi, F.; Gentile, P.; Gioffredi, E.; Labate, G.F.D.; Ciardelli, G. Non-covalently crosslinked chitosan nanofibrous mats prepared by electrospinning as substrates for soft tissue regeneration. Carbohydr. Polym. 2017, 162, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Rowley, J.A.; Mooney, D.J. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Yang, J.-R.; Chiang, N.-J.; Ma, H.; Tsay, R.-Y. Evaluation of decellularized extracellular matrix of skeletal muscle for tissue engineering. Int. J. Artif. Organs 2014, 37, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Gamba, P.G.; Conconi, M.T.; Lo Piccolo, R.; Zara, G.; Spinazzi, R.; Parnigotto, P.P. Experimental abdominal wall defect repaired with acellular matrix. Pediatr. Surg. Int. 2002, 18, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.M.; Lowdell, M.W.; Urbani, L.; Ansari, T.; Burns, A.J.; Turmaine, M.; North, J.; Sibbons, P.; Seifalian, A.M.; Wood, K.J.; et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA 2013, 110, 14360–14365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Petrelli, L.; Pavan, P.G.; Natali, A.N.; Bassetto, F.; Vindigni, V.; De Caro, R. Decellularized Human Skeletal Muscle as Biologic Scaffold for Reconstructive Surgery. Int. J. Mol. Sci. 2015, 16, 14808–14831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, A.K.; Marler, J.; Benvenuto, M.; Willital, G.H.; Vacanti, J.P. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: Preliminary studies. Tissue Eng. 1999, 5, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Willital, G.H.; Vacanti, J.P. Vascularized three-dimensional skeletal muscle tissue-engineering. Biomed. Mater. Eng. 2001, 11, 275–281. [Google Scholar]
- Rimington, R.P.; Capel, A.J.; Christie, S.D.R.; Lewis, M.P. Biocompatible 3D printed polymers via fused deposition modelling direct C2C12 cellular phenotype in vitro. Lab Chip 2017, 17, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Taccola, S.; Pensabene, V.; Mattoli, V.; Fujie, T.; Takeoka, S.; Menciassi, A.; Dario, P. Adhesion and proliferation of skeletal muscle cells on single layer poly(lactic acid) ultra-thin films. Biomed. Microdevices 2010, 12, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Mauck, R.L.; Cooper, J.A.; Yuan, X.; Tuan, R.S. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J. Biomech. 2007, 40, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, Y.S.; Yang, S.H.; Hong, H.-N.; Cho, S.-W.; Cha, S.M.; Pak, J.H.; Kim, C.W.; Kwon, S.W.; Park, C.J. Muscle regeneration by adipose tissue-derived adult stem cells attached to injectable PLGA spheres. Biochem. Biophys. Res. Commun. 2006, 348, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xie, Y.; Zhang, H.; Ye, Z.; Zhang, W. Fabrication of PLGA/MWNTs composite electrospun fibrous scaffolds for improved myogenic differentiation of C2C12 cells. Colloids Surf. B Biointerfaces 2014, 123, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Yamada, K.M. Adhesive recognition sequences. J. Biol. Chem. 1991, 266, 12809–12812. [Google Scholar] [PubMed]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Heher, P.; Maleiner, B.; Prüller, J.; Teuschl, A.H.; Kollmitzer, J.; Monforte, X.; Wolbank, S.; Redl, H.; Rünzler, D.; Fuchs, C. A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain. Acta Biomater. 2015, 24, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.C.; Bettadapur, A.; Santoso, J.W.; McCain, M.L. Fabrication of Micromolded Gelatin Hydrogels for Long-Term Culture of Aligned Skeletal Myotubes. Methods Mol. Biol. 2017, 1668, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Salimath, A.S.; García, A.J. Biofunctional hydrogels for skeletal muscle constructs. J. Tissue Eng. Regen. Med. 2016, 10, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, H.; Rahbar, N. Multi-scale mechanical and transport properties of a hydrogel. J. Mech. Behav. Biomed. Mater. 2014, 37, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Pollot, B.E.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Yissar, V.; Gabai, R.; Shipway, A.N.; Bourenko, T.; Willner, I. Gold Nanoparticle/Hydrogel Composites with Solvent-Switchable Electronic Properties. Adv. Mater. 2001, 13, 1320–1323. [Google Scholar] [CrossRef]
- Ku, S.H.; Park, C.B. Myoblast differentiation on graphene oxide. Biomaterials 2013, 34, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Azcón, J.; Ahadian, S.; Estili, M.; Liang, X.; Ostrovidov, S.; Kaji, H.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Khademhosseini, A.; Matsue, T. Dielectrophoretically Aligned Carbon Nanotubes to Control Electrical and Mechanical Properties of Hydrogels to Fabricate Contractile Muscle Myofibers. Adv. Mater. 2013, 25, 4028–4034. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- McKeon-Fischer, K.D.; Freeman, J.W. Characterization of electrospun poly(L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Vandenburgh, H.H.; Karlisch, P.; Farr, L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell. Dev. Biol. 1988, 24, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.A.; Sen, S.; Sweeney, H.L.; Discher, D.E. Adhesion-contractile balance in myocyte differentiation. J. Cell Sci. 2004, 117, 5855–5863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, W.; Bursac, N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials 2009, 30, 1401–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, T.; Sacks, M.S.; Stankus, J.; Guan, J.; Wagner, W.R. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 2006, 27, 3631–3638. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Childs, S.; Riehle, M.O.; Johnstone, H.J.H.; Affrossman, S.; Curtis, A.S.G. Fibroblast reaction to island topography: Changes in cytoskeleton and morphology with time. Biomaterials 2003, 24, 927–935. [Google Scholar] [CrossRef]
- Yang, H.S.; Ieronimakis, N.; Tsui, J.H.; Kim, H.N.; Suh, K.-Y.; Reyes, M.; Kim, D.-H. Nanopatterned muscle cell patches for enhanced myogenesis and dystrophin expression in a mouse model of muscular dystrophy. Biomaterials 2014, 35, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Sanzari, I.; Callisti, M.; Grazia, A.D.; Evans, D.J.; Polcar, T.; Prodromakis, T. Parylene C topographic micropattern as a template for patterning PDMS and Polyacrylamide hydrogel. Sci. Rep. 2017, 7, 5764. [Google Scholar] [CrossRef] [PubMed]
- Janakiraman, V.; Kienitz, B.L.; Baskaran, H. Lithography Technique for Topographical Micropatterning of Collagen-Glycosaminoglycan Membranes for Tissue Engineering Applications. J. Med. Devices 2007, 1, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geißler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, C.; Raman, R.; Chan, V.; Williams, B.J.; Tolish, M.; Bajaj, P.; Sakar, M.S.; Asada, H.H.; Saif, M.T.A.; Bashir, R. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 10125–10130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omidian, H.; Rocca, J.G.; Park, K. Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate. Macromol. Biosci. 2006, 6, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.J.; Khang, G.; Lee, H.B. Interaction of fibroblasts on polycarbonate membrane surfaces with different micropore sizes and hydrophilicity. J. Biomater. Sci. Polym. Ed. 1999, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kasper, A.M.; Turner, D.C.; Martin, N.R.W.; Sharples, A.P. Mimicking exercise in three-dimensional bioengineered skeletal muscle to investigate cellular and molecular mechanisms of physiological adaptation. J. Cell. Physiol. 2018, 233, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, S.; Madden, L.; Bursac, N. Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles. Ann. Biomed. Eng. 2014, 42, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Genesis, Modulation, and Regeneration of Skeletal Muscle, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Choi, Y.-J.; Kim, T.G.; Jeong, J.; Yi, H.-G.; Park, J.W.; Hwang, W.; Cho, D.-W. 3D Cell Printing of Functional Skeletal Muscle Constructs Using Skeletal Muscle-Derived Bioink. Adv. Healthc. Mater. 2016, 5, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.W.; Powell, H.M. Regulation of electrospun scaffold stiffness via coaxial core diameter. Acta Biomater. 2011, 7, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jun, I.; Shin, Y.M.; Jang, W.; Kim, S.I.; Shin, H. The development of genipin-crosslinked poly(caprolactone) (PCL)/gelatin nanofibers for tissue engineering applications. Macromol. Biosci. 2010, 10, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sreerekha, P.R.; Menon, D.; Nair, S.V.; Chennazhi, K.P. Fabrication of fibrin based electrospun multiscale composite scaffold for tissue engineering applications. J. Biomed. Nanotechnol. 2013, 9, 790–800. [Google Scholar] [CrossRef] [PubMed]
- McKeon-Fischer, K.D.; Flagg, D.H.; Freeman, J.W. Coaxial electrospun poly(ε-caprolactone), multiwalled carbon nanotubes, and polyacrylic acid/polyvinyl alcohol scaffold for skeletal muscle tissue engineering. J. Biomed. Mater. Res. A 2011, 99, 493–499. [Google Scholar] [CrossRef] [PubMed]
- McKeon-Fischer, K.D.; Rossmeisl, J.H.; Whittington, A.R.; Freeman, J.W. In vivo skeletal muscle biocompatibility of composite, coaxial electrospun, and microfibrous scaffolds. Tissue Eng. Part A 2014, 20, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Charvet, B.; Ruggiero, F.; Le Guellec, D. The development of the myotendinous junction. A review. Muscles Ligaments Tendons J. 2012, 2, 53–63. [Google Scholar] [PubMed]
- Kostrominova, T.Y.; Calve, S.; Arruda, E.M.; Larkin, L.M. Ultrastructure of myotendinous junctions in tendon-skeletal muscle constructs engineered in vitro. Histol. Histopathol. 2009, 24, 541–550. [Google Scholar] [PubMed]
- Larkin, L.M.; Calve, S.; Kostrominova, T.Y.; Arruda, E.M. Structure and Functional Evaluation of Tendon–Skeletal Muscle Constructs Engineered In Vitro. Tissue Eng. 2006, 12, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Ladd, M.R.; Lee, S.J.; Stitzel, J.D.; Atala, A.; Yoo, J.J. Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering. Biomaterials 2011, 32, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Merceron, T.K.; Burt, M.; Seol, Y.-J.; Kang, H.-W.; Lee, S.J.; Yoo, J.J.; Atala, A. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 2015, 7, 035003. [Google Scholar] [CrossRef] [PubMed]



| Material | Scaffold Preparation | Shape and Structure of the Scaffold | Mechanical Properties of the Scaffold | Ref. |
|---|---|---|---|---|
| Collagen | Freeze drying | Sponges L = 11, 23 or 51 mm 94% porosity, pore size = 62 μm | For L = 23 mm spec. EM = 0.02 MPa Linear Stiffness = 0.05 N/mm Maximum Stress = 0.005 MPa | [38,39] |
| Collagen/Chondroitin Sulfate | Sponge pore size = 53 μm | Linear Stiffness = 0.025 N/mm | [87] | |
| Collagen/Chondroitin Sulfate | Isotropic sponge pore size = 87 μm Anisotropic pore sizes = 55, 152, 243 μm | ND | [51,55] | |
| Collagen | Extrusion | EDC Crosslinked fiber diameter = 215 µm EDC/EDGE Crosslink diameter = 137 µm | Fiber diameter 215 µm → EM = 19.3 MPa Fiber diameter 137 µm → EM = 46.2 MPa | [52,53] |
| Collagen | ELAC | Collagen thread diameter = 50–100 μm | ND | [33] |
| Collagen | Woven collagen scaffold with 81% of porosity | Stiffness = 23.8 N/mm | [34] | |
| PLGA | Electrospinning | Random nanofibers = 568 nm Aligned fibers = 320, 680 and 1800 nm | Random nanofibers → EM = 107 MPa Aligned fibers → EM = 341–510 MPa | [105,106] |
| PLLA | Aligned nanofiber diameter = 430 nm Random nanofiber diameter = 450 nm | Aligned nanofibers → Stiffness = 3.48 N/mm; EM = 22.76 MPa Random nanofibers → Stiffness = 0.07 N/mm; EM = 0.63 MPa | [30] | |
| PLDLLA | Crimped fiber diameter = 880 nm Amplitude = 5.2 μm Wavelength = 46 μm | Crimped fiber Modulus = 3 MPa | [54] | |
| PEEUR | Aligned or random fiber diameters <1 μm, 1–2 μm or >2 μm | EM = 4.2–9.2 MPa | [45,46] | |
| PCL | Yarned made of twisted aligned fibers (200 μm diameter) | UTS = 17 MPa EM = 30 MPa | [35] | |
| P(LLA-CL)/Collagen | Fiber diameter = 643 nm Final yarn thickness = 150 μm Pore size = 28.5 μm | Yarns EM = 2 MPa Ultimate deformation = 250% | [107] | |
| PLGA | Knitting | Scaffold with 3 yarns. 20 filaments/yarn 25 μm diameter of filament + electrospun nanofibers | Initial failure load = 56.3 N Initial Elastic Stiffness = 5.80 N/mm Initial toe region Stiffness = 0.34 N/mm | [36] |
| Silk | Combined knitted silk fibers and silk sponge pores size from 20 to 100 μm | Maximum Tensile Load = 252 N Tensile Stiffness = 40 N/mm | [37] | |
| Silk/Collagen | Combined knitted silk scaffold and freeze dryed collagen sponge | Failure force = 21.65 N | [97] |
| Cells | Mechanical Stimulation of the Scaffold | Mechanical Properties of Biohybrid Construct | Major Outcomes | Ref. |
|---|---|---|---|---|
| BMSCs from NZ Rabbit | 2 days of static culture and 2.4% strain once every 5 min for 8 h/day for 12 days | Long construct (51 mm): LS = 0.066 N/mm after stimulation. Non-stimulated: LS = 0.047 N/mm | Longest constructs: highest linear stiffness in vitro. Still very weak | [37,38] |
| BMSCs from NZ Rabbit | 2 days of static culture and 2.4% strain 8 h/day for 12 days at 100 or 3000 cycles/day | Stimulated constructs 100 cycles LS = 0.080 N/mm; 3000 cycles LS = 0.032 N/mm | 100 cycles/day: ↑ linear stiffness 3000 cycles/day: ↑ mRNA levels of Col1 and Col3. ECM not shown | [87] |
| Primary horse tenocytes | None | ND | Anisotropic sponges: ↑ cell number, alignment and metabolic activity Pores >150 μm: ↑ cell proliferation and activity Smaller pores with high crosslinking density: ↑ differentiation | [51,55] |
| Sheep patellar tendon fibroblasts | None | ND | EDC/EDGE crosslinking: better mechanical properties, proliferation but ↓ cell viability EDC cross-linked fibers ↑ ECM production | [52] |
| Human MSCs | None | ND | ELAC threads: ↑ cell adhesion, ↓proliferation, ↑ tendon differentiation compared to random threads | [32] |
| Human BMSCs | None | ND | Cells aligned in the 3D structure. Up-regulation of tendon-related markers (TNMD and COL1). New matrix deposition | [33] |
| Human Rotator Cuff Fibroblasts | None | For 600 nm diameter, after 14 days: Aligned Constr: EM = 341 MPa, Random Constructs: EM = 107 MPa | Aligned/random scaffolds: No differences in cell proliferation or cell matrix deposition Nanofiber: ↑ cell proliferation and matrix synthesis Microfiber: ↑ tendon-like gene markers | [105,106] |
| Human TSPC from foetal Achilles Tendon | None | ND | Aligned scaffolds: ↑ tendon differentiation (aligned cells and expression of COL1, SCX, Eya2) | [29] |
| Bovine fibroblasts | Short term: 10% of cyclic uniaxial strain at 1 Hz 3 h/day. Long term: 3 h/day at 1 Hz in alternate days for 2/4 weeks | After 4 weeks on dynamic culture: Crimped structures EM = 33 MPa Uncrimped structures EM = 17 MPa For non-stimulated culture: uncrimped EM = 8.7 MPa | Crimped-like fibers: ↑ collagen accumulation Dynamic culture: ↑ ECM production (collagen and proteoglycans) | [54] |
| C3H10T1/2 | 2 days static culture + 3 days static (50 mN)/dynamic load (4% strain 0.25 Hz for 30 min) | ND | Static load, larger fibers, non-alignment: ↑ tenogenic differentiation | [45,46] |
| Human BMSCs | 5 days of static culture. Cyclic uniaxial strain at 5% elongation at 1 Hz 1 h/day for 7 or 21 days | After 21 days on dynamic culture, UTS = 50 MPa; EM = 110 MPa. Under dynamic culture UTS = 20 MPa; EM = 110 MPa | aligned fibers: ↑ cell alignment Uniaxial cyclic strain: ↑ tendon-related markers (COL1, COL3, TNC, FN)/unloaded cells | [34] |
| Rabbit tendon cells | Static culture for 1 day. Cyclic uniaxial strain at 4% elongation at 0.5 Hz 2 h/day for 14 days | ND | Dynamic culture: ↑ Tendon related markers (COL1, COL3, decorin, TNC, Biglycan and ↓ of bone (Runx2) or cartilage related markers (COL2). Cells aligned in both static or dynamic culture | [107] |
| Pig BMSCs | None | Failure load = 1. 82 N; Elastic Stiffness = 0.64 N/mm; Toe Region Stiffness = 0.05 N/mm | knitted structure + electrospun nanofibers: ↑ cell proliferation, collagen production and tendon-related markers (COL1, Decorin, Biglycan) | [35] |
| Human BMSCs | None | Tensile Load = 257 M Tensile Stiffness = 50 N/mm | Combined silk scaffolds with cells shows higher proliferation, ECM production (COL1, COL3 and GAGs) than knitted silk scaffolds. | [36] |
| Rabbit TSPCs | None | ND | No difference in cells attachment, spreading and proliferation Aligned collagen sponges → aligned ECM deposit | [97] |
| Animal Model, Tissue Site and Duration of Implantation | Mechanical Stimulation before Implantation | Mechanical Properties of the Biohybrid Construct Following Implantation | Biological Outcomes | Ref. |
|---|---|---|---|---|
| Rabbit patellar tendon 12 weeks | 2.4% strain every 5 min for 8 h/day for 12 days prior implantation | Stimulated repair: LS = 241.6 N/mm; EM = 441.2 MPa. Non-stimulated repair: LS = 88.6 N/mm; EM = 343.2 MPa | Stimulated repair constructs: ↑ mechanical properties over time than non-stimulated repair | [38] |
| Sheep patellar tendon 3 or 6 months | None | After 6 months: EDC cross linked: EM = 73 MPa EDC/EDGE cross linked: EM = 68 MPa | EDC cross-linked fibers: ↑ mechanical properties, integration, resorption and tissue ingrowth after 6 months | [53] |
| -Mice muscle for 1 or 6 weeks -Mice skin for 1 week | None | None | -Cytotoxicity model: aligned cells with more oriented bundles of collagen compared to random scaffolds -Subcutaneous model: ↑ concentration of collagen with aligned morphology in aligned scaffolds | [30] |
| -In vivo: Mice back for 2, 4 or 8 weeks -In situ: Rabbit tendon for 4 or 12 weeks | In situ: Static or dynamic culture, 4% elongation at 0.5 Hz 2 h/day, 14 days | In situ: EM = 426.69 MPa for dynamic group EM = 41.5 MPa for static group | -In vivo: Mechanical stimulation: ↑ neo-tendon tissue formation with aligned ECM deposition -In situ: Dynamic culture: ↑ alignment of cells and matrix deposition. Larger collagen fibers on pre-stimulated construct | [107] |
| Rabbit tendon 12 weeks | None | Failure force = 139.85 N Stress at failure = 4.34 MPa Energy = 0.42 J Stiffness = 26.67 N/mm | Combined knitted and collagen-aligned sponge: ↑ ovoid cells, larger and denser collagen fibers | [97] |
| Material | Scaffold Preparation | Shape and Structure of the Scaffold | Mechanical Properties of the Scaffold | Ref. |
|---|---|---|---|---|
| Collagen I | Hydrogel (Layer) | Membrane Flexcell | EM = 930 kPa | [174] |
| Collagen | Sheet -smooth | ND | [173] | |
| Collagen I—Matrigel | Layer | ND | [200] | |
| Fibrin | Layer | ND | [175] | |
| Collagen I | 3D cylinder hydrogel with inner diameters: 0.90 and 0.53 mm | ND | [176] | |
| Oxidized alginate/gelatin cross-linking | Layer | EM = 1 and 10 kPa | [177] | |
| PMDS/NCO-sP(EO-stat-PO) hydrogel/fibronectin coating | Fibronectin lines micropattern (30 μm wide parallel lines with 40 μm spacing) coating on hydrogel | EM ~1 MPa | [225] | |
| PDMS/laminin coating | Micropatterned waves with 3, 6 and 12 μm in periodicity | ND | [178] | |
| PDMS/fibronectin coating | Fibronectin geometrical cues: linear, 30°, circular micropatterns | EM = 100 and 500 Pa | [220] | |
| poly-l-lactide/trimethylene carbonate | Micropatterns with groove widths (5, 10, 25, 50, 100 μm) and depths (0.5, 1, 2.5, 5 μm) | ND | [180] | |
| Gelatin methacryloyl | Hydrogel (3D matrix) | Hydrogel slabs cross sections: 2000 μm × 2000 μm, 1000 μm × 1000 μm, 500 μm × 500 μm | Compressive modulus = 1 to 17 kPa | [182] |
| Gelatin methacrylate | Micropatterns with groove-ridges: 100 μm/50 μm; 100 μm/100 μm | ND | [183] | |
| Mix of matrigel and fibrin | 3D matrix: 1.5 mm thick—hexagonal holes lengths = 0.6, 1.2, or 1.8 mm | ND | [204] | |
| Mix of collagen and matrigel | 3D matrix | ND | [203] | |
| Fibrin | None | ND | [202] | |
| ECM proteins | 3D matrix | EM = from 200 to 500 kPa; Passive tension = from 860 to 1150 μN | [286] | |
| Polycarbonate polymer and titanium with gold nanoparticulates | Hydrogel (3D porous sponge) | Micropatterns with ridges, grooves, arrays of holes (5–75 μm) | ND | [181] |
| l-lactide/e-caprolactone copolymer (70/30) | Porous sponge = 3 cm diameter, 2–3 mm thickness with an average pore size of about 320 μm | ND | [201] | |
| Atelocollagen | Porous sponge = pore diameters with a range of 50 to 100 μm | ND | [243] | |
| Collagen | Porous sponge | ND | [199] | |
| Polyurethane | Electrospinning | Smooth film or random or aligned fibers Aligned fiber size = 600 nm–10 µm | EM = 0.5–1–22 MPa | [184] |
| Polyesterurethane (DegraPol®) | Highly oriented fiber (10 µm diameter) Scaffold thickness = 200 µm | ND | [185] | |
| PCL | Highly oriented fibers = 438–520 nm range | Non-aligned scaffolds = EM 2.1 MPa | [260] | |
| PLGA | 1500 rpm: 0.6–0.9 μm range oriented with standard deviation: 19.5° 300 rpm: 0.4–0.8 μm range random with standard deviation: 74.7° | ND | [186] | |
| ß-PVDF | Fiber diameter = ~200 nm Films with a thickness = ~110 µm | ND | [187] | |
| Collagen I | Spring-shape | ND | [188] | |
| Chitosan/PVA | Random structure: diameter = 137 nm, pore size = 1.9 µm2 | Break strain = 83.42%, Peak stress = 6.63 MPa | [205] | |
| PCL | Parallel -oriented with wavy micropatterns: period. = 90um—depth = 14um—fiber diam. = 148 nm random orientation: size fibers = 265 nm aligned fibers: size fibers = 354 nm | EM = 36 MPA; UTS = 15 MPa; Elongation to break = 44% EM = 7 MPa; UTS = 4 MPa; Elongation to break = 161% EM =17 mMPa; UTS = 14 MPa; Elongation to break = 64% | [189] | |
| PCL blends with PLGA or decorin | Aligned fiber diameters from 0.4–0.7 µm to 0.7–2.7 µm, for 15% w/v and 20% w/v of polymer solution | ND | [190] | |
| PCL/PANi: (100/0); (85/15); (70/30) | Random 3D interconnected pores or oriented fibers Fiber diameters: PLCL/PANi (100/0) = 516 nm PLCL/PANi (85/15) = 499 nm PLCL/PANi (70/30) = 466 nm | Tensile strain—Elongation at break—EM—conductivity: PLCL/PANi (100/0): 18.2 MPa—248%—4.74 MPa PLCL/PANi (85/15): 16.7 MPa—176%—6.8 MPa—0.160 ± 0.046 S/cm PLCL/PANi (70/30): 14.1 MPa—160%—6.41 MPa—0.296 S/cm | [191] | |
| Polyurethane/carbon nanotubes | Thickness = 36–64 µm range; Fiber diameter = 441–1533 nm range; Pore area = 2.5–12.3 µm2 | EM = 6.1–41.0 MPa range Tensile strength = 9.95–45.02 MPa range; Elongation at break = 115–300% range | [192] | |
| Gelatin crosslinked by GTA, +/−0.5 or 5 mg/mL MWNTs | Fiber diameter from 18 kV = 250 to 900 nm and from 15 kV = 300 to 600 nm | EM (20% Gelatin) = 509 ± 37 kPa EM (20% gelatin −0.5 mg/mL MWNTs) =1170 kPa EM (20% gelatin −5 mg/mL MWNTs) = 1170 kPa | [194] | |
| PLGA/collagen with graphene oxide nanoparticules | Randomly oriented average diameter = 440 nm | Hydrophilicity angle contact = 85°; Surface energy = 32.35 mN/m; Tensile strenghs = 16.8 MPa; E = 460 MPa | [195] | |
| PCL/collagen sputter-coated with gold nanoparticules | Fiber diameters = from 296 to 334 nm Fiber orientation:
| Tensile strength—Elongation at break EM: Random parallel: 4.01 MPa—53%—4.33 MPa Random perpendicular: 3.86 MPa—53%—4.07 MPa Aligned parallel: 4.88 MPa—42.33%—4.43 MPa Aligned perpendicular: 3.06 MPa—91.67%—42.93 MPa | [265] | |
| Fibers:PCL/silk fibroin/polyaniline Hydrogel: PEG | Aligned fiber diameters within hydrogel = 600 to 900 nm Yarn diameters within hydrogel = 50, 100, 165 µm | Tensile stress = 1.49 to 4.02 cN by yarn diameter: 25 to 165 µm Strain of yarns with diameters from 76% to 107%, | [196] | |
| PCL/multiwalled carbon nanotubes (MWCNT) Hydrogel: PAA/PVA | Fiber diameter averages: PCL: 1.032 µm PCL-MWCNT: 1.704 µm PCL-MWCNT-Hydrogel:1.861 µm | Electrical conductivity PCL: 0.026 S/cm PCL-MWCNT:0.043 S/cm PCL-MWCNT-Hydrogel: 0.039.011 S/cm | [296] | |
| PCL Hydrogel: PEG | Random, parallel, perpendicular fibers versus hydrogel pattern; Hydrogel pattern: 100 and 200 μm width | ND | [197] |
| Cells | Mechanical and/or Electrical Stimulation | Biological Outcomes | Ref. |
|---|---|---|---|
| C2C12 | Mechanical: uniaxial cyclic tensile strain (CTS)—semi-sinusoidal tensile stretching pulses with a duration of 1 s. Peak amplitude 15% | Cell alignment perpendicular to the direction of strain ↑ myotube/myoblast ratio and % of myosin-positive myotubes | [174] |
| C2C12 | Mechanical: 24 h of static culture Electrical: period 1 s, duration 0.1 s for 72 h, amplitude: 0.1 V to 12 V | Pulses lower than 8 V: ↑ cell adherence and proliferation Pulses of 0.1 V: ↑ cell differentiation Cell repetitive contraction at 8 days | [173] |
| MPCs/C2C12 | Electrical: 4 V/cm, 6 ms pulses, frequency 2 Hz for 48 h | ↑ sarcomere assembly and expression of late muscle maturation markers Faster maturation of myotubes in 3D model system than in 2D MPCs more mature than C2C12 and more susceptible to the electrical stimulation | [200] |
| MPCs/C2C12 | Mechanical: 2 days uniaxial ramp stretch of 0–2% followed by an uniaxial intermittent stretch regime of 2–6% (3 h on, 3 h off) | ↓ maturation into functional muscle fibers | [175] |
| C2C12 | Mechanical: Cyclic stretching of 60 Hz −5% amplitude for 4 days | ↑ degree of cell orientation and differentiation. Formation of a necrotic core in larger diameter rode | [176] |
| MSCs | - | Coverage of the total surface hydrogels OA/GEL (30/70) after 14 day culture | [177] |
| C2C12 | Mechanical: orientation relative to the cyclic strain direction: 0°–45°–90°, amplitude 7% at 0.5 Hz for 4 days | Alignment of the actin stress fibers relative to the strain direction Significant effect on stress fiber orientation under geometric constraints of 30 μm width | [225] |
| C2C12 | - | Wave periodicity (6 µm) of scaffold: ↑ alignment of moyblasts and myotubes | [178] |
| C2C12 | Electrical: 20 V, 50 ms pulse, 1 Hz | 30° hybrid structure: ↑ differentiation into myotubes with the highest fusion index | [220] |
| C2C12 | - | ↑ cell differentiation and maturation with 25 μm grooves width and 0.5–1 μm depth after 7 days of culture | [180] |
| C2C12 | - | GelMA 3 and 4%: ↑ myogenesis Hydrogel structures (500 μm × 500 μm) and (1000 μm × 1000 μm) ↑ cell parallel orientation | [182] |
| C2C12 | Electrical: 48 h of stimulation at 22 mA,1 Hz, and 2 ms | Surface topography with ridge width 50 µm: ↑ myotube orientation compared to width of 100 µm Electrical stimulation ↑ myoblast alignment and myotube diameter | [183] |
| Neonatal rat skeletal myoblasts | - | Elongated pores: ↑ cell alignment Tissue networks: ↑ fraction of myogenin-positive nuclei, and cell maturation into myotubes | [204] |
| Primary human skeletal cells | Mechanical: 3 sets (5% strain for 2 days,10% strain for 2 days and 15% strain for 4 days) of 5 stretch/relaxation cycles, each separated by 30 s of rest, with 28 min of rest after the third set | Repetitive stretch/relaxation cycles: ↑ myofiber diameter, area percentage and aligned multinucleated myofibers | [203] |
| Primary rat myoblast | Electrical: biphasic stimulation 6.8 mA; 4 ms. Electric bursts lasted for 250 ms, delivered at intervals every 4 s | ↓ expression of the MRFs, MyoD and myogenin and AChR-ε | [202] |
| C2C12 | Electrical: bipolar pulses: 20 V, amplitude (21.6-V cm−1 field strength) and 50 ms pulse | IGF-1: ↑ rate of fusion, maturation and myotube density Electrical stimulation triggered contraction | [286] |
| C2C12/primary myoblast | - | Microscale topography: modulates myoblast alignment | [181] |
| Human myoblast | - | ↑ desmin and MyoD expression and myotube formation | [201] |
| MPCs | Electrical: Pulses 70 mV/cm for 3 ms, frequency 33.3 mHz | ↑ expression of MyoD and desmin compare to non-stimulated control and ↑ total amount and release rate of NOX | [199] |
| C2C12 | Electrical: 20 V, 1 Hz, for 1 h with 5 h of rest Synchronized electromechanical: pre-stretching mechanical protocol: 5% cyclic strain at 1 Hz, followed by electrical stimulation | ↑ degree of myotube striation when applied during post differentiation period compared to prior one Synchronized elecromechanical stimulation ↑ degree of myotube striation compared to unstimulated control | [184] |
| C2C12 | Mechanical: 5 days of static culture (24 h of stretching at 0.02 mm/h, up to 960 μm displacement) followed by stretching pattern (frequency 0.5 Hz, amplitude 1 mm, 30 sec rest, followed by 28 min rest) | Cyclic stretching pattern stimulation: ↑ myosin accumulation | [185] |
| C2C12 | - | Parallel electrospun fibers ↑ myoblast alignment, myosin expression and sarcomeric protein organization | [186] |
| C2C12 | - | Negative poled ß-PVDF ↑ cell adhesion and proliferation. Oriented ß-PVDF fibers ↑ cell alignment | [187] |
| C2C12 | - | Stained MHC-positive cells at day 7, multi-nucleated with parallel orientation along the microfiber at day 10 Myoblasts showed typical sarcomeric cross-striations The entire tissue continuously pulsated by autonomous contraction | [188] |
| Rabbit MSCs | - | Hybrid (chitosan/PVA) composition: ↑ myogenesis | [205] |
| C2C12 | - | Periodic grooves: ↑ myotube formation and orientation | [189] |
| C2C12 | - | Aligned PCL/PLGA 50% fibers: ↑ cell growth and differentiation versus to randomly oriented fibers Decorin addition: ↑ cell fusion, myotube length but ↓ myotube alignment | [190] |
| C2C12 | - | PLCL/PANi (85/15) and (70/30): ↑ myotube length and width and ↑ expression of myogenin, troponin T and MHC genes | [191] |
| C2C12 | Electrical: 10 µA at 10 Hz, 6 h/day, 21 days | Modulation of myotube maturation depend on the conductivity of the scaffolds | [192] |
| C2C12 | Electrical: 5 V, 1 Hz, 1 ms for 2 days | ↑ speed and the rate of myotube formation and length ↑ myogenin and FAK gene expression Increasing carbon nanotube concentration ↑ maturation and contractibility of myotubes | [194] |
| C2C12 | - | GO-PLGA-Col hybrid scaffold composition ↑ cell attachment and proliferation, myogenic differentiation, myoblast fusion and myotube maturation | [195] |
| C2C12 | - | Hybrid scaffold/hydrogel: ↑ formation of 3D aligned and elongated myotube ↑ Cell adherence, alignment and elongation with 50 and 100 µm yarns embedded in hydrogels | [196] |
| C2C12 | - | PCL-carbon nanotubes-hydrogel: ↑ multinucleated cellular formation | [296] |
| C2C12 | - | Aligned nonofibers: ↑ cells elongation compared to random and perpendicular nanofibers 100 μm pattern sizes on parallel fibrous scaffolds ↑ MHC expression and myogenesis | [197] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beldjilali-Labro, M.; Garcia Garcia, A.; Farhat, F.; Bedoui, F.; Grosset, J.-F.; Dufresne, M.; Legallais, C. Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges. Materials 2018, 11, 1116. https://doi.org/10.3390/ma11071116
Beldjilali-Labro M, Garcia Garcia A, Farhat F, Bedoui F, Grosset J-F, Dufresne M, Legallais C. Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges. Materials. 2018; 11(7):1116. https://doi.org/10.3390/ma11071116
Chicago/Turabian StyleBeldjilali-Labro, Megane, Alejandro Garcia Garcia, Firas Farhat, Fahmi Bedoui, Jean-François Grosset, Murielle Dufresne, and Cécile Legallais. 2018. "Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges" Materials 11, no. 7: 1116. https://doi.org/10.3390/ma11071116





