Influence of Water Solute Exposure on the Chemical Evolution and Rheological Properties of Asphalt
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Preparation of Solutions
2.3. Water Solute Immersing Tests
2.4. Characterization Methods
2.4.1. Experimental Program Plan
2.4.2. Four Compositions Analysis
2.4.3. Fourier Transform Infrared (FTIR)
2.4.4. Dynamic Shear Rheometer (DSR) Test
3. Results and Discussion
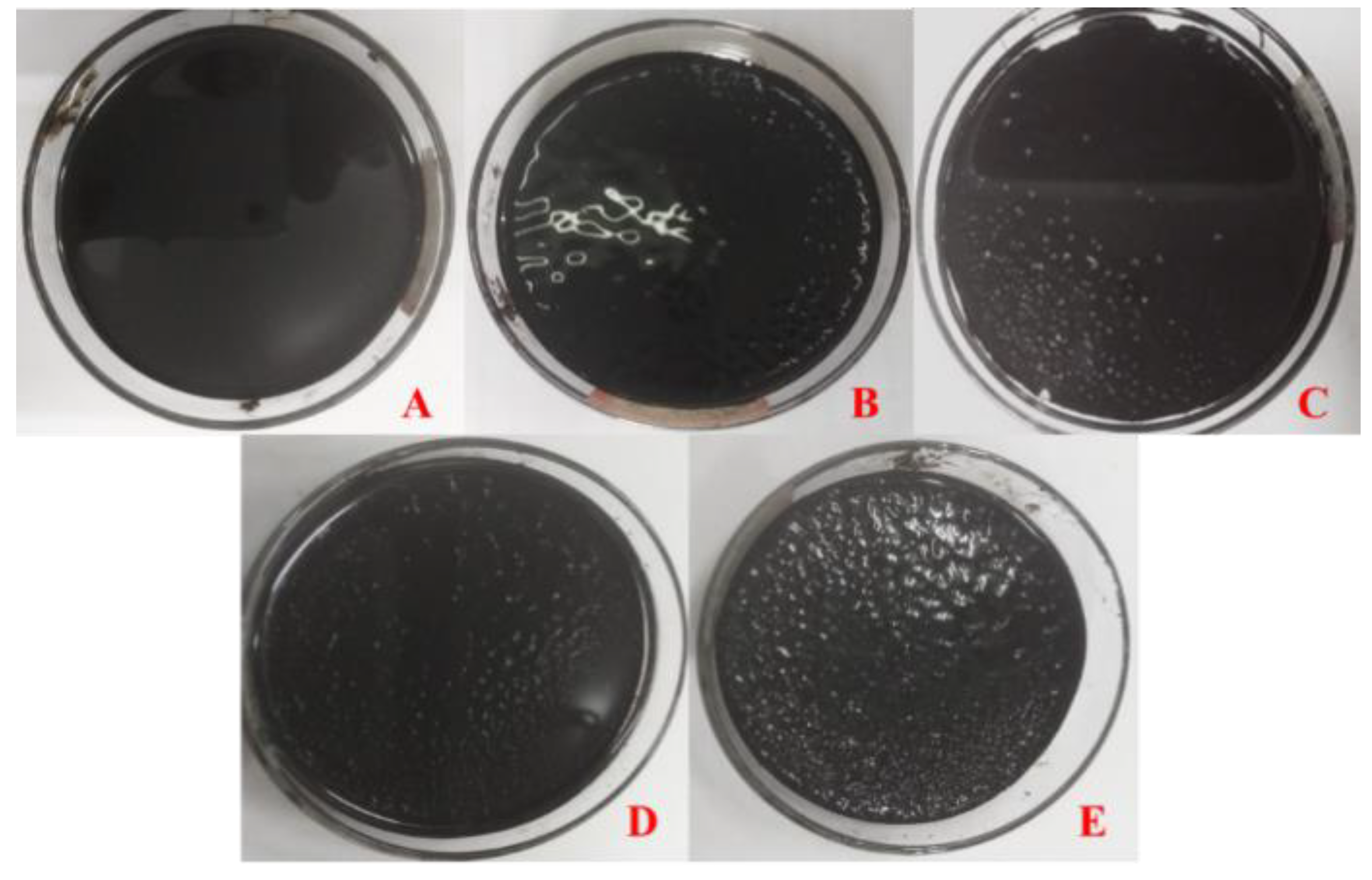
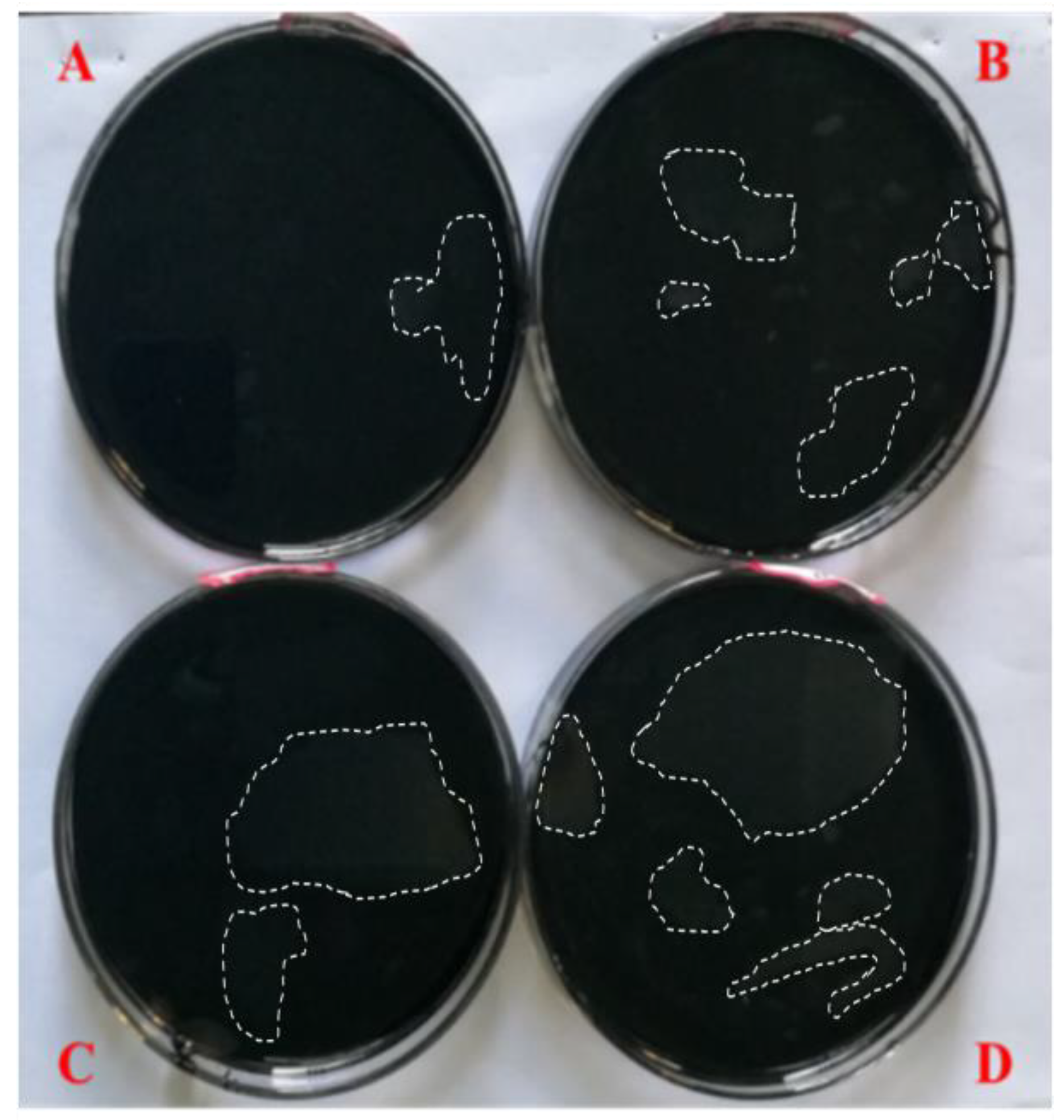
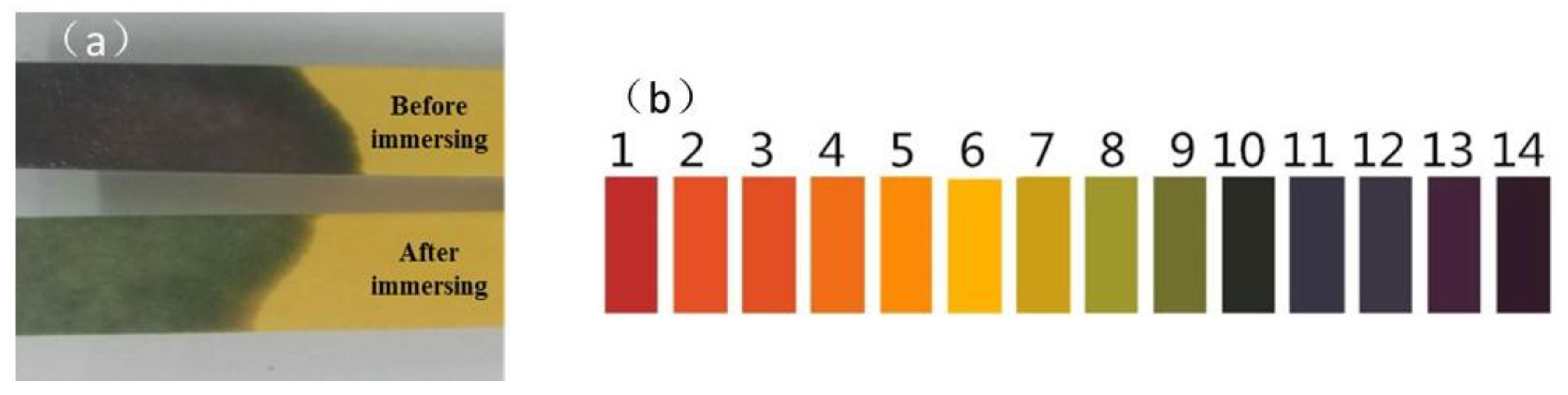
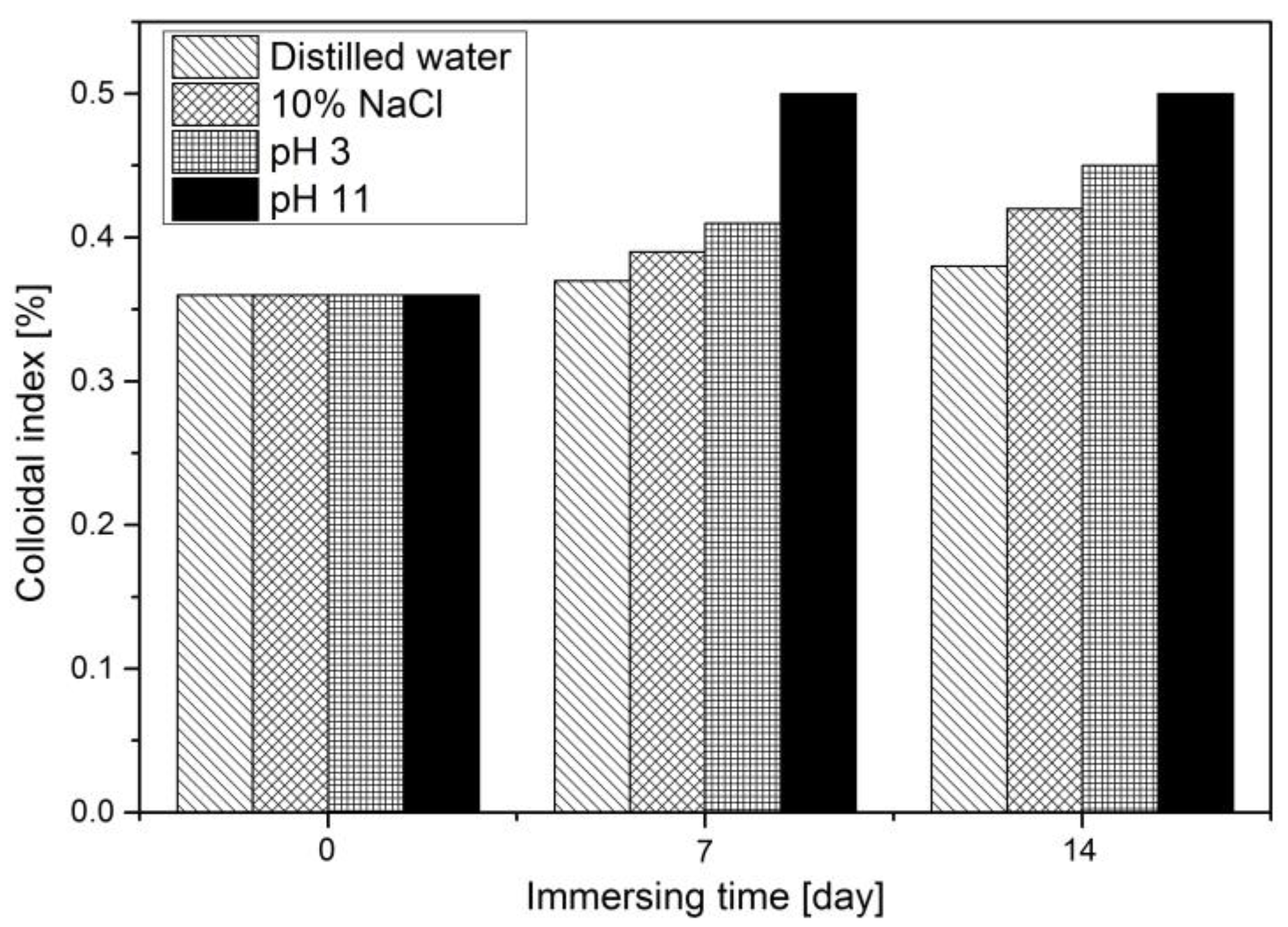
3.1. Water Solutions’ Effect on the Appearance of Asphalt
3.2. Four Component Fractions Analysis
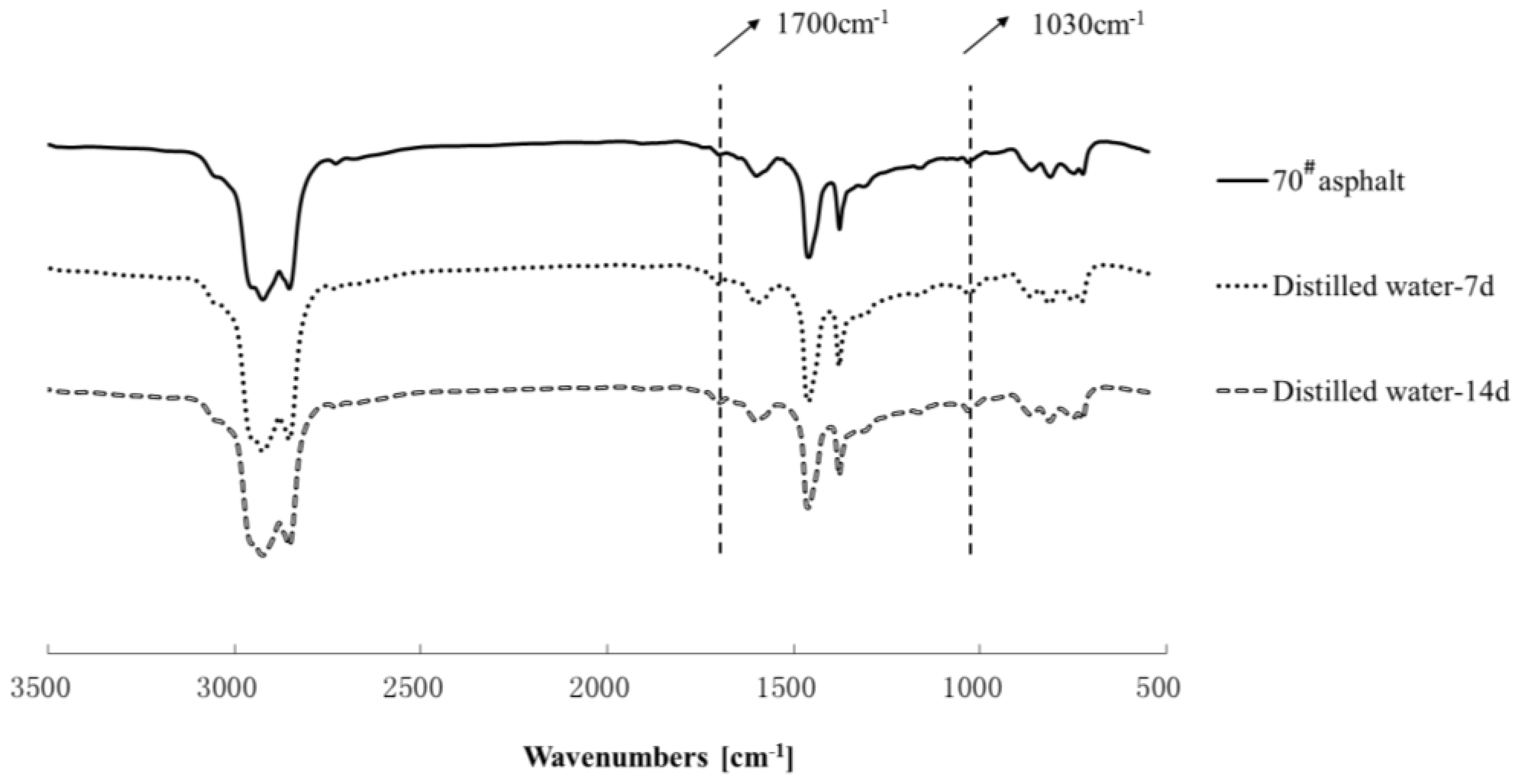
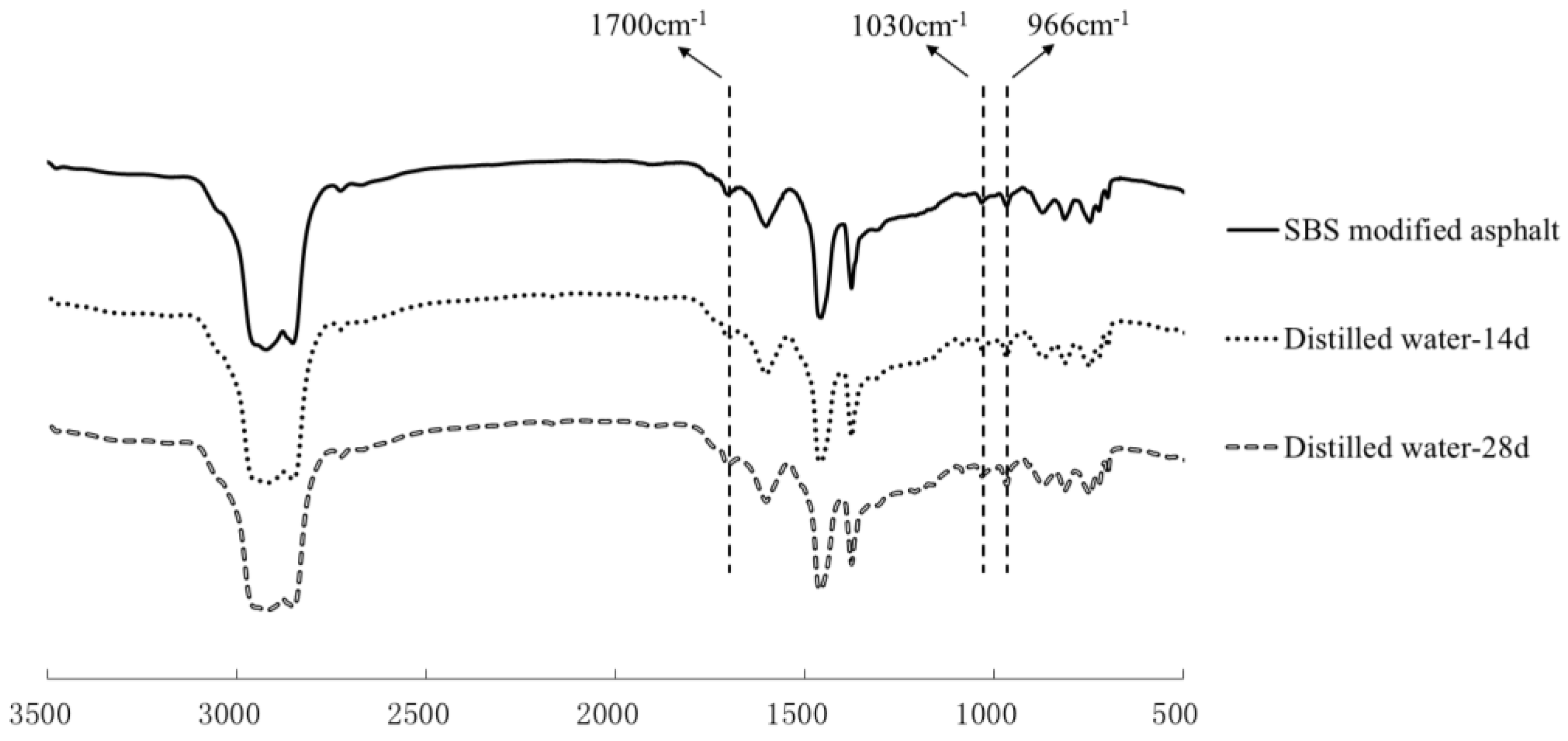
3.3. Chemical Structure Analysis
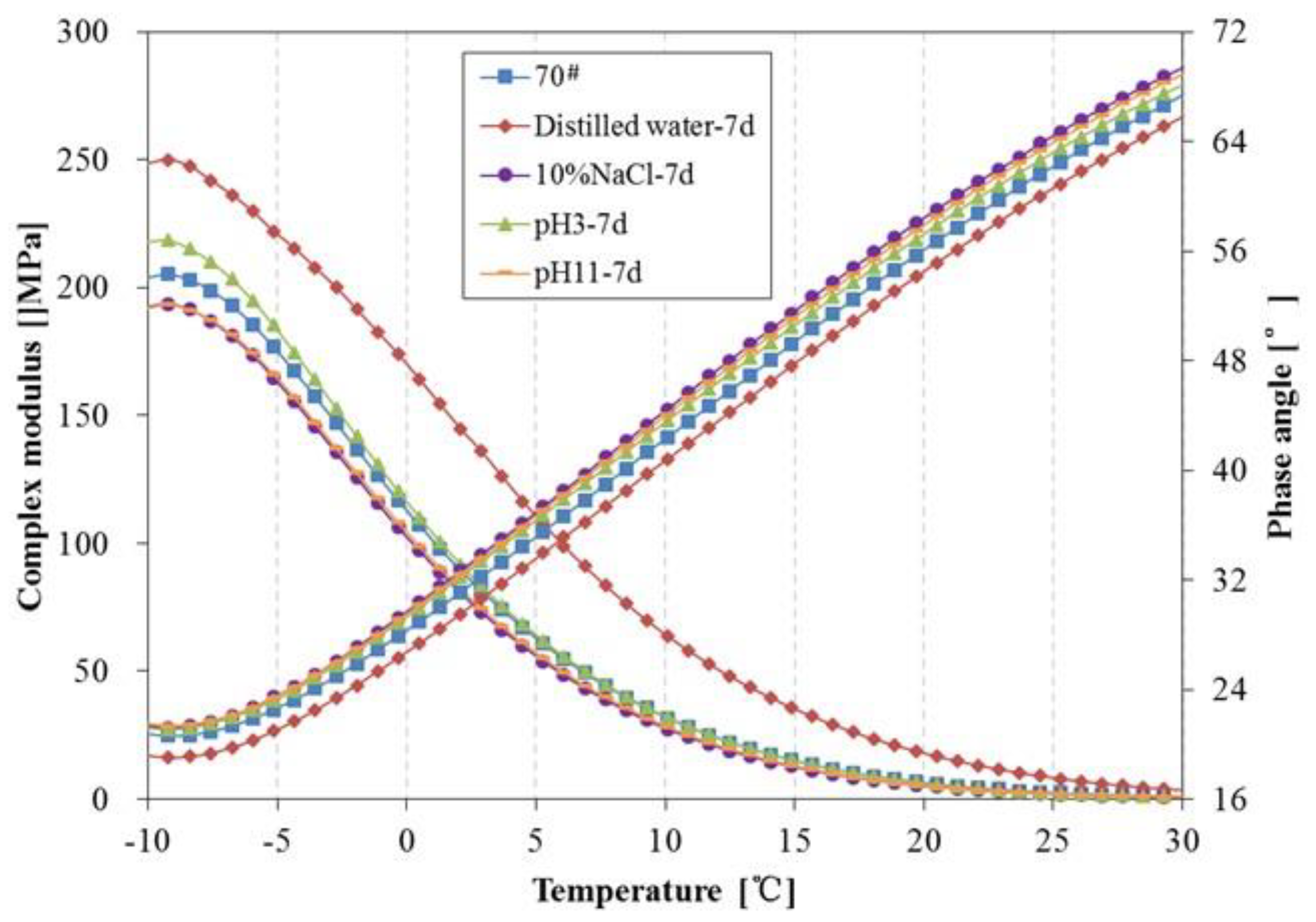
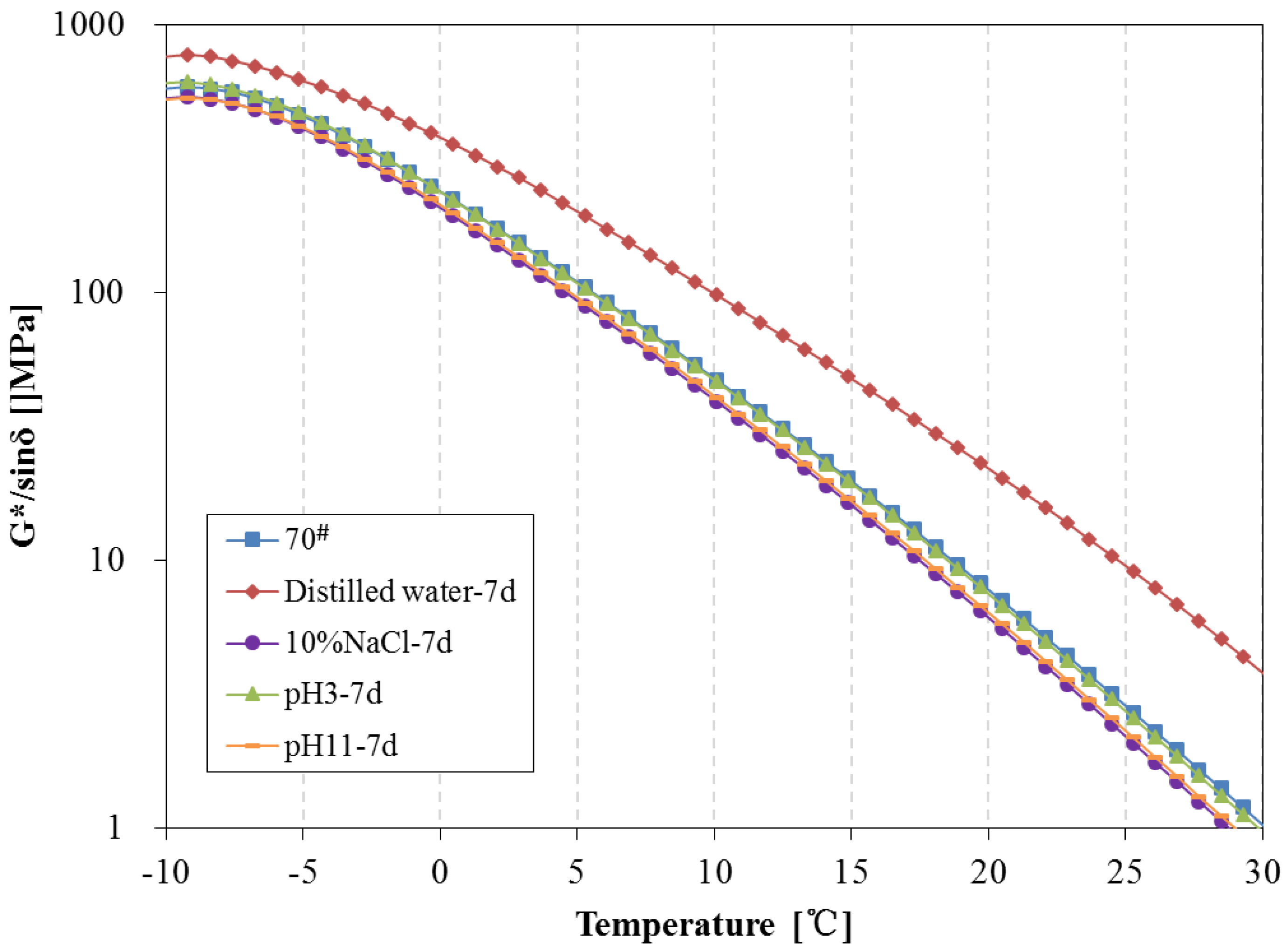
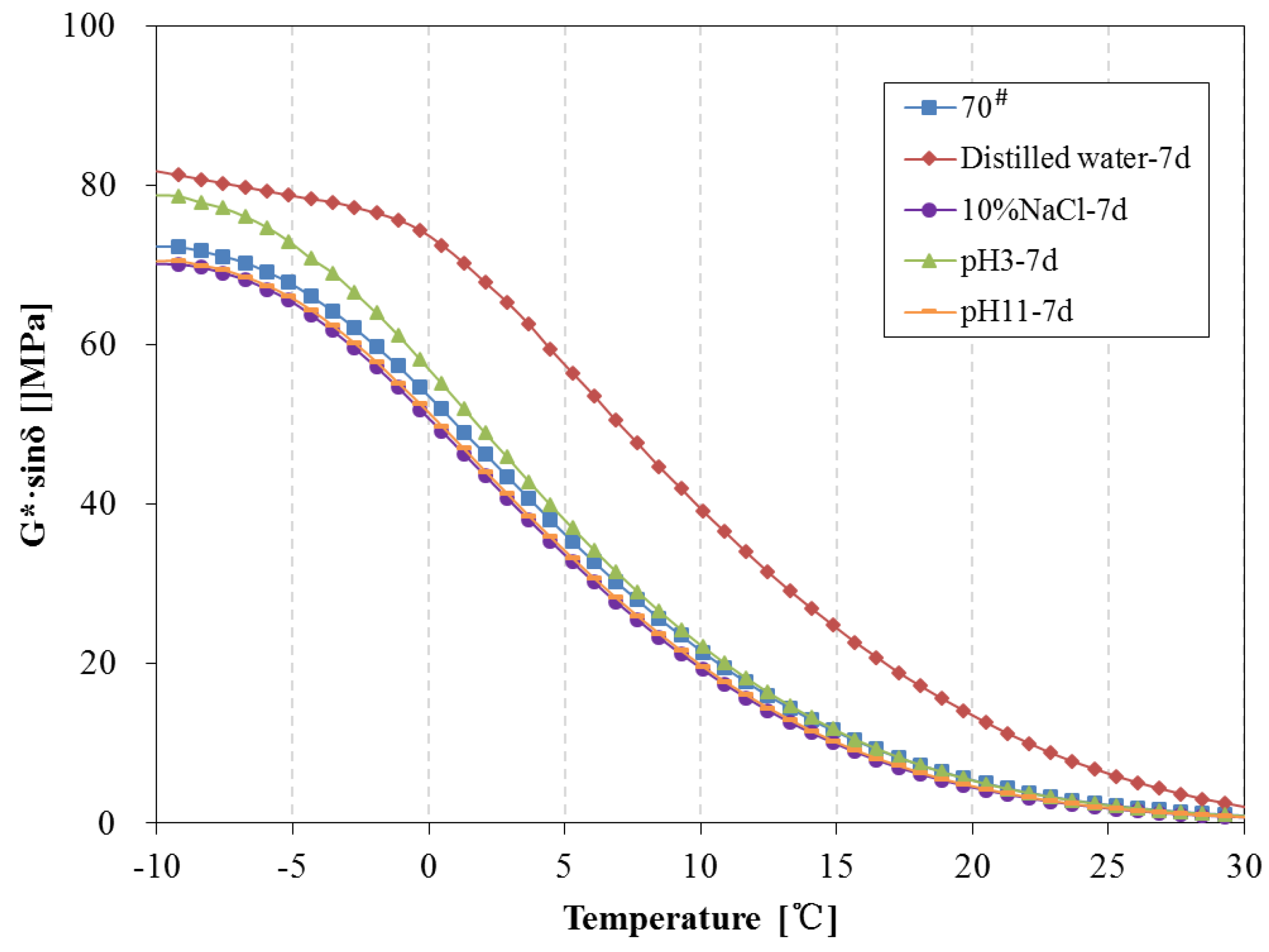
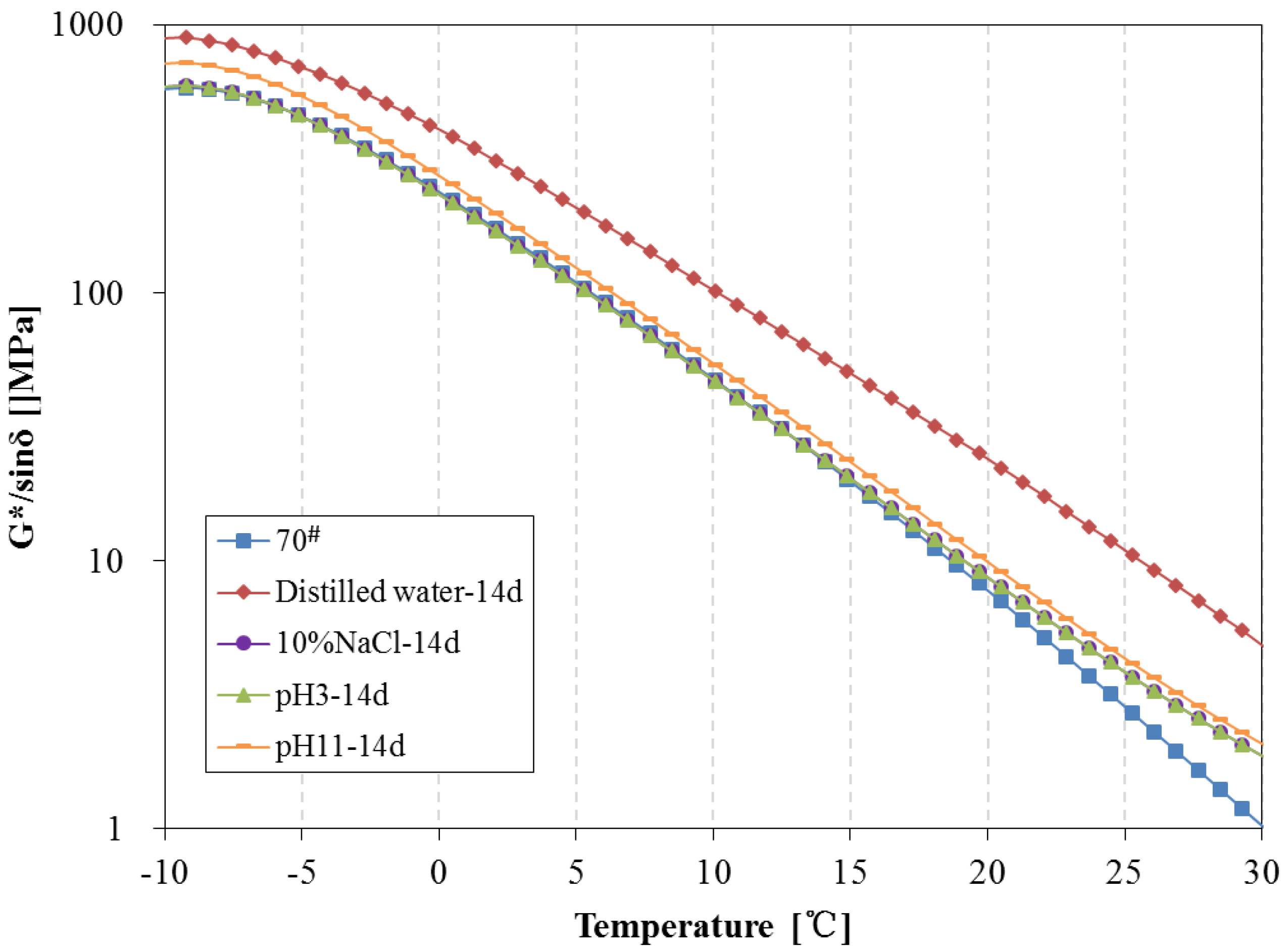
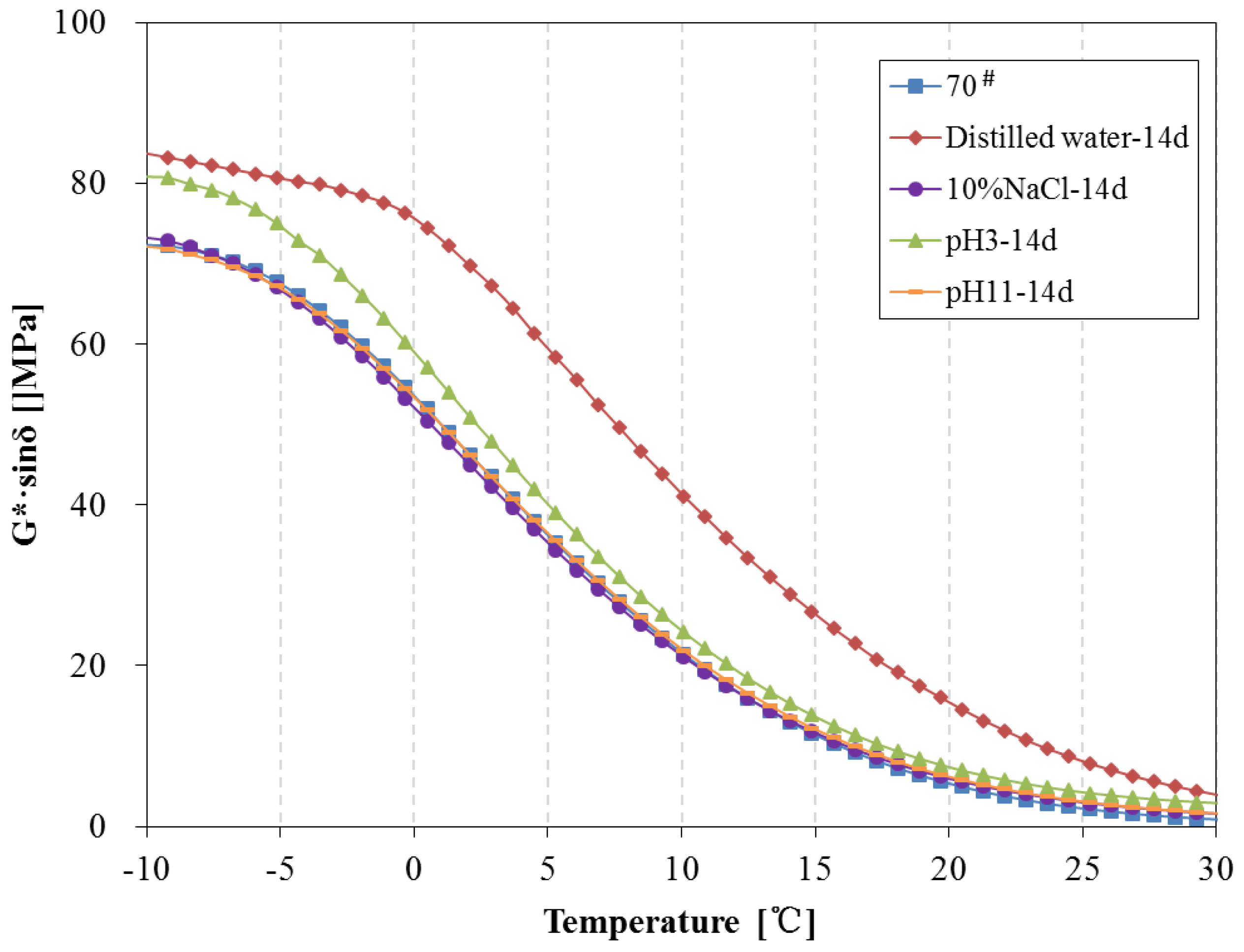
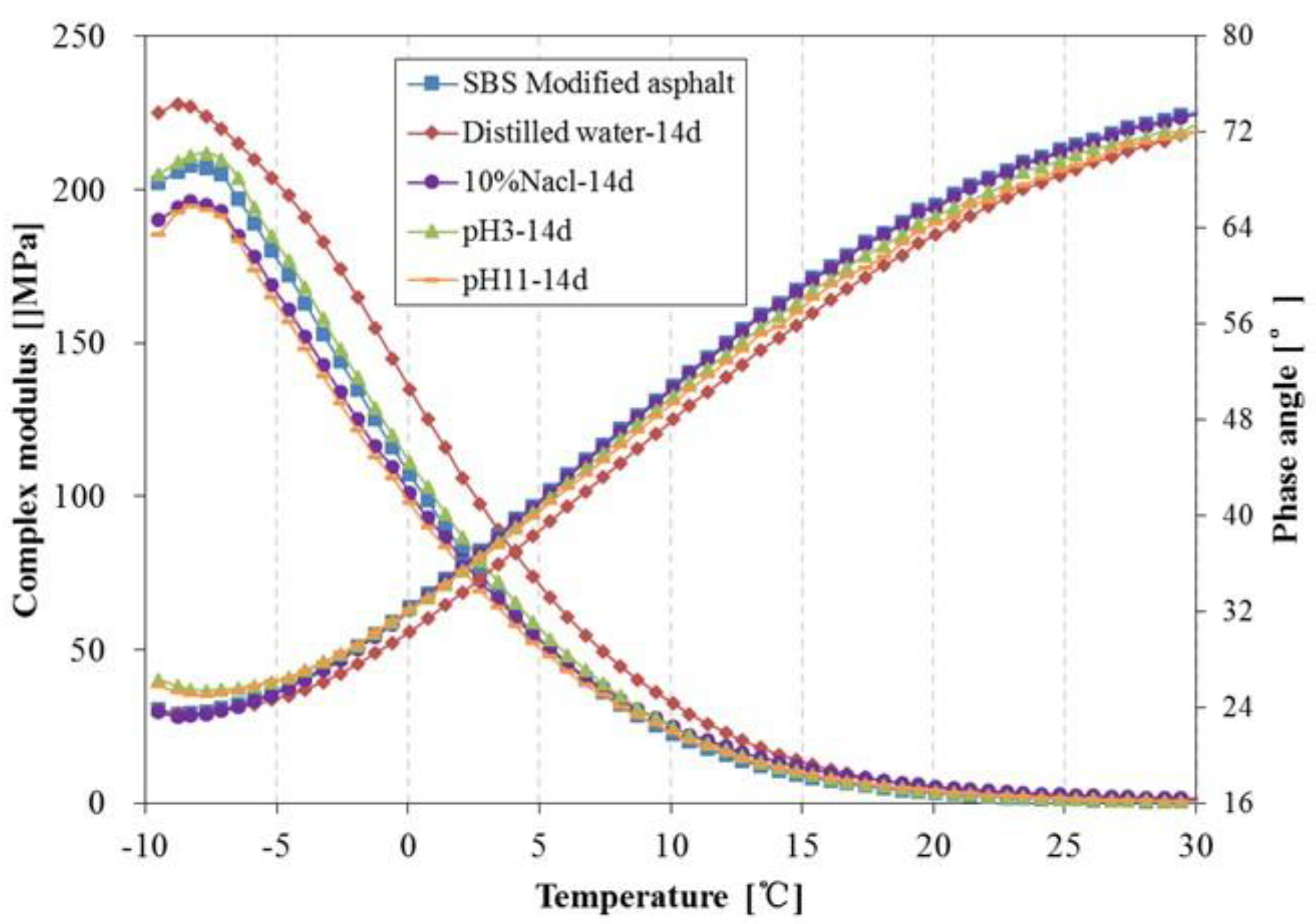
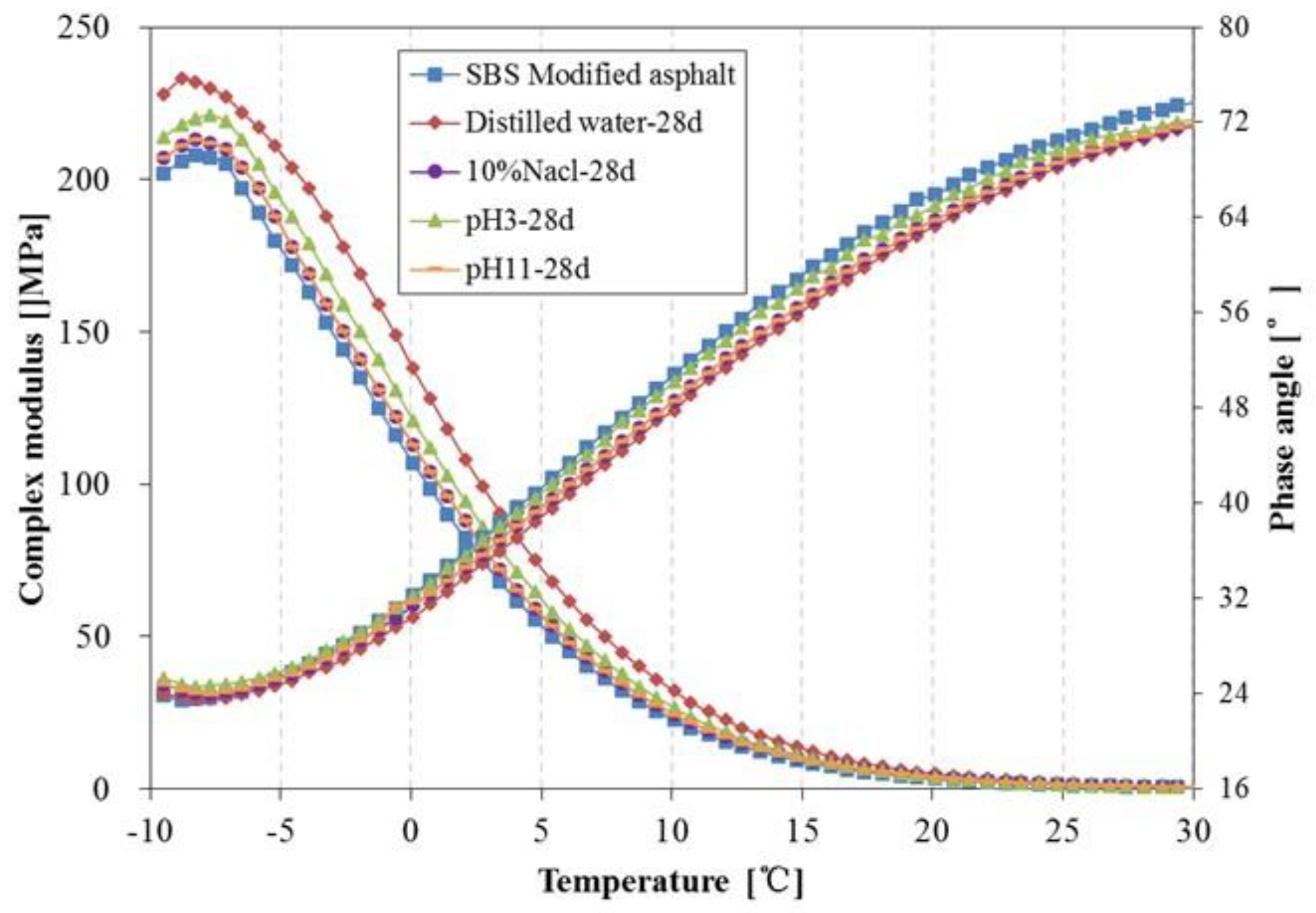
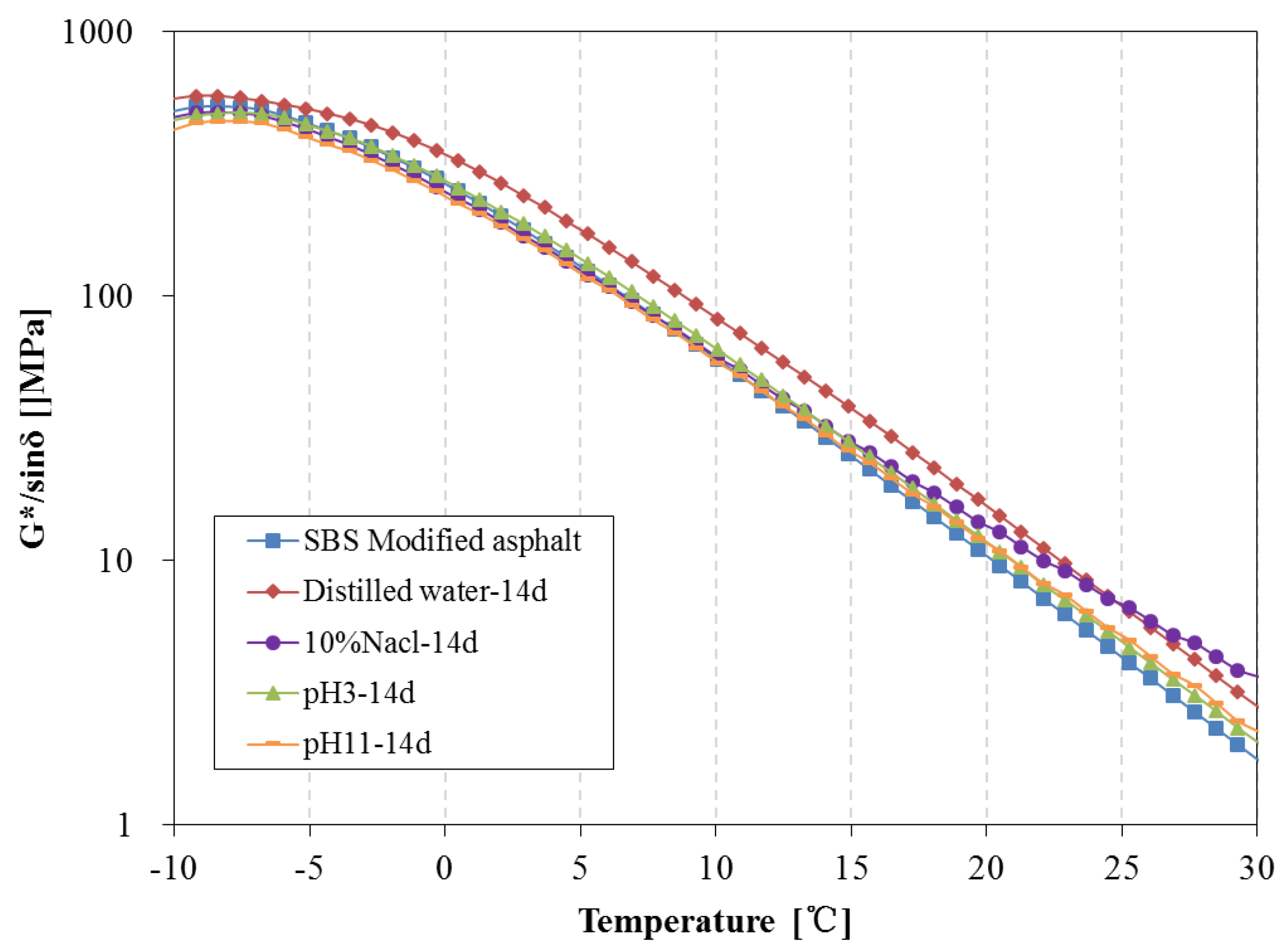
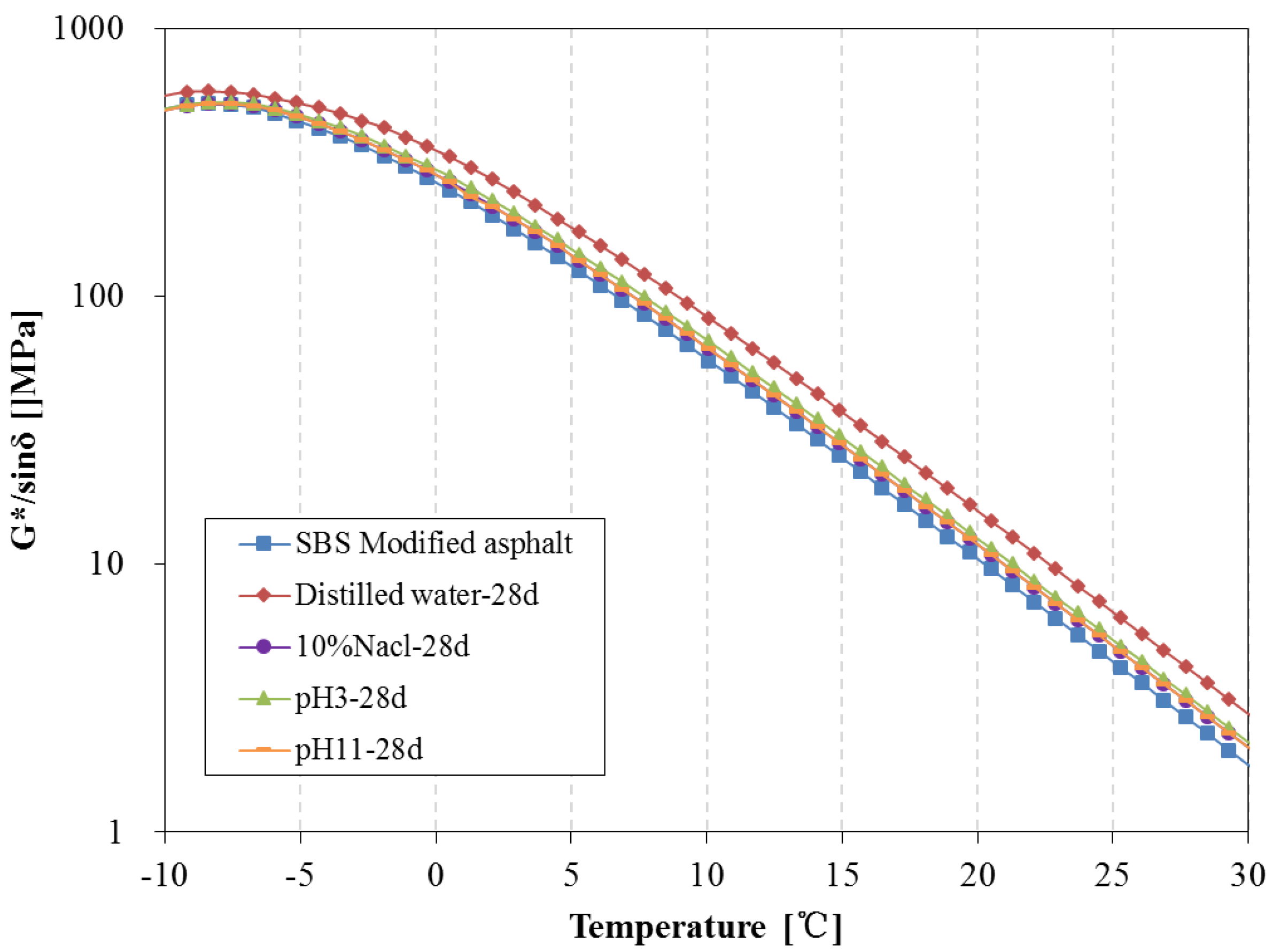
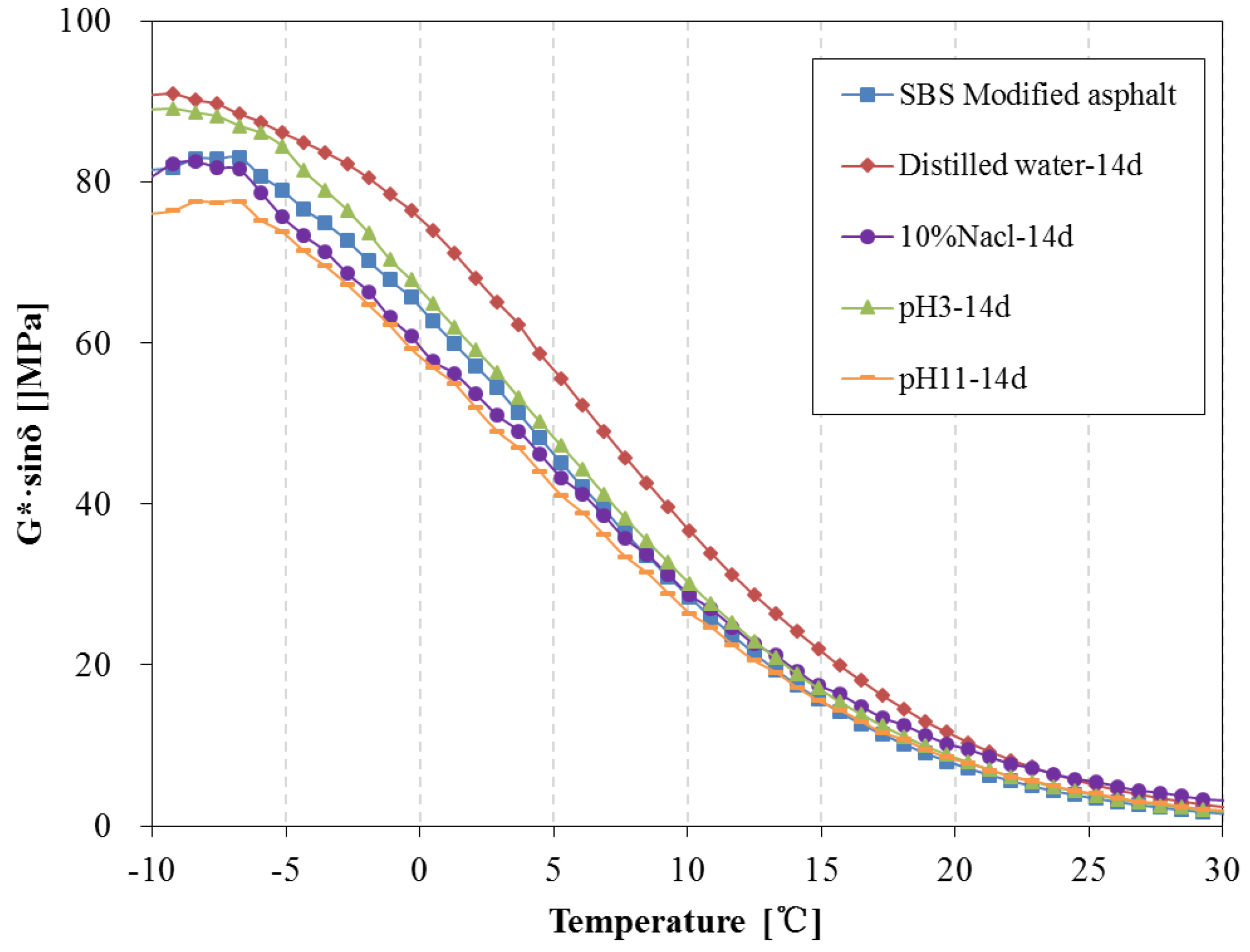
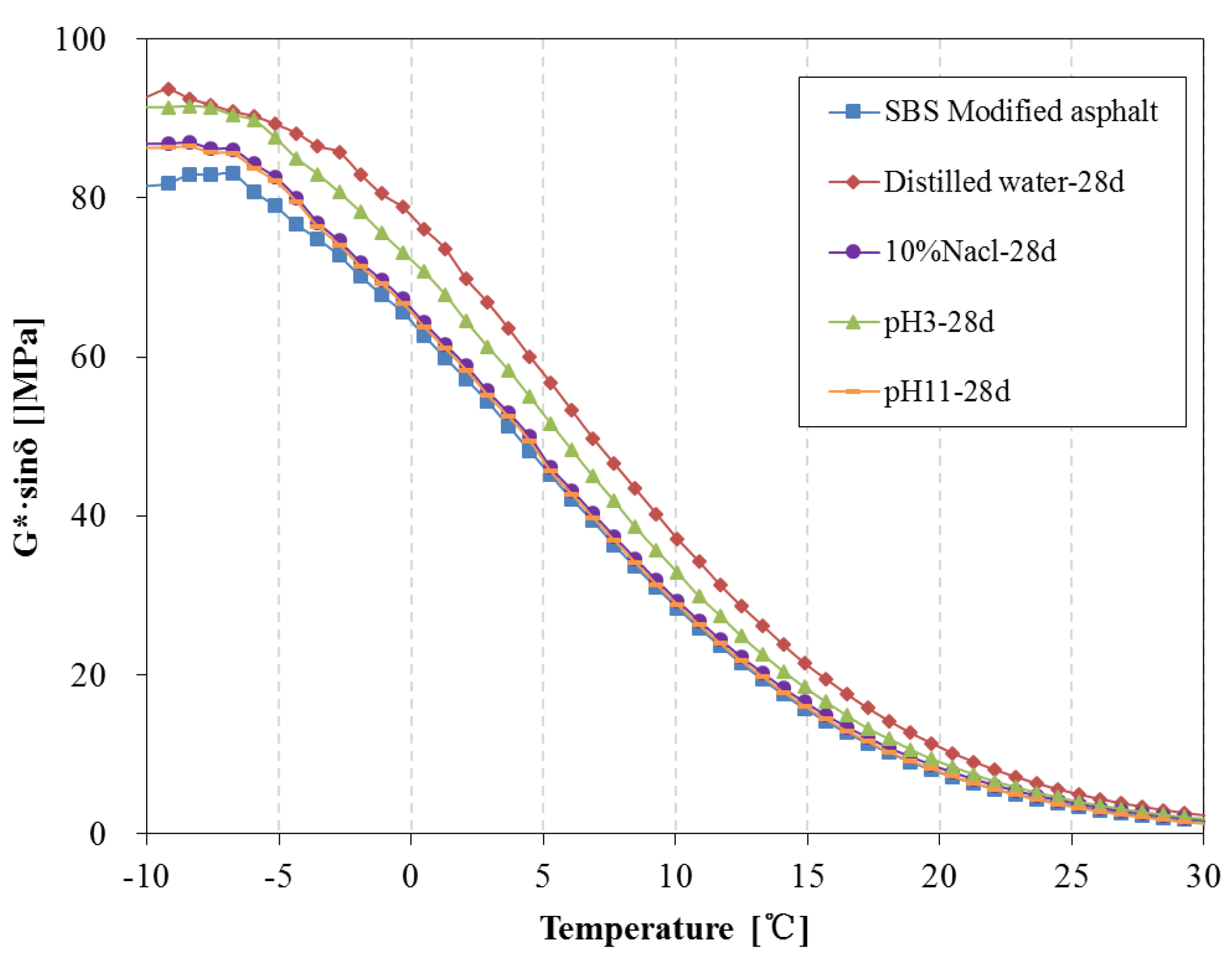
3.4. Rheological Properties Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Asphalt Pavement Association. Black and Green: Sustainable Asphalt, Now and Tomorrow; Recycled Materials: Lanham, MD, USA, 2016. [Google Scholar]
- Hung, A.M.; Goodwin, A.; Fini, E.H. Effects of water exposure on bitumen surface microstructure. Constr. Build. Mater. 2017, 135, 682–688. [Google Scholar] [CrossRef]
- Behiry, E.M. Laboratory evaluation of resistance to moisture damage in asphalt mixtures. Ain Shams Eng. J. 2013, 4, 351–363. [Google Scholar] [CrossRef]
- Noguera, J.A.H.; Quintana, H.A.R.; Gómez, W.D.F. The influence of water on the oxidation of asphalt cements. Constr. Build. Mater. 2014, 71, 451–455. [Google Scholar] [CrossRef]
- Ma, T.; Huang, X.; Mahmoud, E.; Garibaldy, E. Effect of moisture on the aging behavior of asphalt binder. Int. J. Miner. Metall. Mater. 2011, 18, 460–466. [Google Scholar] [CrossRef]
- Kang, A.H.; Zhou, X.; Mei-Ping, W.U.; Sun, L.J. Aging of TOR asphalt rubber in combination of environmental factors. J. Nanjing Univ. Sci. Technol. 2012, 36, 724–728. [Google Scholar]
- Liu, K.; Deng, L.; Zheng, J. Nanoscale study on water damage for different warm mix asphalt binders. Int. J. Pavement Res. Technol. 2016, 9, 405–413. [Google Scholar] [CrossRef]
- López-Montero, T.; Miró, R. Differences in cracking resistance of asphalt mixtures due to ageing and moisture damage. Constr. Build. Mater. 2016, 112, 299–306. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Cheng, G.; Zhang, K. The deterioration and performance improvement of long-term mechanical properties of warm-mix asphalt mixtures under special environmental conditions. Constr. Build. Mater. 2017, 135, 622–631. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Chemo-physical analysis and molecular dynamics (MD) simulation of moisture susceptibility of nano hydrated lime modified asphalt mixtures. Constr. Build. Mater. 2015, 101, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Little, D.N.; Lytton, R.L.; Holste, J.C.; Davis, R.; Brown, S.; Hobson, K.; Dunning, M.; Mcdaniel, B.; Newman, K. Use of surface free energy properties of the asphalt-aggregate system to predict moisture damage potential. Asph. Paving Technol. Assoc. Asph. Paving Technol. Proc. Tech. Sess. 2002, 71, 59–88. [Google Scholar]
- Huang, S.C.; Turner, T.F.; Pauli, A.T.; Miknis, F.P.; Branthaver, J.F.; Robertson, R.E. Evaluation of different techniques for adhesive properties of asphalt-filler systems at interfacial region. J. ASTM Int. 2005, 2, 1–15. [Google Scholar] [CrossRef]
- Kakar, M.R.; Hamzah, M.O.; Valentin, J. A review on moisture damages of hot and warm mix asphalt and related investigations. J. Clean. Prod. 2015, 99, 39–58. [Google Scholar] [CrossRef]
- Feng, D.; Yi, J.; Wang, D.; Chen, L. Impact of salt and freeze–thaw cycles on performance of asphalt mixtures in coastal frozen region of China. Cold Reg. Sci. Technol. 2010, 62, 34–41. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, J.; Liu, Y.; Qiang, X.; Feng, L. Influence of freeze–thaw cycles on properties of asphalt-modified epoxy repair materials. Constr. Build. Mater. 2013, 41, 580–585. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, J. Influence of coastal area salinity on road surface resistance to permanent deformation performance. J. Dalian Jiaotong Univ. 2016, 37, 69–72. [Google Scholar]
- Ma, Q.; Wu, J.; Qin, K. Tests and analyses of the influence of chlorine salt on freezing-thawing splitting tensile strength of asphalt concrete. J. Glaciol. Geocryol. 2013, 35, 1202–1208. [Google Scholar]
- Feng, X.J.; Tang, X.; Xiong, X. Study on influence of acid rain on pavement performances of asphalt mixtures. J. Wuhan Univ. Technol. 2015, 37, 39–43. [Google Scholar]
- Zhang, Q.; Hu, J.R.; Peng, Y.H. Analysis of chemical mechanism for aggregates corroded by acid rain in asphalt mixtures. Highway 2004, 2, 104–106. [Google Scholar]
- Dendooven, L.; Alcántara-Hernández, R.J.; Valenzuela-Encinas, C.; Luna-Guido, M.; Perez-Guevara, F.; Marsch, R. Dynamics of carbon and nitrogen in an extreme alkaline saline soil: A review. Soil. Biol. Biochem. 2010, 42, 865–877. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Shao, M. Investigation on snow-melting performance of asphalt mixtures incorporating with salt-storage aggregates. Constr. Build. Mater. 2017, 142, 187–198. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, X.J.; Li, Y.L.; Tong, S.J. the simulation study on effect of acid rain on the strength and void rate of asphalt mixture. J. China Foreign Highw. 2005, 25, 78–80. [Google Scholar]
- Zhang, Q.; Meng, X.R.; Yan, W. Analysis of the chemical mechanism of influence of precipitation on components of asphalt binder. J. Xian Univ. Archit. Technol. 2010, 42, 669–673. [Google Scholar]
- Liu, X.; Wu, S.; Liu, G.; Li, L. Effect of ultraviolet aging on rheology and chemistry of LDH-modified bitumen. Materials 2015, 8, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.; Zhai, Y.; Liao, K.; Yang, P.; Yan, F.; Wei, Y. A study on change of family composition and properties of Liaoshu paving asphalt on aging. Petrol. Sci. Technol. 2001, 19, 651–660. [Google Scholar]
- Ouyang, C.; Wang, S.; Zhang, Y.; Zhang, Y. Improving the aging resistance of asphalt by addition of Zinc dialkyldithiophosphate. Fuel 2006, 85, 1060–1066. [Google Scholar] [CrossRef]
- Redelius, P.; Soenen, H. Relation between bitumen chemistry and performance. Fuel 2015, 140, 34–43. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Fourier transform infrared spectroscopy characterization of aging-related properties of original and nano-modified asphalt binders. Constr. Build. Mater. 2015, 101, 1078–1087. [Google Scholar] [CrossRef]
- Tarefder, R.A.; Arisa, I. Molecular dynamic simulations for determining change in thermodynamic properties of asphaltene and resin because of aging. Energy Fuels 2011, 25, 2211–2222. [Google Scholar] [CrossRef]
- Lu, X.; Isacsson, U. Effect of ageing on bitumen chemistry and rheology. Constr. Build. Mater. 2002, 16, 15–22. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Ali, M.F. Studies on the aging behavior of the arabian asphalts. Fuel 1999, 78, 1005–1015. [Google Scholar] [CrossRef]
- Caro, S.; Diaz, A.; Rojas, D.; Nuñez, H. A Micromechanical model to evaluate the impact of air void content and connectivity in the oxidation of asphalt mixtures. Constr. Build. Mater. 2014, 61, 181–190. [Google Scholar] [CrossRef]

| Properties | Units | 70# Asphalt | SBS Modified Asphalt | Test Specification |
|---|---|---|---|---|
| Penetration (25 °C, 10 g, 5 s) | 0.1 mm | 68 | 56 | ASTM D5-61 |
| Softening point | °C | 47.2 | 74.0 | ASTM D36-26 |
| Ductility | cm | 63.2 | 68.0 | ASTM D113 |
| Sample | Saturates (%) | Aromatics (%) | Resins (%) | Asphaltenes (%) | |
|---|---|---|---|---|---|
| Original sample | 15.35 | 38.88 | 34.44 | 11.33 | |
| Distilled water | 7 days | 15.11 | 38.77 | 34.15 | 11.97 |
| 14 days | 15.02 | 37.79 | 34.68 | 12.51 | |
| 10% NaCl salt solution | 7 days | 15.57 | 39.04 | 32.83 | 12.56 |
| 14 days | 15.36 | 37.56 | 33.02 | 14.06 | |
| pH3 acid solution | 7 days | 11.00 | 39.28 | 31.78 | 17.94 |
| 14 days | 12.29 | 38.32 | 30.49 | 18.89 | |
| pH11 alkali solution | 7 days | 16.73 | 39.03 | 27.70 | 16.54 |
| 14 days | 13.57 | 38.64 | 28.11 | 19.68 | |
| Results | Original Sample | Distilled Water | 10% NaCl Saline Solution | pH3 Acid Solution | pH11 Alkaline Solution | ||||
|---|---|---|---|---|---|---|---|---|---|
| 7 days | 14 days | 7 days | 14 days | 7 days | 14 days | 7 days | 14 days | ||
| Carbonyl index | 0.0003 | 0.0009 | 0.0012 | 0.0009 | 0.0051 | 0.0106 | 0.0121 | 0.0148 | 0.0151 |
| Sulphoxide index | 0.0348 | 0.0387 | 0.0412 | 0.0567 | 0.0598 | 0.0651 | 0.0662 | 0.0717 | 0.0778 |
| Results | Original Sample | Distilled Water | 10% NaCl Saline Solution | pH3 Acid Solution | pH11 Alkaline Solution | ||||
|---|---|---|---|---|---|---|---|---|---|
| 14 days | 28 days | 14 days | 28 days | 14 days | 28 days | 14 days | 28 days | ||
| Carbony1 index | 0.0108 | 0.0109 | 0.0135 | 0.0110 | 0.0135 | 0.0117 | 0.0201 | 0.0129 | 0.0216 |
| Sulphoxide index | 0.0198 | 0.0202 | 0.0218 | 0.0220 | 0.0259 | 0.0223 | 0.0257 | 0.0233 | 0.0265 |
| Butadiene index | 0.0252 | 0.0245 | 0.0237 | 0.0229 | 0.0229 | 0.0228 | 0.0205 | 0.0220 | 0.0207 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, L.; Zhang, X.; Wu, S.; Ye, Y.; Li, Y. Influence of Water Solute Exposure on the Chemical Evolution and Rheological Properties of Asphalt. Materials 2018, 11, 983. https://doi.org/10.3390/ma11060983
Pang L, Zhang X, Wu S, Ye Y, Li Y. Influence of Water Solute Exposure on the Chemical Evolution and Rheological Properties of Asphalt. Materials. 2018; 11(6):983. https://doi.org/10.3390/ma11060983
Chicago/Turabian StylePang, Ling, Xuemei Zhang, Shaopeng Wu, Yong Ye, and Yuanyuan Li. 2018. "Influence of Water Solute Exposure on the Chemical Evolution and Rheological Properties of Asphalt" Materials 11, no. 6: 983. https://doi.org/10.3390/ma11060983





