Trial of an All-Ceramic SnO2 Gas Sensor Equipped with CaCu3Ru4O12 Heater and Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of CaCu3Ru4O12-Based Conducting Paste
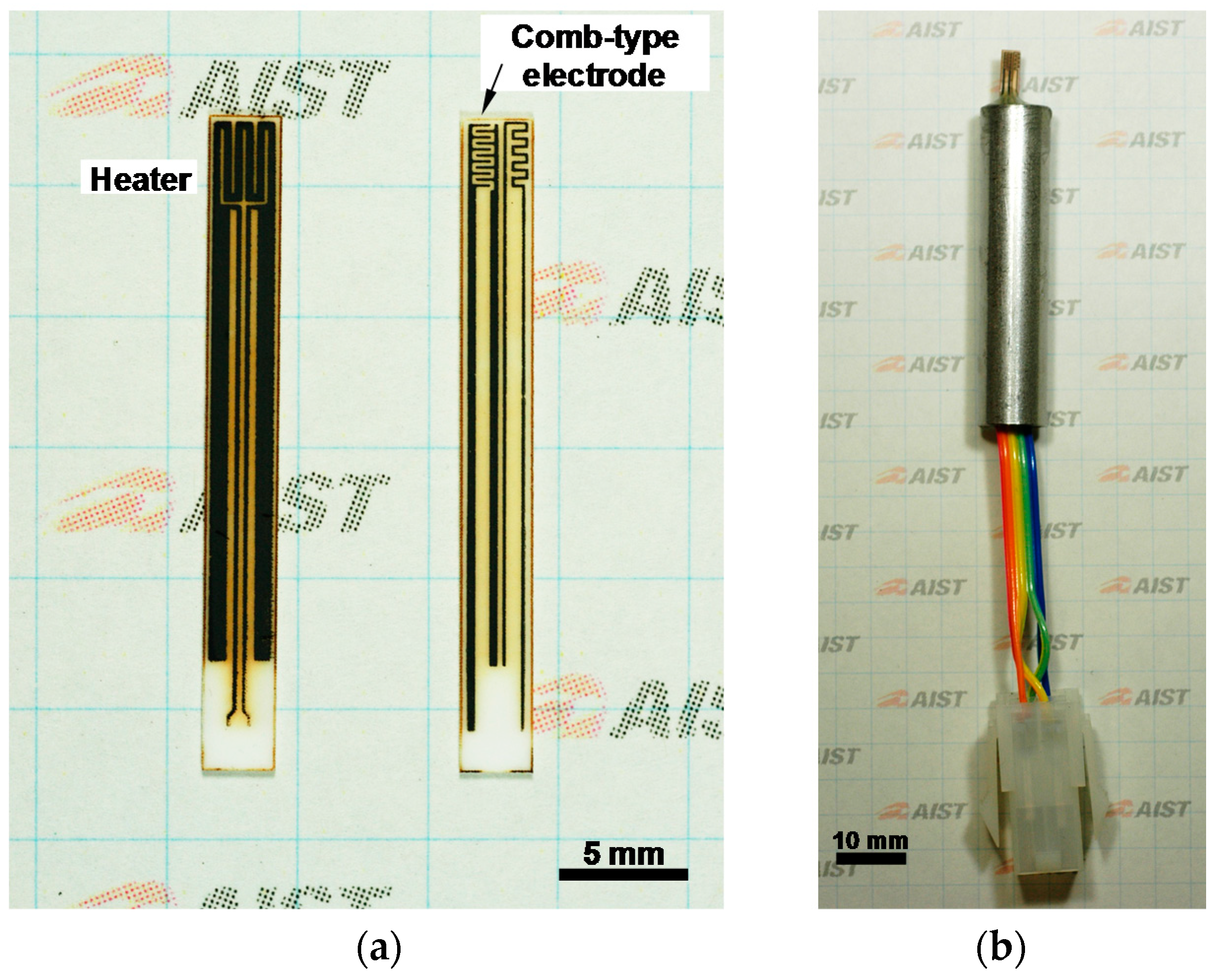
2.2. Fabrication of All-Ceramic Gas Sensor
2.3. Evaluation of Materials and Sensor
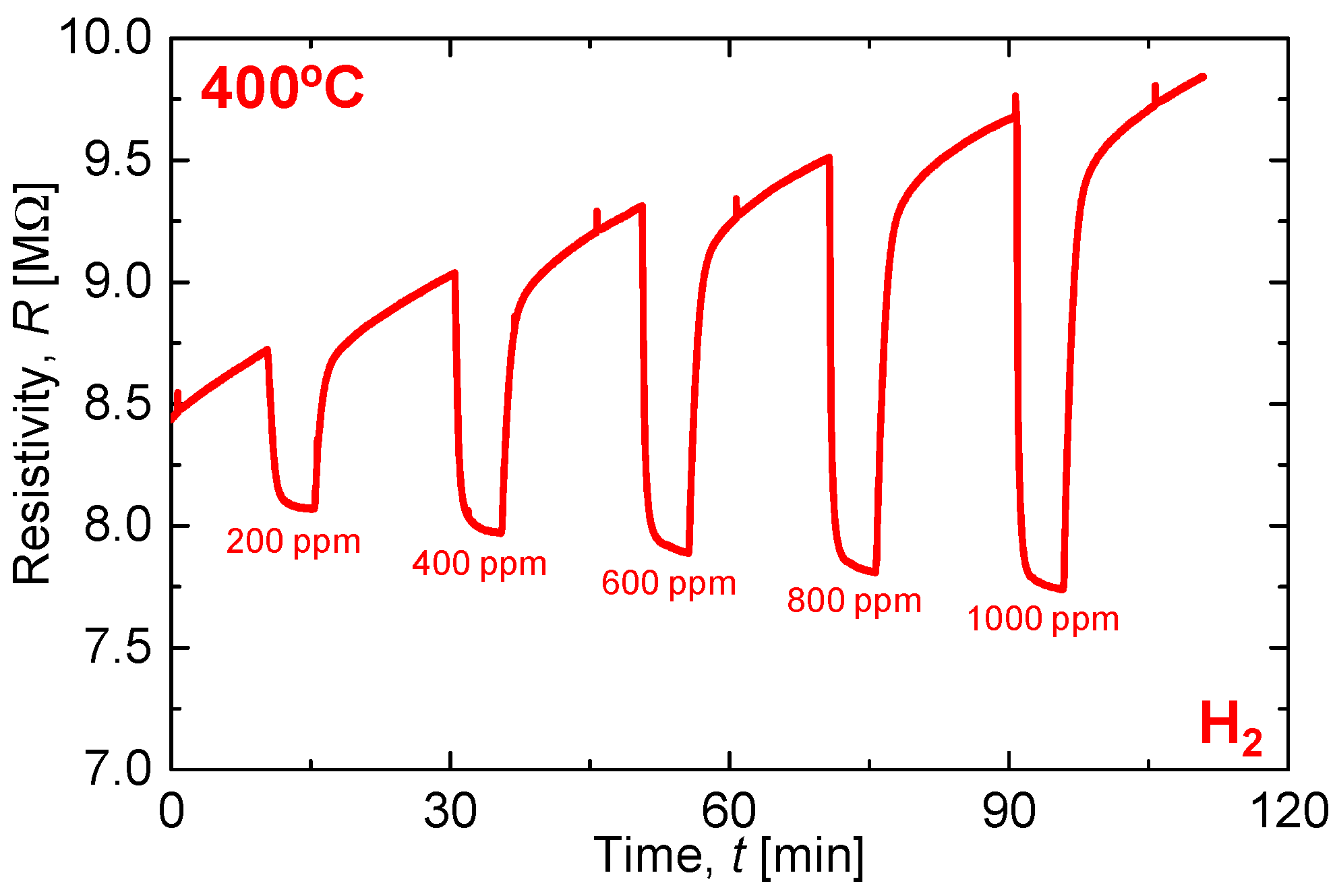
3. Results and Discussion
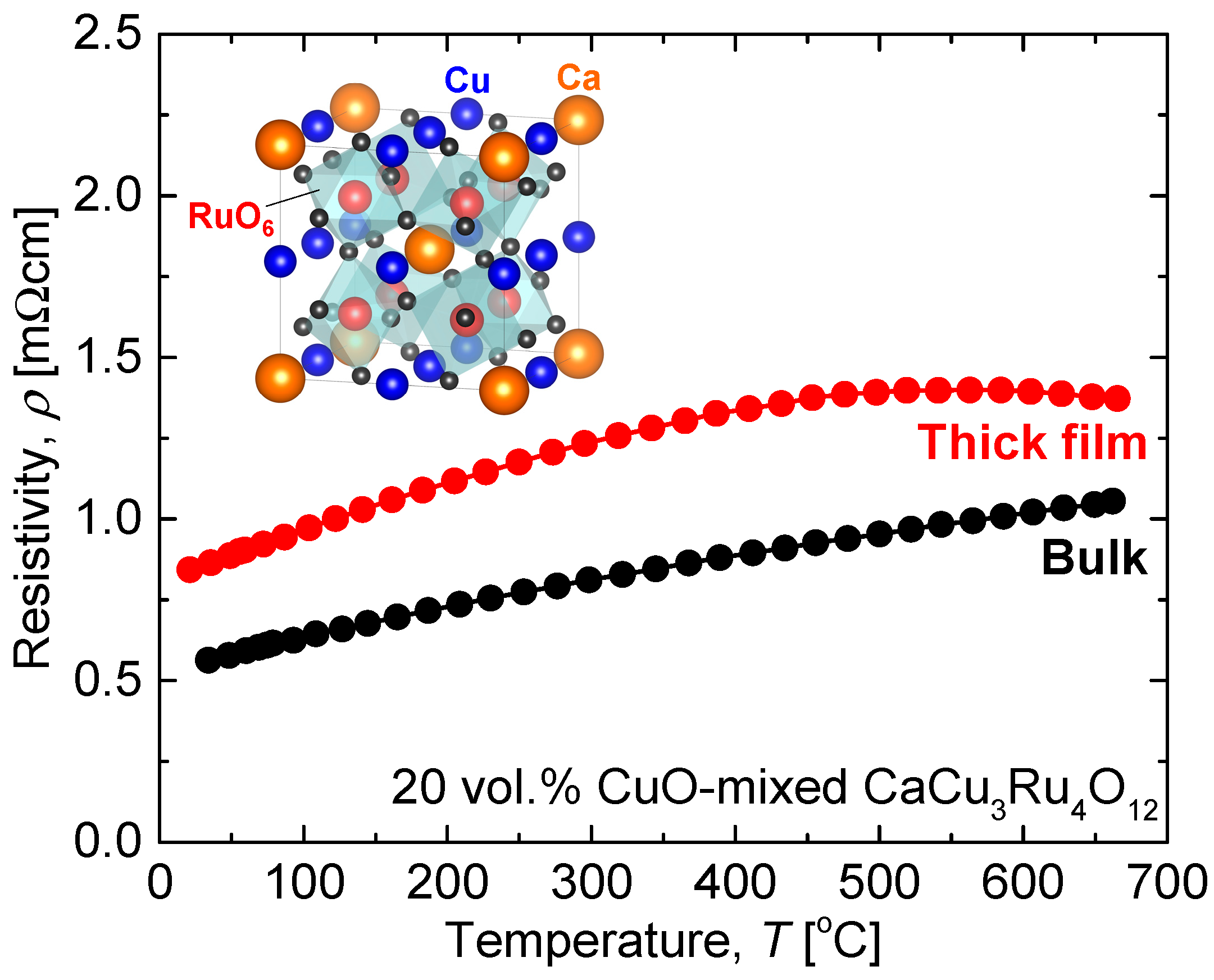
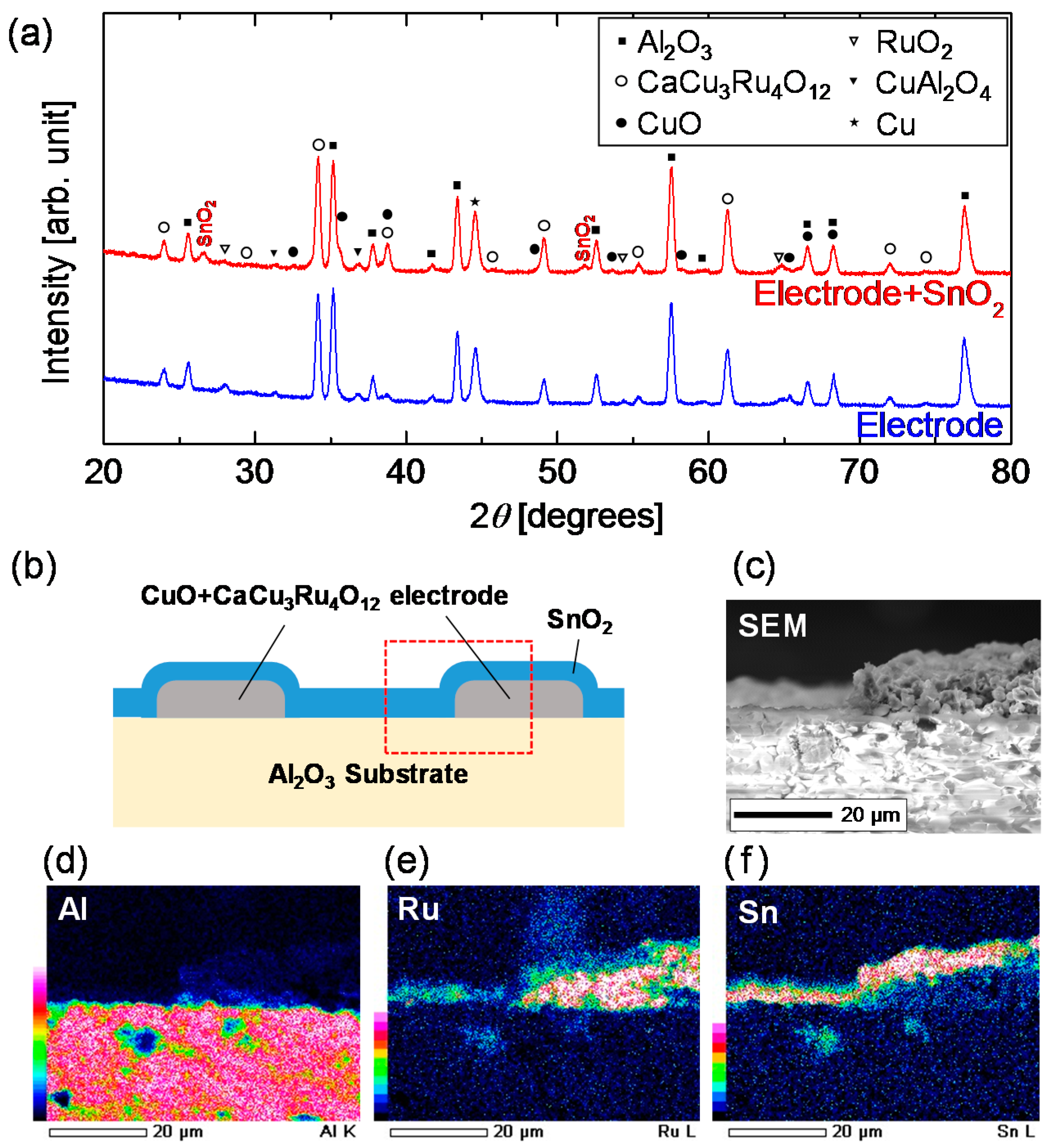
3.1. Chemical Stability of CaCu3Ru4O12
3.2. Thermal Stability of CaCu3Ru4O12
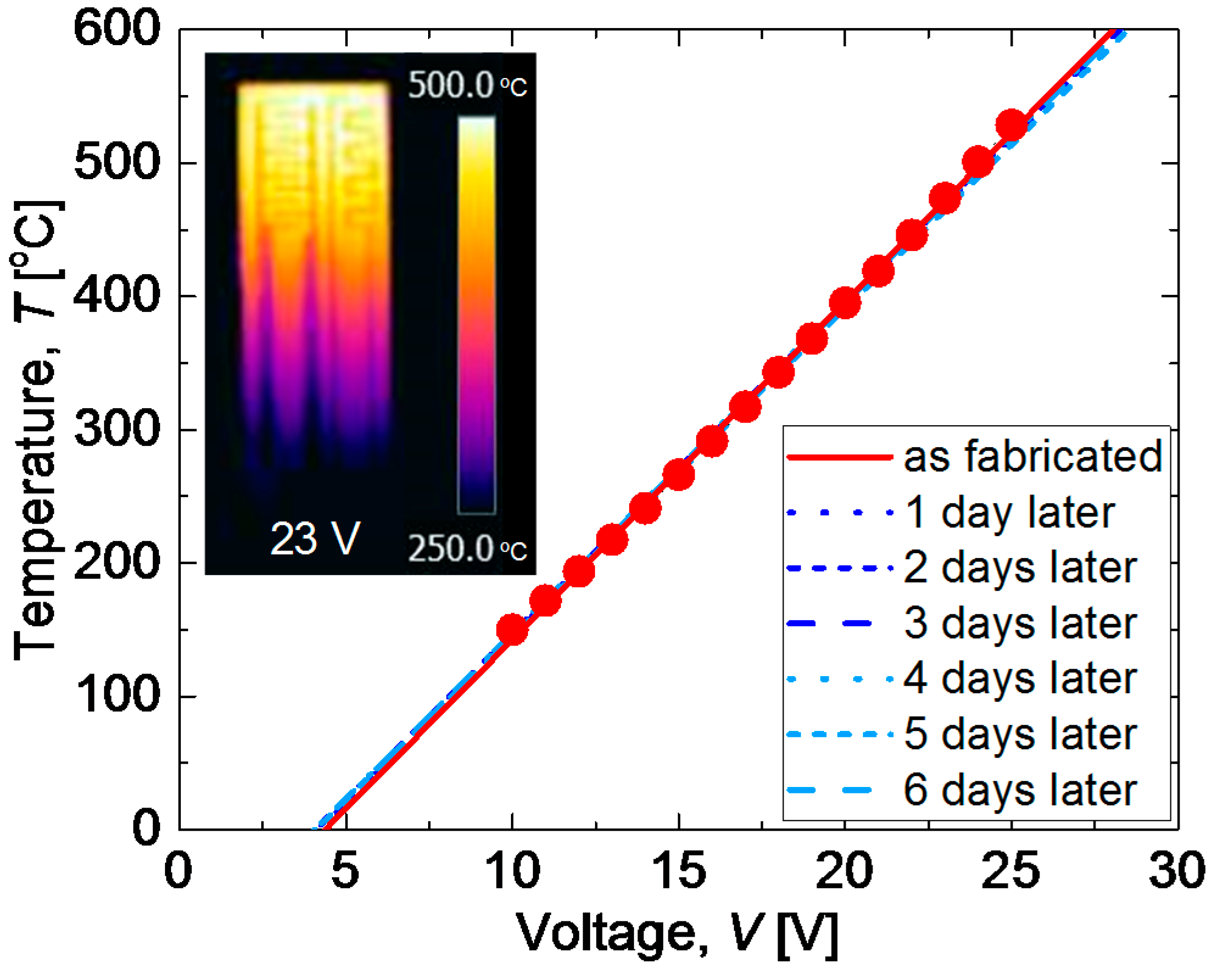
3.3. Demonstration of All-Ceramics Gas Sensor
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Itoh, T.; Izu, N.; Akamatsu, T.; Shin, W.; Miki, Y.; Hirose, Y. Elimination of Flammable Gas Effects in Cerium Oxide Semiconductor-Type Resistive Oxygen Sensors for Monitoring Low Oxygen Concentrations. Sensors 2015, 15, 9427–9437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, T.; Miwa, T.; Tsuruta, A.; Akamatsu, T.; Izu, N.; Shin, W.; Park, J.; Hida, T.; Eda, T.; Setoguchi, Y. Development of an Exhaled Breath Monitoring System with Semiconductive Gas Sensors, a Gas Condenser Unit, and Gas Chromatograph Columns. Sensors 2016, 16, 1891. [Google Scholar] [CrossRef] [PubMed]
- Simon, I.; Bârsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B 2001, 73, 1–26. [Google Scholar] [CrossRef]
- Burgués, J.; Marco, S. Low Power Operation of Temperature-Modulated Metal Oxide Semiconductor Gas Sensors. Sensors 2018, 18, 339. [Google Scholar] [CrossRef] [PubMed]
- Bogue, R. Towards the trillion sensors market. Sens. Rev. 2014, 34, 137–142. [Google Scholar] [CrossRef]
- Güntner, A.T.; Koren, V.; Chikkadi, K.; Righettoni, M.; Pratsinis, S.E. E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens. 2016, 1, 528–535. [Google Scholar] [CrossRef]
- Shin, W.; Nishibori, M.; Houlet, L.F.; Itoh, T.; Izu, N.; Matsubara, I. Fabrication of thermoelectric gas sensors on micro-hotplates. Sens. Actuators B 2009, 139, 340–345. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.X.; Wu, X.D.; Foltyn, S.R.; Findikoglu, A.T.; Tiwari, P.; Zheng, J.P.; Jow, T.R. Heteroepitaxial growth of highly conductive metal oxide RuO2 thin films by pulsed laser deposition. Appl. Phys. Lett. 1995, 67, 1677–1679. [Google Scholar] [CrossRef]
- Ryden, W.; Lawson, A.; Sartain, C. Electrical Transport Properties of IrO2 and RuO2. Phys. Lett. B 1970, 1, 1494–1500. [Google Scholar] [CrossRef]
- Pearsall, T.P.; Lee, C.A. Electronic transport in ReO3: DC conductivity and Hall effect. Phys. Rev. B 2015, 91, 045106. [Google Scholar] [CrossRef]
- Hébert, S.; Daou, R.; Maignan, A. Thermopower in the quadruple perovskite ruthenates. Phys. Rev. B 1974, 10, 2190. [Google Scholar] [CrossRef]
- Kobayashi, W.; Terasaki, I.; Takeya, J.; Tsukada, I.; Ando, Y. A Novel Heavy-Fermion State in CaCu3Ru4O12. J. Phys. Soc. Jpn. 2004, 73, 2373–2376. [Google Scholar] [CrossRef]
- Tsuruta, A.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. High electrical conductivity of composite ceramics consisting of insulating oxide and ordered perovskite conducting oxide. Phys. Status Solidi A 2017, 214, 1600968. [Google Scholar] [CrossRef]
- Tsuruta, A.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. Element Strategy Using Ru-Mn Substitution in CuO-CaCu3Ru4O12 Composite Ceramics with High Electrical Conductivity. Crystals 2017, 7, 213. [Google Scholar] [CrossRef]
- Ebbinghaus, S.G.; Weidenkaff, A.; Cava, R.J. Structural Investigations of ACu3Ru4O12 (A = Na, Ca, Sr, La, Nd)—Comparison between XRD-Rietveld and EXAFS Results. J. Solid State Chem. 2002, 167, 126–136. [Google Scholar] [CrossRef]
- Todd, R.I.; Zapata-Solvas, E.; Bonilla, R.S.; Sneddon, T.; Wilshaw, P.R. Electrical characteristics of flash sintering: Thermal runaway of Joule heating. J. Eur. Ceram. Soc. 2015, 35, 1865–1877. [Google Scholar] [CrossRef]
- Okamoto, T.; Huybrechts, B.; Takata, M. Electric Field Sensitive Moving Hot Spot in GdBa2Cu3O7-δ Ceramics. Jpn. J. Appl. Phys. 1994, 33, L1212–L1214. [Google Scholar] [CrossRef]
- Itoh, T.; Nakashima, T.; Akamatsu, T.; Izu, N.; Shin, W. Nonanal gas sensing properties of platinum, palladium, and gold-loaded tin oxide VOCs sensors. Sens. Actuators B 2013, 187, 135–141. [Google Scholar] [CrossRef]
- Miyazaki, H.; Iwakiri, S.; Hirao, K.; Fukuda, S.; Izu, N.; Yoshizawa, Y.; Hyuga, H. Effect of high temperature cycling on both crack formation in ceramics and delamination of copper layers in silicon nitride active metal brazing substrates. Ceram. Int. 2017, 43, 5080–5088. [Google Scholar] [CrossRef]

| Material | ρ500 (μΩ∙cm) | α30–500 (%/°C) | Cost ($/kg) |
|---|---|---|---|
| Pt | 27.5 | 0.324 | 50,000 |
| CaCu3Ru4O12 | 937.4 | 0.135 | 950 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuruta, A.; Itoh, T.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. Trial of an All-Ceramic SnO2 Gas Sensor Equipped with CaCu3Ru4O12 Heater and Electrode. Materials 2018, 11, 981. https://doi.org/10.3390/ma11060981
Tsuruta A, Itoh T, Mikami M, Kinemuchi Y, Terasaki I, Murayama N, Shin W. Trial of an All-Ceramic SnO2 Gas Sensor Equipped with CaCu3Ru4O12 Heater and Electrode. Materials. 2018; 11(6):981. https://doi.org/10.3390/ma11060981
Chicago/Turabian StyleTsuruta, Akihiro, Toshio Itoh, Masashi Mikami, Yoshiaki Kinemuchi, Ichiro Terasaki, Norimitsu Murayama, and Woosuck Shin. 2018. "Trial of an All-Ceramic SnO2 Gas Sensor Equipped with CaCu3Ru4O12 Heater and Electrode" Materials 11, no. 6: 981. https://doi.org/10.3390/ma11060981






