Nanoscale Electrical Potential and Roughness of a Calcium Phosphate Surface Promotes the Osteogenic Phenotype of Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation and CP Deposition
2.2. Surface Characterization
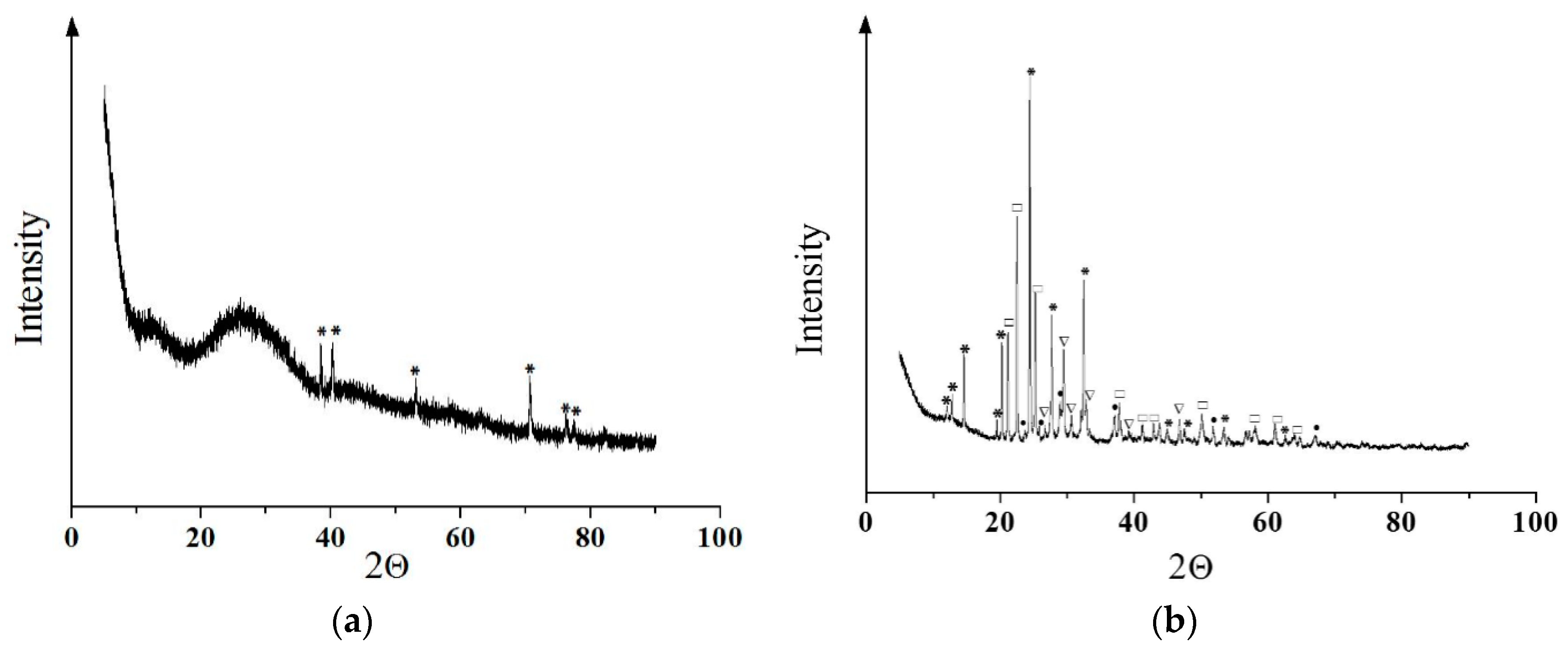
2.2.1. Surface and Microstructure Observation and Phase Composition
2.2.2. Microroughness Measurements
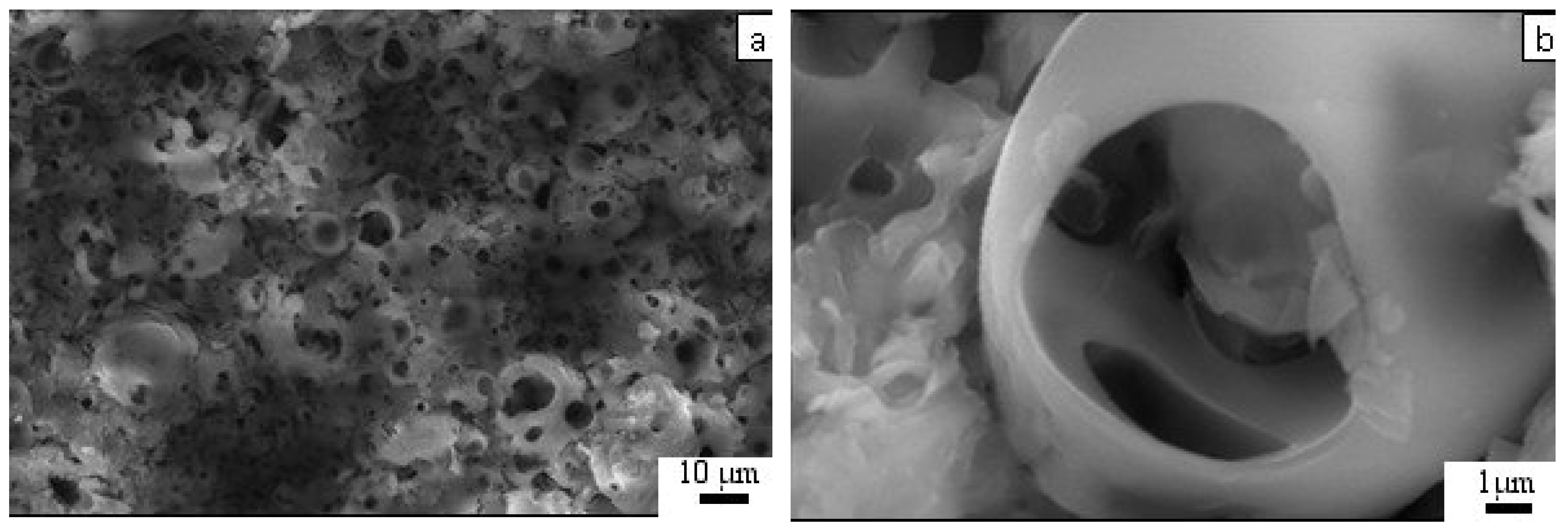
2.2.3. Optical Microscopy and SEM
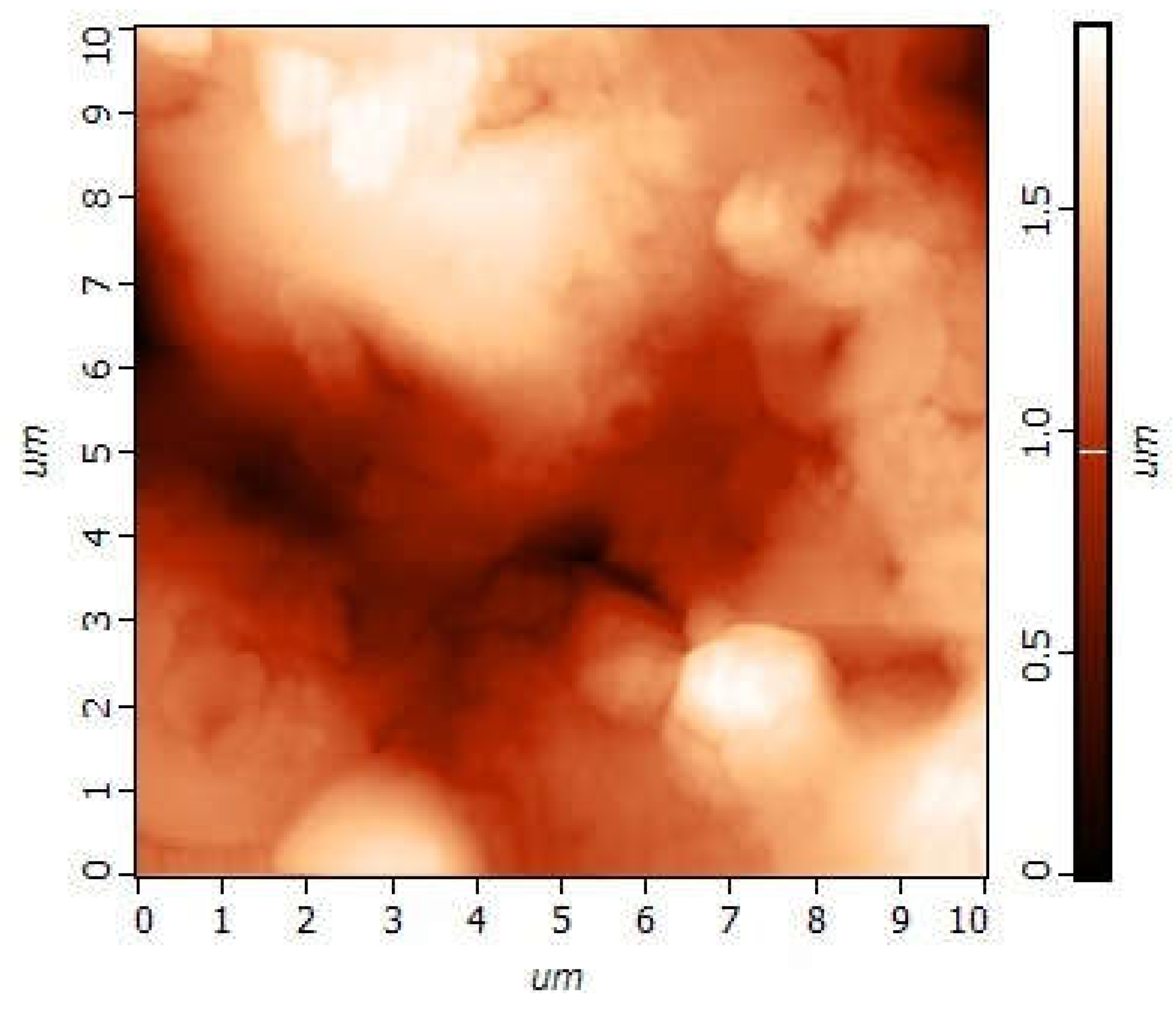
2.2.4. Atomic Force and Kelvin Probe Microscopy
2.3. Surface EP
2.3.1. Surface Electric Charge at the Macroscale
2.3.2. Electron Work Function
2.4. Biological Testing
2.4.1. Cell Culture and In Vitro Staining
2.4.2. Cytochemical Staining of HLPSCs for ALP via the Diazocoupling Technique
2.4.3. Immunocytochemical Detection of OCN in HLPSCs
2.4.4. Computer Morphometry
2.5. Statistical Analysis
3. Results
3.1. AMSC and HLPSC Response to CP Coating
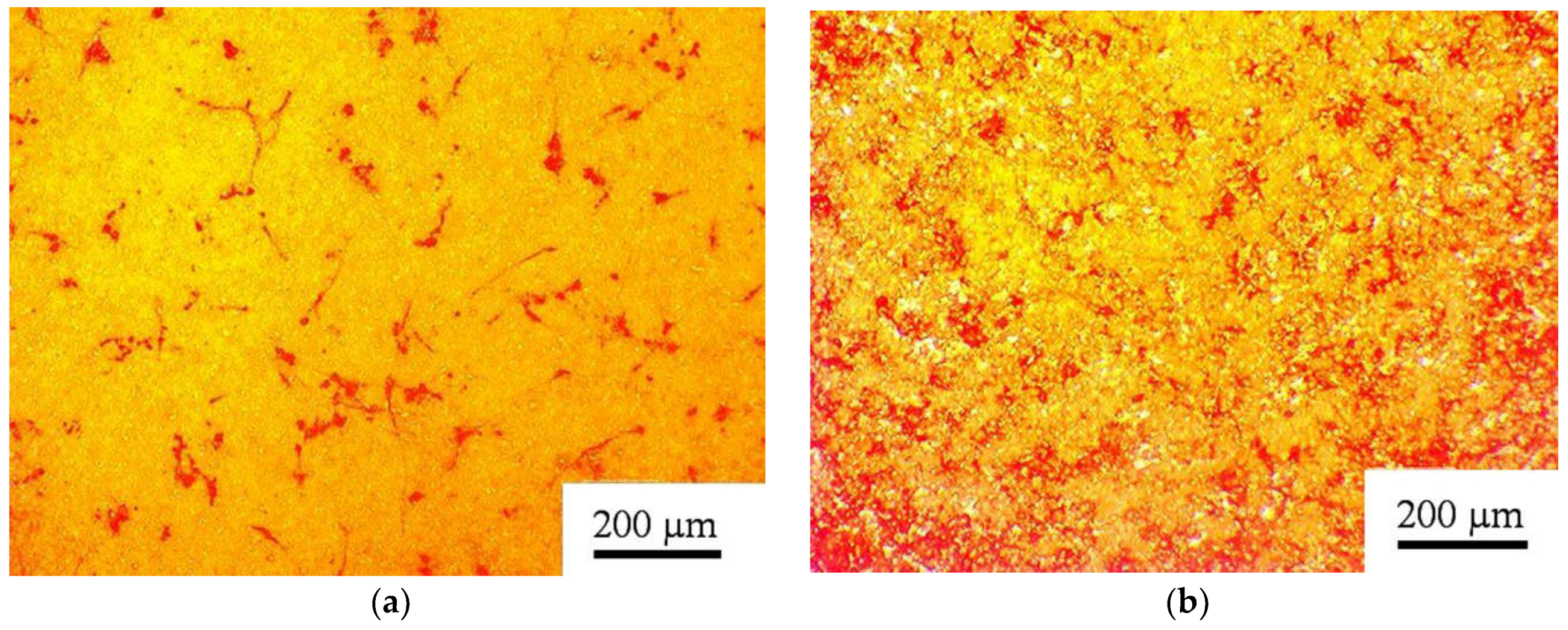
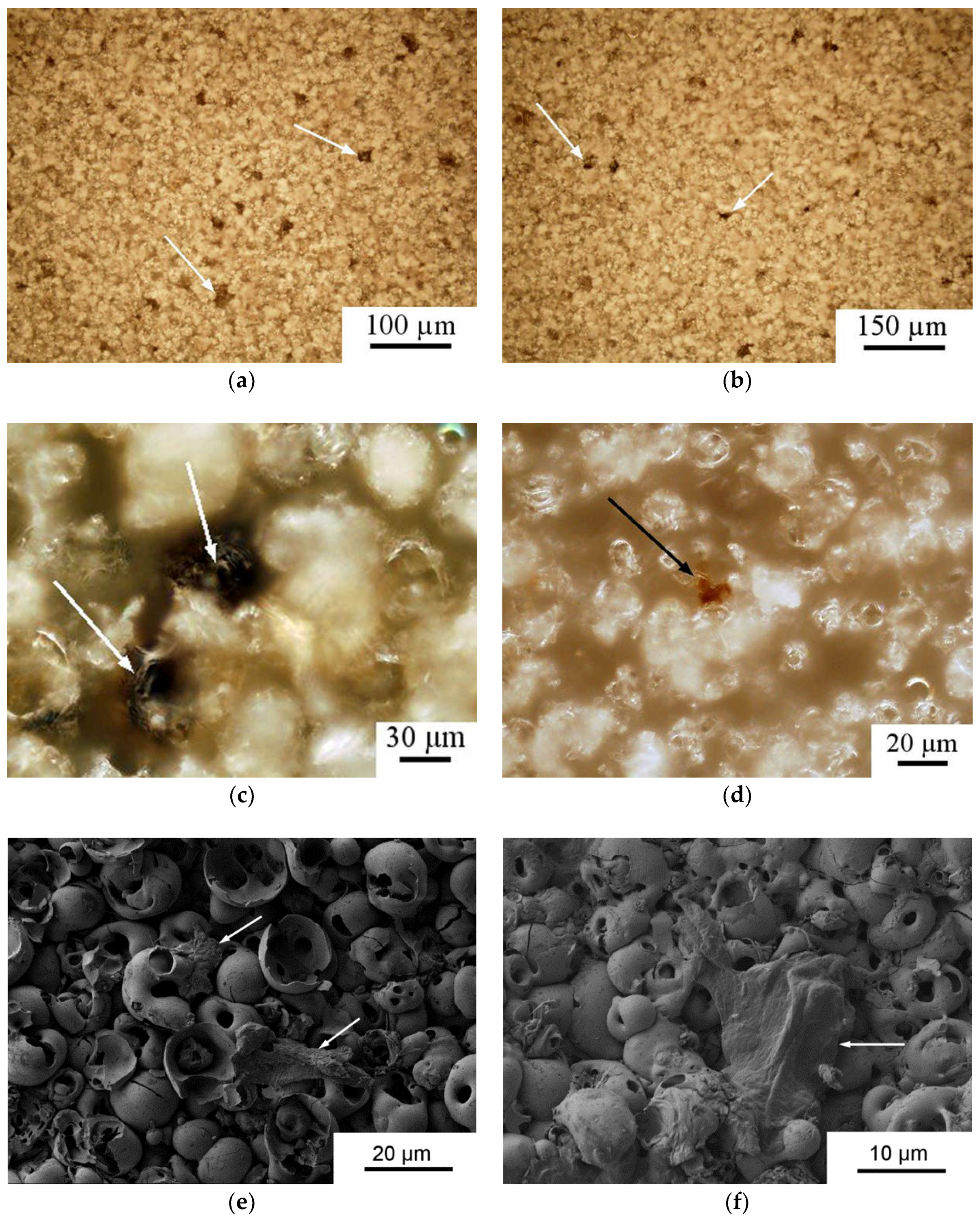
3.2. Coating Phase Composition and Morphology
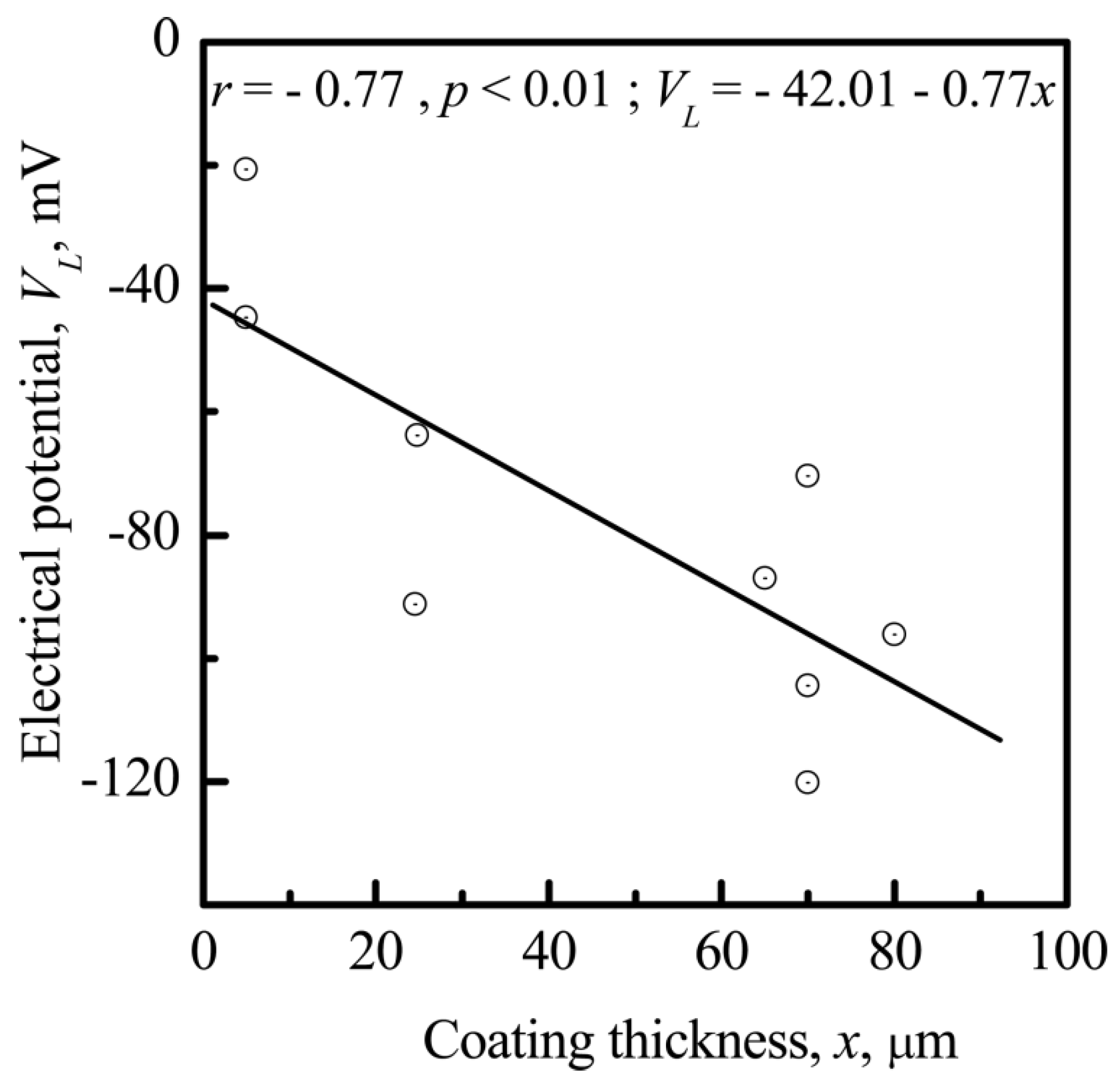
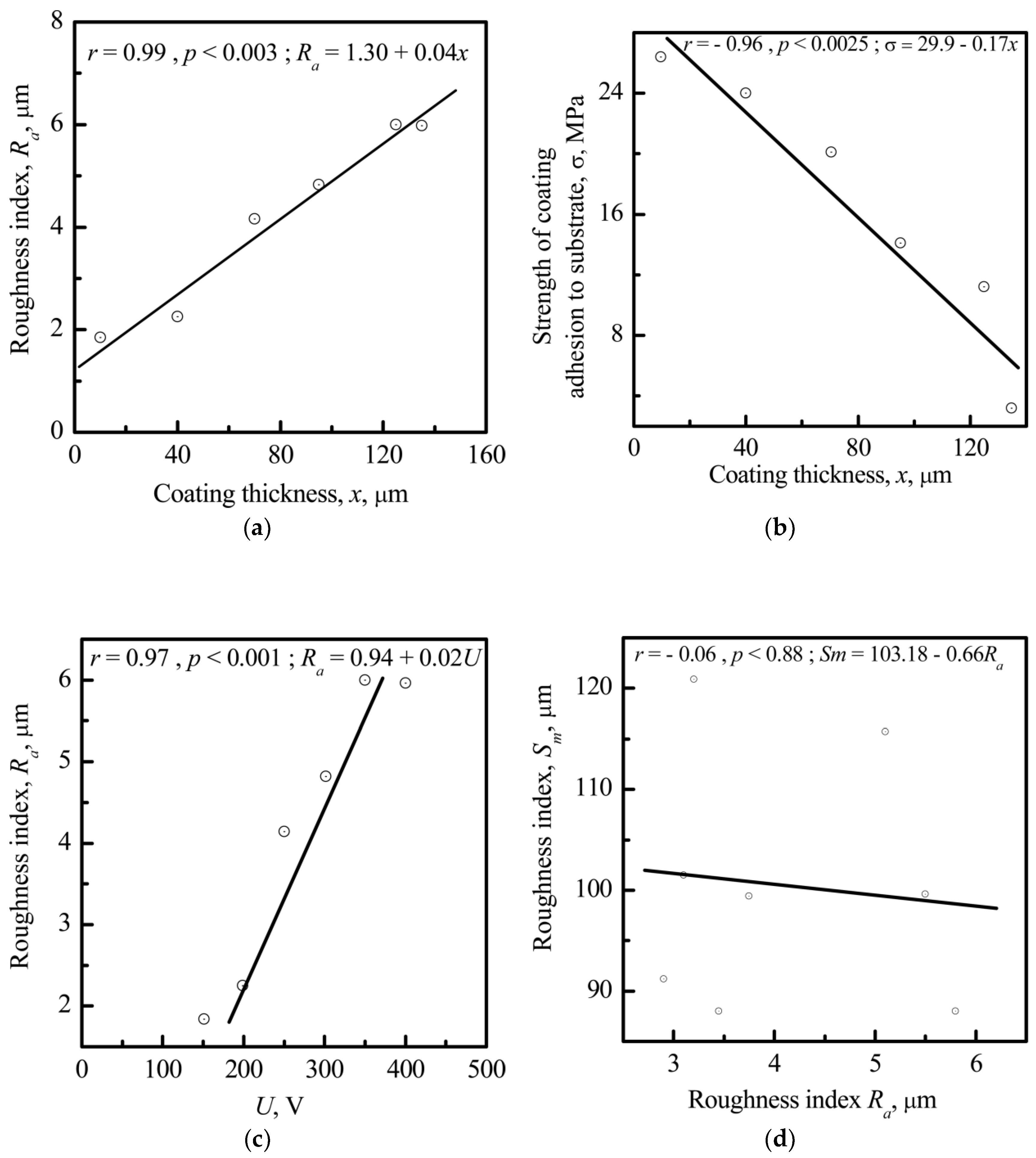
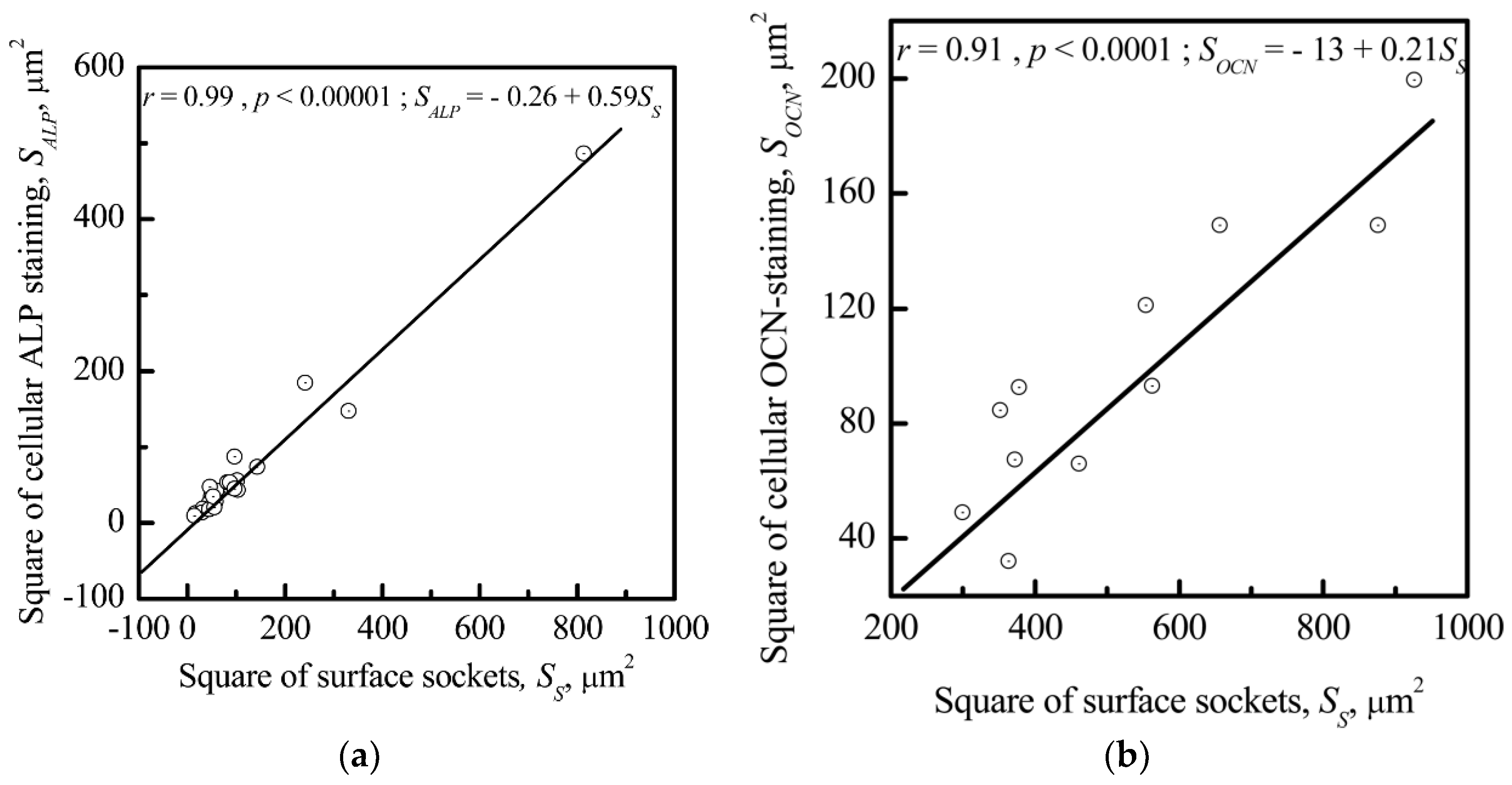
3.3. Relationship between Electrostatic, Geometrical, and Cytological Properties of the CP Coating
3.3.1. Microscale
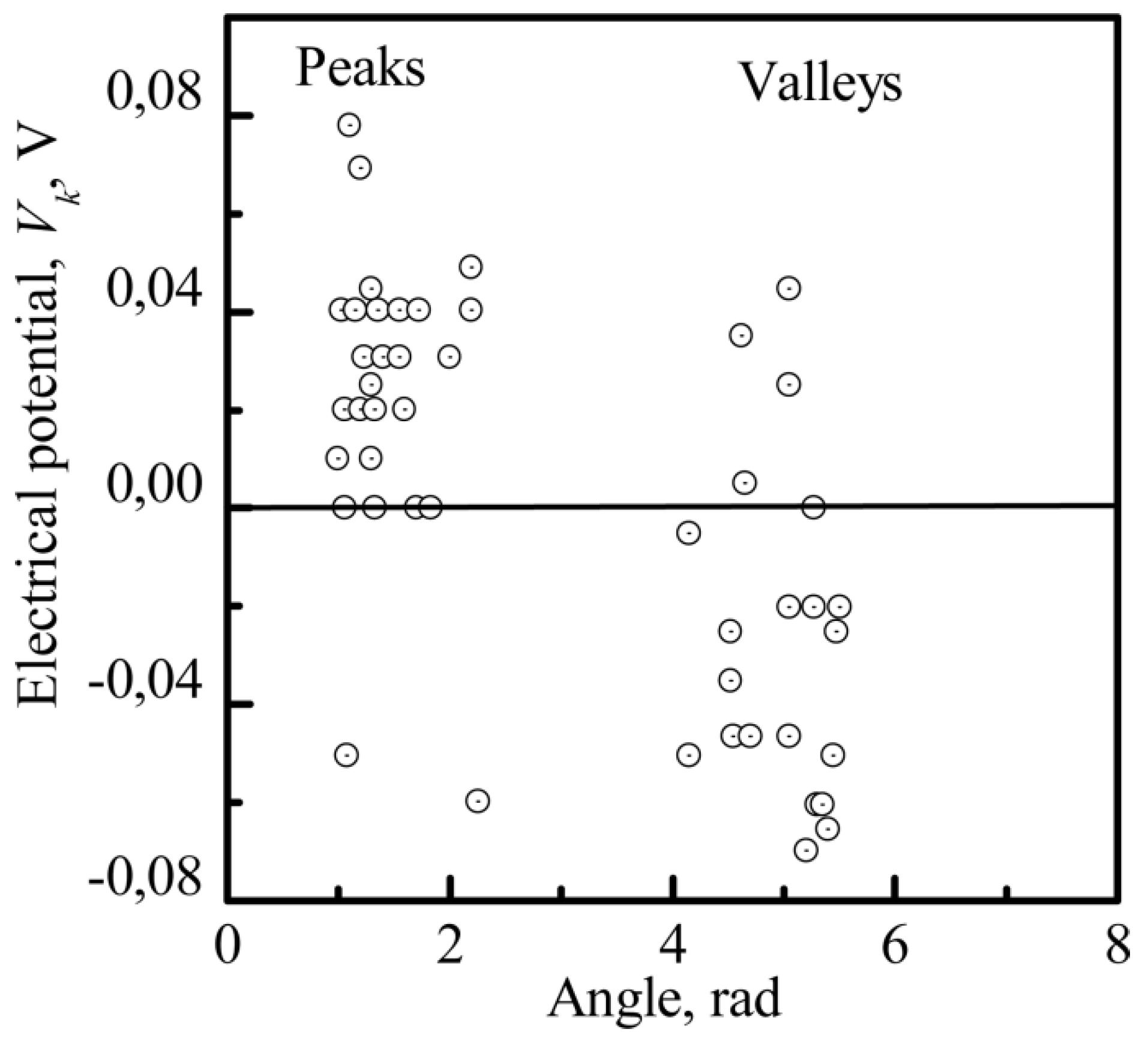
3.3.2. The Nanoscale
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Elsevier Science Publishing Co., Inc.: San Diego, CA, USA, 2012. [Google Scholar]
- Derjaguin, B.V.; Landau, L.D. Theory of the stability of strongly charged liophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. 1941, 14, 633–662. [Google Scholar]
- London, F. Properties’ and application of molecular forces. Z. Phys. Chem. 1930, 11, 222–251. [Google Scholar]
- Aronov, D.; Molotskii, M.; Rosenman, G. Charge-induced wettability modification. Appl. Phys. Lett. 2007, 90, 1–3. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakamura, S.; Yamashita, K. Enhanced osteobonding by negative surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 2001, 57, 477–484. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Coutinho, J.; Bystrova, A.V.; Dekhtyar, Y.D.; Pullar, R.C.; Poronin, A.; Palcevskis, E.; Dindune, A.; Alkan, B.; Durucan, C.; et al. Computational study of hydroxyapatite structures, properties and defects. J. Phys. D Appl. Phys. 2015, 48. [Google Scholar] [CrossRef]
- Chen, J.Z.; Shi, Y.L.; Wang, L.; Yan, F.Y.; Zhang, F.Q. Preparation and properties of hydroxyapatite-containing titania coating by micro-arc oxidation. Mater. Lett. 2006, 60, 2538–2543. [Google Scholar] [CrossRef]
- Frauchiger, V.M.; Schlottig, F.; Gasser, B.; Textor, M. Anodic plasma-chemical treatment of CP titanium surfaces for biomedical applications. Biomaterials 2004, 25, 593–606. [Google Scholar] [CrossRef]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Dekhtyar, Y.; Dvornichenko, M.V.; Karlov, A.V.; Khlusov, I.A.; Polyaka, N.; Sammons, R.; Zaytsev, K.V. Electrically functionalized hydroxyapatite and calcium phosphate surfaces to enhance immobilization and proliferation of osteoblasts in vitro and modulate osteogenesis in vivo. IFMBE Proc. 2009, 25, 245–248. [Google Scholar]
- Martínez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Pollock, J.F.; Schaffer, D.V.; Healy, K.E. Designing synthetic materials to control stem cell phenotype. Curr. Opin. Chem. Biol. 2007, 11, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.M.Y.; Leipzig, N.D.; Shoichet, M.S. Promoting neuron adhesion and growth. Mater. Today 2008, 11, 36–43. [Google Scholar] [CrossRef]
- Palcevskis, E.; Dindune, A.; Dekhtyar, Y.; Polyaka, N.; Veljović, D.; Sammons, R.L. The influence of surface treatment by hydrogenation on the biocompatibility of different hydroxyapatite materials. IOP Conf. Ser. Mater. Sci. Eng. 2011, 23, 012032. [Google Scholar] [CrossRef]
- Curtis, A.; Varde, M. Control of Cell Behavior: Topological Factors. J. Natl. Cancer Inst. 1964, 33, 15–62. [Google Scholar] [PubMed]
- Sniadecki, N.J.; Desai, R.A.; Ruiz, S.A.; Chen, C.S. Nanotechnology for cell-substrate interactions. Ann. Biomed. Eng. 2006, 34, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; de Groot, K.; Chen, W.; Li, Y.; Zhang, X. Osseous substance formation induced in porous calcium phosphate ceramics in soft tissues. Biomaterials 1994, 15, 31–34. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 2000, 49, 155–166. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005, 1, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Giljean, S.; Ponche, A.; Bigerelle, M.; Anselme, K. Statistical approach of chemistry and topography effect on human osteoblast adhesion. J. Biomed. Mater. Res.-A 2010, 94, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Bigerelle, M. On the relation between surface roughness of metallic substrates and adhesion of human primary bone cells. Scanning 2014, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Giljean, S.; Bigerelle, M.; Anselme, K. Roughness statistical influence on cell adhesion using profilometry and multiscale analysis. Scanning 2014, 36, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Schwartz, Z.; Nasazky, E.; Boyan, B.D. Surface microtopography regulates osteointegration: The role of implant surface microtopography in osteointegration. Alpha Omegan 2005, 98, 9–19. [Google Scholar] [PubMed]
- Meyer, U.; Büchter, A.; Wiesmann, H.P.; Joos, U.; Jones, D.B. Basic reactions of osteoblasts on structured material surfaces. Eur. Cells Mater. 2005, 9, 39–49. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Khlusova, M.Y.; Zaitsev, K.V.; Kolokol’tsova, T.D.; Sharkeev, Y.P.; Pichugin, V.F.; Legostaeva, E.V.; Trofimova, I.E.; Klimov, A.S.; Zhdanova, A.I. Pilot in vitro study of the parameters of artificial niche for osteogenic differentiation of human stromal stem cell pool. Bull. Exp. Biol. Med. 2011, 150, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R. Effects of nanotopography on stem cell phenotypes. World J. Stem Cells 2009, 1, 55. [Google Scholar] [CrossRef] [PubMed]
- Heubach, J.F.; Graf, E.M.; Leutheuser, J.; Bock, M.; Balana, B.; Zahanich, I.; Christ, T.; Boxberger, S.; Wettwer, E.; Ravens, U. Electrophysiological properties of human mesenchymal stem cells. J. Physiol. 2004, 554, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Large-scale biophysics: Ion flows and regeneration. Trends Cell Biol. 2007, 17, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-R. Functional ion channels in stem cells. World J. Stem Cells 2011, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, K.; Frant, M.; Bossert, J.; Hildebrand, G.; Liefeith, K.; Jandt, K.D. Surface functionalized titanium thin films: Zeta-potential, protein adsorption and cell proliferation. Colloids Surf. B Biointerfaces 2006, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Wu, S.H.; Yao, M.; Lu, C.W.; Lin, Y.S.; Hung, Y.; Mou, C.Y.; Chen, Y.C.; Huang, D.M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.; Blanco, L.; Khraisat, A.; Tresguerres, I.F. Influence of Titanium Surface Charge on Fibroblast Adhesion. Clin. Implant Dent. Relat. Res. 2006, 8, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nagai, A.; Hentunen, T.; Salonen, J.; Sekijima, Y.; Okura, T.; Hashimoto, K.; Toda, Y.; Monma, H.; Yamashita, K. Surface electric fields increase osteoblast adhesion through improved wettability on hydroxyapatite electret. ACS Appl. Mater. Interfaces 2009, 1, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Ahmad, Z.; Huang, J.; Edirisinghe, M.J.; Jayasinghe, S.N.; Ireland, D.C.; Brooks, R.A.; Rushton, N.; Bonfield, W.; Best, S.M. Electrosprayed nanoapatite: A new generation of bioactive material. Key Eng. Mater. 2008, 361–363, 597. [Google Scholar] [CrossRef]
- Costa, D.O.; Prowse, P.D.H.; Chrones, T.; Sims, S.M.; Hamilton, D.W.; Rizkalla, A.S.; Dixon, S.J. The differential regulation of osteoblast and osteoclast activity bysurface topography of hydroxyapatite coatings. Biomaterials 2013, 34, 7215–7226. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces 2006, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-M.; Chung, T.-H.; Hung, Y.; Lu, F.; Wu, S.-H.; Mou, C.-Y.; Yao, M.; Chen, Y.-C. Internalization of mesoporous silica nanoparticles induces transient but not sufficient osteogenic signals in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2008, 231, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Aronov, D.; Rosenman, G.; Karlov, A.; Shashkin, A. Wettability patterning of hydroxyapatite nanobioceramics induced by surface potential modification. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Aronov, D.; Rosen, R.; Ron, E.Z.; Rosenman, G. Tunable hydroxyapatite wettability: Effect on adhesion of biological molecules. Process Biochem. 2006, 41, 2367–2372. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Karlov, A.V.; Sharkeev, Y.P.; Pichugin, V.F.; Kolobov, Y.P.; Shashkina, G.A.; Ivanov, M.B.; Legostaeva, E.V.; Sukhikh, G.T. Osteogenic potential of mesenchymal stem cells from bone marrow in situ: Role of physicochemical properties of artificial surfaces. Bull. Exp. Biol. Med. 2005, 140, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Elter, P.; Thull, R. Protein adsorption on nanostructured implant surfaces: A model calculation for the prediction of preferred adsorption sites. Biomaterials 2006, 7, 138–144. [Google Scholar]
- Sharkeev, Y.P.; Legostaeva, E.V.; Eroshenko, Y.A.; Khlusov, I.A.; Kashin, O.A. The Structure and Physical and Mechanical Properties of a Novel Biocomposite Material, Nanostructured Titanium–Calcium-Phosphate Coating. Compos. Interfaces 2009, 16, 535–546. [Google Scholar] [CrossRef]
- Legostaeva, E.V.; Kulyashova, K.S.; Komarova, E.G.; Epple, M.; Sharkeev, Y.P.; Khlusov, I.A. Physical, chemical and biological properties of micro-arc deposited calcium phosphate coatings on titanium and zirconium-niobium alloy. Materwiss. Werksttech. 2013, 44, 188–197. [Google Scholar] [CrossRef]
- Leach, R.K. The Measurement of Surface Texture Using Stylus Instruments; National Physical Laboratory Teddington: Middlesex, UK, 2001; p. 97. [Google Scholar]
- Eguchi, M. On the permanent electret. Philos. Mag. 1925, 49, 178–192. [Google Scholar] [CrossRef]
- Gostischev, E.A. A Noncontacting Manner to Determine the Potentials of Charged Surface of Objects and the Device for Its Realization. Russian Federation Patent 2223511, 10 February 2004. [Google Scholar]
- Akmene, R.J.; Balodis, A.J.; Dekhtyar, Y.D.; Markelova, G.N.; Matvejevs, J.V.; Rozenfelds, L.B.; Sagaloviąs, G.L.; Smirnovs, J.S.; Tolkaąovs, A.A.; Upmiņš, A.I. Exoelectron emission specrometre complete set of surface local investigation. Poverhn. Fiz. Him. Meh. 1993, 8, 125–128. (In Russian) [Google Scholar]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Khlusov, I.A.; Shevtsova, N.M.; Khlusova, M.Y. Detection in vitro and quantitative estimation of artifi cial microterritories which promote osteogenic differentiation and maturation of stromal stem cells. Methods Mol. Biol. 2013, 1035, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9780470528129. [Google Scholar]
- Bystrov, V.S.; Paramonova, E.; Dekhtyar, Y.; Katashev, A.; Karlov, A.; Polyaka, N.; Bystrova, A.V.; Patmalnieks, A.; Kholkin, A.L. Computational and experimental studies of size and shape related physical properties of hydroxyapatite nanoparticles. J. Phys. Condens. Matter 2011, 23, 65302. [Google Scholar] [CrossRef] [PubMed]
- Dellatore, S.M.; Garcia, A.S.; Miller, W.M. Mimicking stem cell niches to increase stem cell expansion. Curr. Opin. Biotechnol. 2008, 19, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Doyonnas, R.; Havenstrite, K.; Koleckar, K.; Blau, H.M. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr. Biol. 2009, 1, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, D.; Fonseca, A.V.; Alakel, N.; Fierro, F.A.; Muller, K.; Bornhauser, M.; Ehninger, G.; Corbeil, D.; Ordemann, R. Hematopoietic stem cells in co-culture with mesenchymal stromal cells–modeling the niche compartments in vitro. Haematologica 2010, 95, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Peerani, R.; Rao, B.M.; Bauwens, C.; Yin, T.; Wood, G.A.; Nagy, A.; Kumacheva, E.; Zandstra, P.W. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007, 26, 4744–4755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlusov, I.A.; Dekhtyar, Y.; Khlusova, M.Y.; Gostischev, E.A.; Sharkeev, Y.P.; Pichugin, V.F.; Legostaeva, E.V. Novel concepts of “niche-relief” and “niche-voltage” for stem cells as a base of bone and hematopoietic tissues biomimetic engineering. IFMBE Proc. 2013, 38, 99–102. [Google Scholar]
- Terleeva, O.P.; Sharkeev, Y.P.; Slonova, A.I.; Mironov, I.V.; Legostaeva, E.V.; Khlusov, I.A.; Matykina, E.; Skeldon, P.; Thompson, G.E. Effect of microplasma modes and electrolyte composition on micro-arc oxidation coatings on titanium for medical applications. Surf. Coat. Technol. 2010, 205, 1723–1729. [Google Scholar] [CrossRef]
- De Groot, K. Bioceramics consisting of calcium phosphate salts. Biomaterials 1980, 1, 47–50. [Google Scholar] [CrossRef]
- De Bruijn, J.D. Calcium Phosphate Biomaterials: Bone-Bonding and Biodegradation Properties; Leiden University: Leiden, The Netherlands, 1993. [Google Scholar]
- Ikeda, N.; Kawanabe, K.; Nakamura, T. Quantitative comparison of osteoconduction of porous, dense A-W glass-ceramic and hydroxyapatite granules (effects of granule and pore sizes). Biomaterials 1999, 20, 1087–1095. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous biphasic calcium phosphate ceramics: Influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar] [CrossRef]
- Sous, M. Cellular biocompatibility and resistance to compression of macroporous β-tricalcium phosphate ceramics. Biomaterials 1998, 19, 2147–2153. [Google Scholar] [CrossRef]
- Yuan, H.; Kurashina, K.; De Bruijn, J.D.; Li, Y.; De Groot, K.; Zhang, X. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 1999, 20, 1799–1806. [Google Scholar] [CrossRef]
- Lobo, S.E.; Arinzeh, T.L. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials (Basel) 2010, 3, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Ferrier, J.; Ross, S.M.; Kanehisa, J.; Aubin, J.E. Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical field. J. Cell. Physiol. 1986, 129, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, N.; Hamamoto, Y.; Nakajima, T.; Ozawa, H. Histological, histocytochemical and ultrastructural study on the effects of surface charge on bone formation in the rabbit mandible. Arch. Oral Biol. 1995, 40, 97–106. [Google Scholar] [CrossRef]
- Smith, I.O.; Baumann, M.J.; McCabe, L.R. Electrostatic interactions as a predictor for osteoblast attachment to biomaterials. J. Biomed. Mater. Res. A 2004, 70, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Lossdorfer, S.; Wang, L.; Zhao, G.; Lohmann, C.H.; Cochran, D.L.; Schwartz, Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur. Cells Mater. 2003, 6, 22–27. [Google Scholar] [CrossRef]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. Rep. 2009, 5, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Yin, L. Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks. AJP Heart Circ. Physiol. 2004, 287, H1276–H1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobasnick, G.; Curtis, A. Chloride channels and cell topographic reaction. Eur. Cells Mater. 2001, 2, 49–61. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Clark, R.; Moses, R.L. Cell adhesion to biomaterials: Correlations between surface charge, surface roughness, adsorbed protein, and cell morphology. J. Long-Term Eff. Med. Implant. 1995, 5, 209–231. [Google Scholar]








| Group Studied (n = 5) | AMSC Viability, % | HLPSC Viability, % |
|---|---|---|
| Control cell culture | 88 (88–94) | 91 (88–92) |
| Cell culture in contact with the CP-coated sample | 87 (81–90) | 90 (88–91) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlusov, I.A.; Dekhtyar, Y.; Sharkeev, Y.P.; Pichugin, V.F.; Khlusova, M.Y.; Polyaka, N.; Tyulkin, F.; Vendinya, V.; Legostaeva, E.V.; Litvinova, L.S.; et al. Nanoscale Electrical Potential and Roughness of a Calcium Phosphate Surface Promotes the Osteogenic Phenotype of Stromal Cells. Materials 2018, 11, 978. https://doi.org/10.3390/ma11060978
Khlusov IA, Dekhtyar Y, Sharkeev YP, Pichugin VF, Khlusova MY, Polyaka N, Tyulkin F, Vendinya V, Legostaeva EV, Litvinova LS, et al. Nanoscale Electrical Potential and Roughness of a Calcium Phosphate Surface Promotes the Osteogenic Phenotype of Stromal Cells. Materials. 2018; 11(6):978. https://doi.org/10.3390/ma11060978
Chicago/Turabian StyleKhlusov, Igor A., Yuri Dekhtyar, Yurii P. Sharkeev, Vladimir F. Pichugin, Marina Y. Khlusova, Nataliya Polyaka, Fedor Tyulkin, Viktorija Vendinya, Elena V. Legostaeva, Larisa S. Litvinova, and et al. 2018. "Nanoscale Electrical Potential and Roughness of a Calcium Phosphate Surface Promotes the Osteogenic Phenotype of Stromal Cells" Materials 11, no. 6: 978. https://doi.org/10.3390/ma11060978





