Characterization and Microstructure of Linear Electrode-Electrospun Graphene-Filled Polyvinyl Alcohol Nanofiber Films
Abstract
:1. Introduction
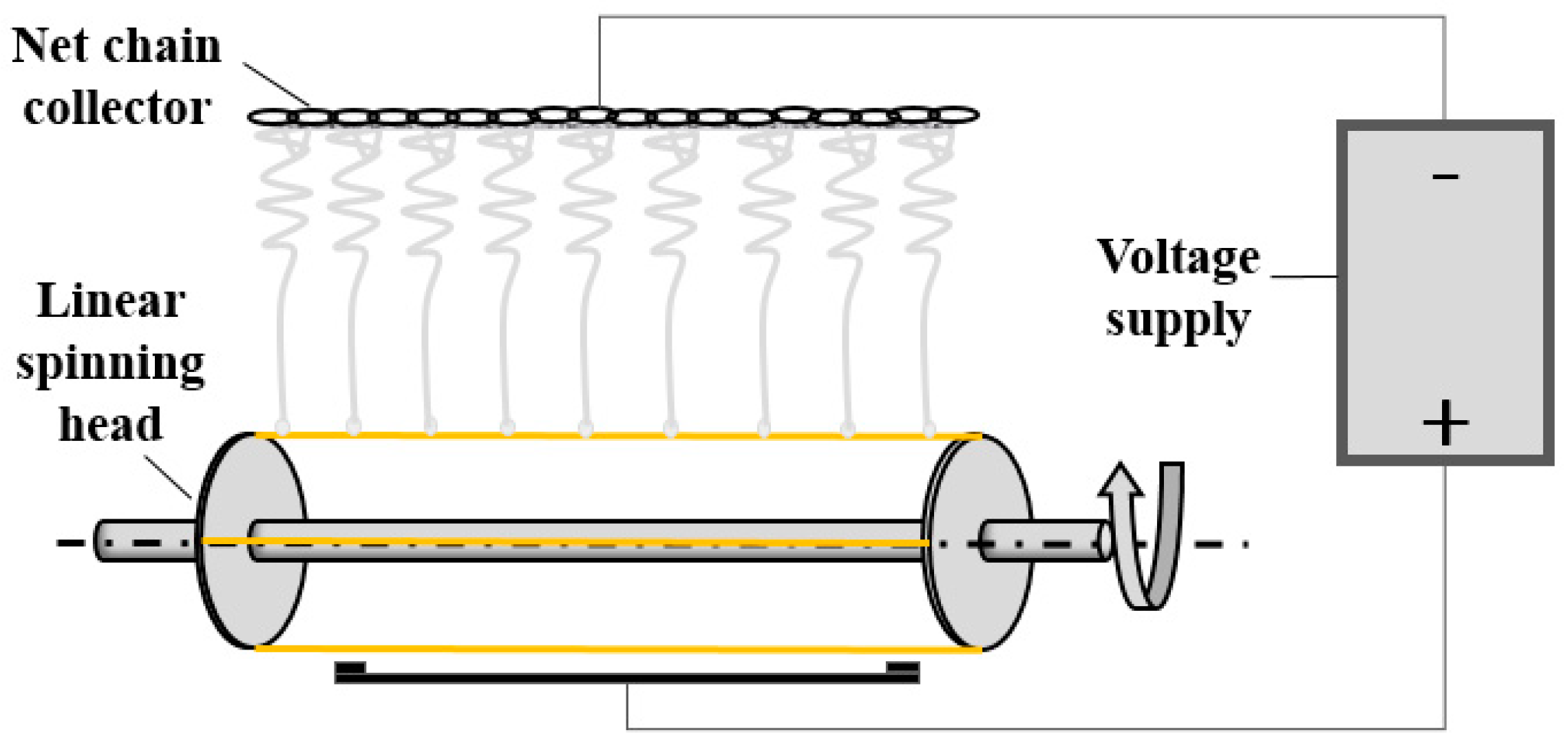
2. Experiments
2.1. Materials
2.2. Preparation of PVA/GR Nanofiber Films
2.3. Morphology and Characterizations of PVA/GR Nanofiber Film
3. Results and Discussion
3.1. Properties of GR/PVA Mixtures
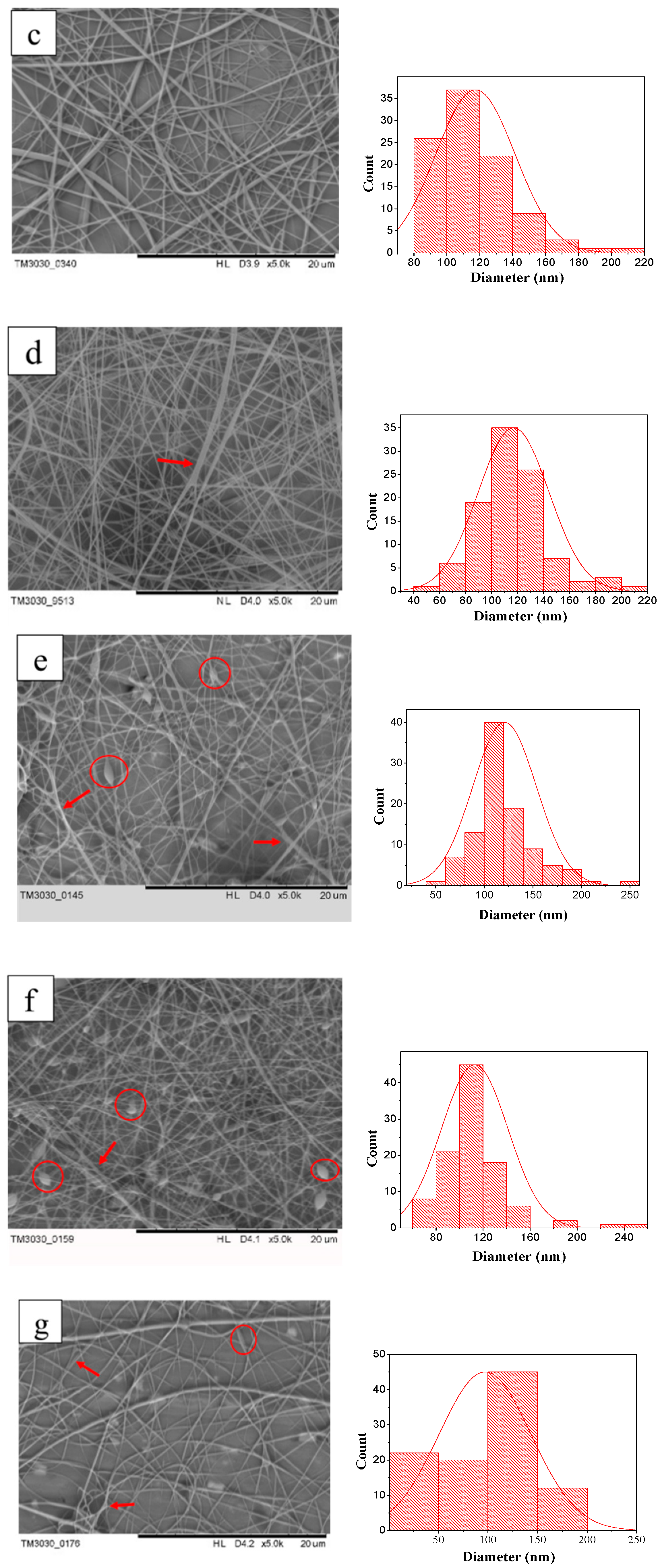
3.2. Morphology and Diameter of PVA/GR Nanofiber Films
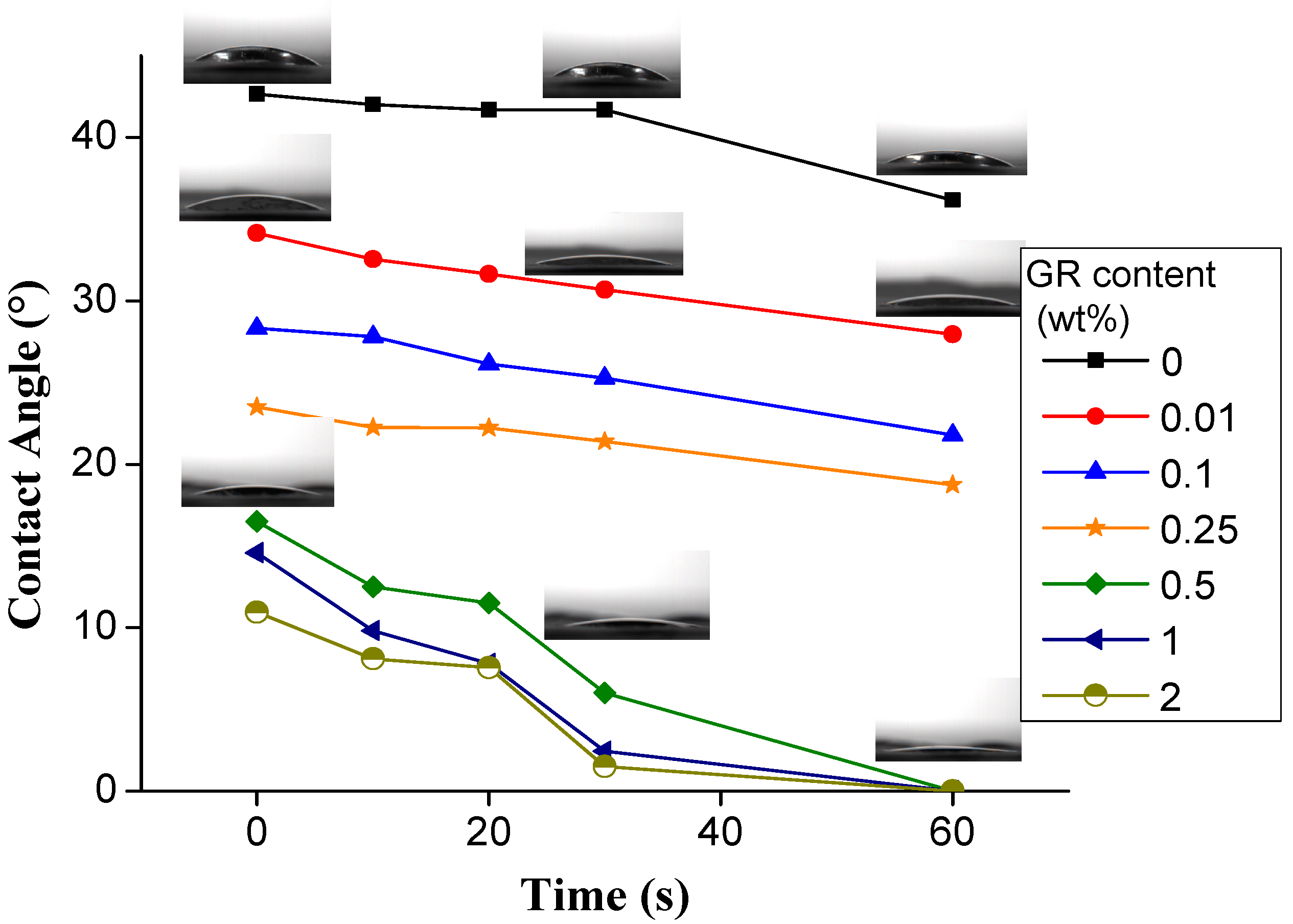
3.3. Dynamic Hydrophilicity of PVA/GR Nanofiber Films
3.4. Thermal Stability of PVA/GR Nanofiber Films
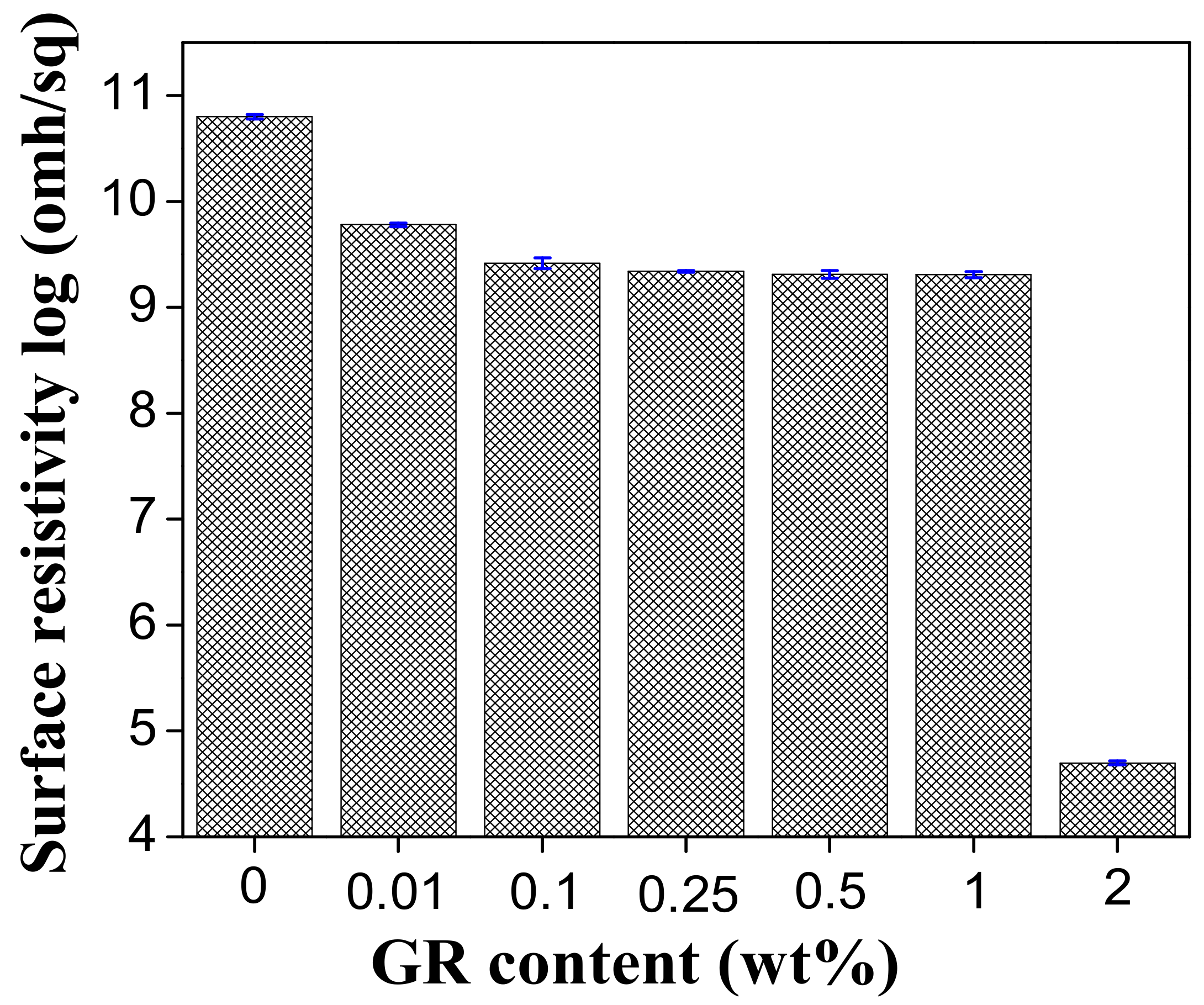
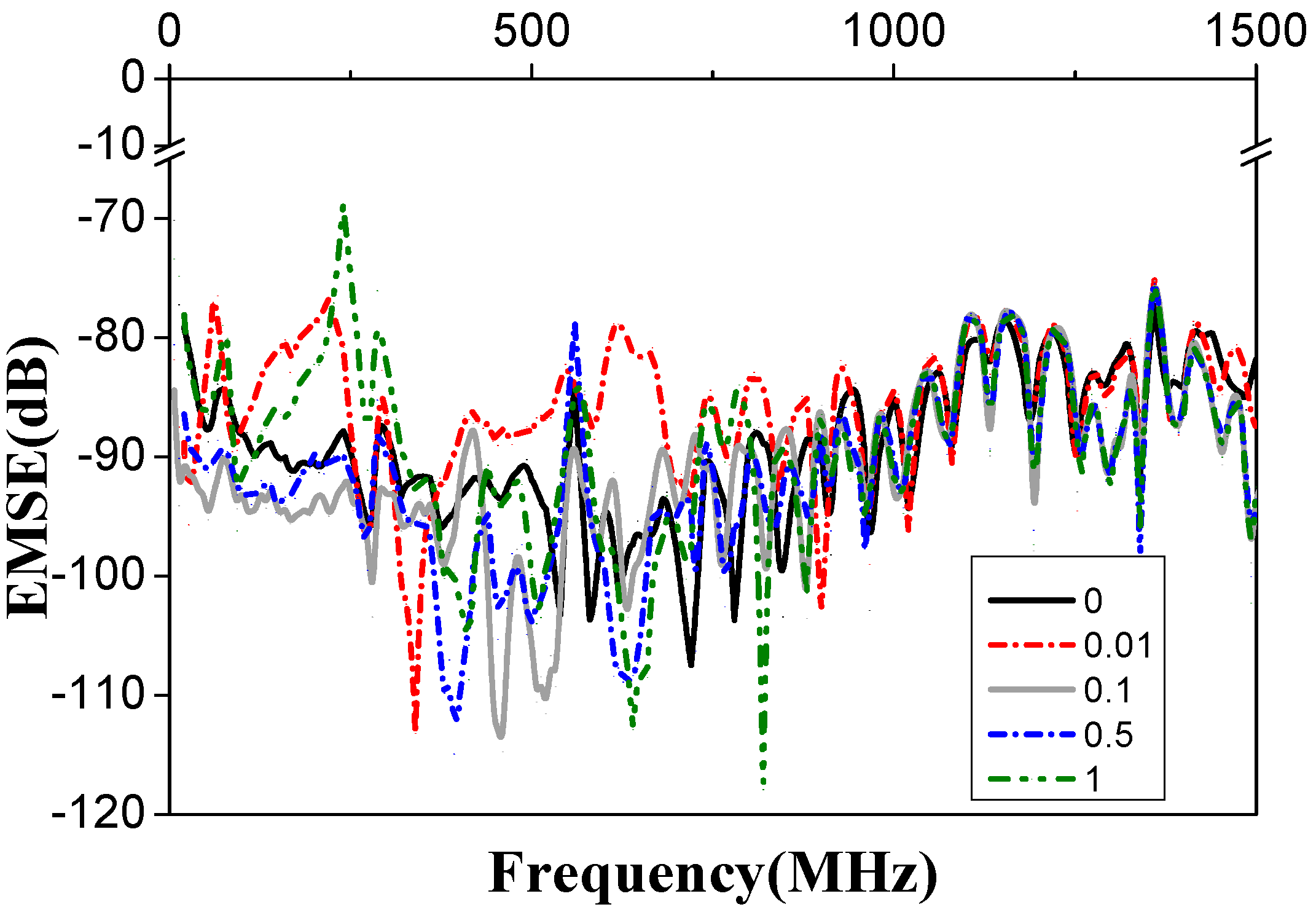
3.5. Electrical Property of PVA/GR Nanofiber Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Surudžić, R.; Janković, A.; Mitrić, M.; Matić, I.; Juranić, Z.D.; Živković, L. The effect of graphene loading on mechanical, thermal, and biological properties of poly(vinyl alcohol)/graphene nanocomposites. J. Ind. Eng. Chem. 2016, 34, 250–257. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhang, Y.; Zhu, Y. Preparation and characterization of graphene oxide reinforced PVA film with boric acid as crosslinker. J. Appl. Polym. Sci. 2015, 132, 42000. [Google Scholar] [CrossRef]
- Jose, J.; Al-Harthi, M.A.; Alma’Adeed, M.A.; Bhadra Dakua, J.; De, S.K. Effect of graphene loading on thermomechanical properties of poly(vinyl alcohol)/starch blend. J. Appl. Polym. Sci. 2015, 132, 755–760. [Google Scholar] [CrossRef]
- Rastogi, P.K.; Ganesan, V. Palladium nanoparticles incorporated organic–inorganic hybrid material for electrocatalytic oxygen reduction. Energy Environ. 2015, 4, 221–225. [Google Scholar] [CrossRef]
- Schlierf, A.; Cha, K.; Schwab, M.G.; Samorı, P.; Palermo, V. Exfoliation of graphene with an industrial dye: Teaching an old dog new tricks. 2D Mater. 2014, 1, 035006. [Google Scholar] [CrossRef]
- Prüsse, U.; Morawsky, V.; Dierich, A. Encapsulation of microscopic catalysts in polyvinyl alcohol hydrogel beads. Stud. Surf. Sci. Catal. 1998, 118, 137–146. [Google Scholar]
- Huang, C.; Peng, S.; Wang, Y.; Chen, W.; Lin, J. Microstructure and characterization of electrospun poly(vinyl alcohol) nanofiber scaffolds filled with graphene nanosheets. J. Appl. Polym. Sci. 2015, 132, 41891. [Google Scholar] [CrossRef]
- Song, W.; Markel, D.C.; Wang, S. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, X.; Cai, C. Biodegradable branched polyesters poly(vinyl sulfonate-covinyl alcohol)-graft poly(d,l-lactic-coglycolic acid) as a negatively charged polyelectrolyte platform for drug delivery: Synthesis and characterization. Macromolecules 2008, 41, 2791–2799. [Google Scholar] [CrossRef]
- Noori, M.; Ravari, F.; Ehsani, M. Preparation of PVA nanofibers reinforced with magnetic graphene by electrospinning method and investigation of their degradation kinetics using master plot analyses on solid state. J. Therm. Anal. Calorim. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Ismail, Z.; Abdullah, A.H.; Abidin, A.S.Z. Application of graphene from exfoliation in kitchen mixer allows mechanical reinforcement of PVA/graphene film. Appl. Nanosci. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly(vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Fasolino, A.; Los, J.H.; Katsnelson, M.I. Intrinsic ripples in graphene. Nat. Mater. 2007, 6, 858–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maravi, S.; Bajpai, J.; Bajpai, A.K. Improving mechanical and electrical properties of poly(vinyl alcohol-g-acrylic acid) nanocomposite films by reinforcement of thermally reduced graphene oxide. Polym. Sci. Ser. A 2017, 59, 1–13. [Google Scholar] [CrossRef]
- Dong, L.X.; Chen, Q. Properties, synthesis, and characterization of graphene. Propellants Explos. Pyrotech. 2015, 24, 159–162. [Google Scholar] [CrossRef]
- Moradi, M.; Mohandesi, J.A.; Haghshenas, D.F. Mechanical properties of the poly(vinyl alcohol) based nanocomposites at low content of surfactant wrapped graphene sheets. Polymer 2015, 60, 207–214. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Graphene: A revolution in nanobiotechnology. J. Res. Nanobiotechnol. 2012, 1, 19–30. [Google Scholar]
- Ghugare, S.V.; Chiessi, E.; Fink, R. Structural investigation on thermoresponsive PVA/Poly(methacrylate-co-N-isopropylacrylamide) microgels across the volume phase transition. Macromolecules 2011, 44, 4470–4478. [Google Scholar] [CrossRef]
- Tyagi, M.G.; Albert, A.P.; Tyagi, V.; Hema, R. Graphene nanomaterials and applications in bio-medical sciences. Word J. Pharm. Pharm. Sci. 2013, 3, 339–345. [Google Scholar]
- Nirmala, R.; Kalpana, D.; Jin, W.J.; Oh, H.J.; Lee, J.H.; Navamathavan, R. Multifunctional baicalein blended poly(vinyl alcohol) composite nanofibers via electrospinning. Colloids Surf. A 2011, 384, 605–611. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. High-flux microfiltration filters based on electrospun polyvinylalcohol nanofibrous membranes. Polymer 2014, 54, 548–556. [Google Scholar] [CrossRef]
- Cooper, A.; Oldinski, R.; Ma, H.; Bryers, J.D.; Zhang, M. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohyd. Polym. 2013, 92, 254–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.L.; Baji, A.; Tien, H.W.; Yang, Y.K.; Yang, S.Y.; Wu, S.Y. Self-assembly of silver–graphene hybrid on electrospun polyurethane nanofibers as flexible transparent conductive thin films. Carbon 2012, 50, 3473–3481. [Google Scholar] [CrossRef]
- Berry, S.M.; Warren, S.P.; Hilgart, D.A.; Schworer, A.T.; Pabba, S.; Gobin, A.S. Endothelial cell scaffolds generated by 3d direct writing of biodegradable polymer microfibers. Biomaterials 2011, 32, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Lukas, D.; Sarkar, A.; Pokorny, P. Self-organization of jets in electrospinning from free liquid surface: A generalized approach. J. Appl. Phys. 2008, 103, 084309. [Google Scholar] [CrossRef]
- Yarin, A.L.; Zussman, E. Upward needleless electrospinning of multiple nanofibers. Polymer 2004, 45, 2977–2980. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.H.; Yu, J.Y. Bubble-electrospinning: A novel method for making nanofibers. J. Phys. Conf. Ser. 2008, 96, 012001. [Google Scholar] [CrossRef]
- Çallıoğlu, F.C.; Jirsak, O.; Dayık, M. Investigation into the relationships between independent and dependent parameters in roller electrospinning of polyurethane. Text. Res. J. 2012, 83, 718–729. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Lin, T.; Wang, X. Needleless electrospinning of nanofibers with a conical wire coil. Polym. Eng. Sci. 2010, 49, 1582–1586. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, S.; Qin, X. High throughput of quality nanofibers via one stepped pyramid-shaped spinneret. Mater. Lett. 2013, 106, 56–58. [Google Scholar] [CrossRef]
- Niu, H.; Lin, T.; Wang, X. Needleless electrospinning. I. A comparison of cylinder and disk nozzles. J. Appl. Polym. Sci. 2010, 114, 3524–3530. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Wang, X.; Lin, T. Needleless electrospinning of uniform nanofibers using spiral coil spinnerets. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- He, J.H.; Liu, Y.; Xu, L.; Yu, J.Y.; Sun, G. Biomimic fabrication of electrospun nanofibers with high-throughput. Chaos Soliton Fractals 2008, 37, 643–651. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2016, 111, 752–763. [Google Scholar] [CrossRef]
- Li, T.T.; Yan, M.; Wu, Z.H. The spinnability study of the dynamic linear electrode electrospun PVA nanofibers. Mater. Rev. 2018. in press (In Chinese) [Google Scholar]
- Pan, Y.J.; Lin, J.H.; Chiang, K.C. Biomedical applications of antibacterial nanofiber mats made of electrospinning with wire electrodes. Appl. Sci. 2016, 6, 46. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lou, C.W.; Pan, Y.J. Fabrication of poly (vinyl alcohol) nanofibers by wire electrode-incorporated electrospinning. Fibers Polym. 2016, 17, 1217–1226. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Xu, X. Modified Wenzel and Cassie equations for wetting on rough surfaces. SIAM J. Appl. Math. 2016, 76, 2353–2374. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.J.; Sun, G.H.; Wang, X.S. Generalized Wenzel equation of spherical nanodroplets within a homogeneous and rough conical cavity. J. Comput. Theor. Nanosci. 2016, 13, 5322–5326. [Google Scholar] [CrossRef]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and characterization of antibacterial electrospun chitosan/poly (vinyl alcohol)/graphene oxide composite nanofibrous membrane. Appl. Surf. Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Da, S.X.; Wang, J.; Geng, H.Z. High adhesion transparent conducting films using graphene oxide hybrid carbon nanotubes. Appl. Surf. Sci. 2017, 392, 1117–1125. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Li, J. Ultrasonic-microwave assisted synthesis of three-dimensional polyvinyl alcohol carbonate/graphene oxide sponge and studies of surface resistivity and thermal stability. Ultrason. Sonochem. 2018, 42, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Ma, Z. Preparation of polyvinyl alcohol graphene oxide phosphonate film and research of thermal stability and mechanical properties. Ultrason. Sonochem. 2018, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, K.; Thilagavathi, G. Electromagnetic shielding effectiveness of copper/PET composite yarn fabrics. Indian J. Fibre Text. 2012, 37, 133–137. [Google Scholar]
- Li, B.W.; Shen, Y.; Yue, Z.X. Enhanced microwave absorption in nickel/hexagonal-ferrite/polymer composites. Appl. Phys. Lett. 2006, 89, 132504. [Google Scholar] [CrossRef]
- Li, X.H.; Li, X.; Liao, K.N. Thermally annealed anisotropic graphene aerogels and their electrically conductive epoxy composites with excellent electromagnetic interference shielding efficiencies. ACS Appl. Mater. Interfaces 2016, 8, 33230–33239. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Lin, Z.I.; Pan, Y.J.; Chen, C.K.; Huang, C.L.; Huang, C.H. Improvement in mechanical properties and electromagnetic interference shielding effectiveness of PVA-based composites: Synergistic effect between graphene nano-sheets and multi-walled carbon nanotubes. Macromol. Mater. Eng. 2016, 301, 199–211. [Google Scholar] [CrossRef]



| Content of Graphene (wt %) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.01 | 0.1 | 0.25 | 0.5 | 1 | 2 | |
| Viscosity (mPa∙s) | 254.0 | 330.0 | 427.0 | 485.0 | 287.0 | 155.5 | 133.5 |
| Electric conductivity (mS/cm) | 0.67 | 1.67 | 1.99 | 1.93 | 1.97 | 2.03 | 1.98 |
| GR Content (wt %) | 0 | 0.01 | 0.1 | 0.25 | 0.5 | 1 | 2 |
|---|---|---|---|---|---|---|---|
| Maximum decomposition temp. (°C) | 248.9 | 255.2 | 253.2 | 254.7 | 247.7 | 242.9 | 238.6 |
| Residual mass at 700 °C (%) | 9.36 | 12.4 | 15.26 | 21.4 | 20.57 | 23.57 | 33.64 |
| Mass at 350 °C (%) | 38.0 | 60.1 | 60.4 | 63.1 | 62.0 | 62.6 | 66.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-T.; Yan, M.; Jiang, Q.; Peng, H.-K.; Lin, J.-H.; Lou, C.-W. Characterization and Microstructure of Linear Electrode-Electrospun Graphene-Filled Polyvinyl Alcohol Nanofiber Films. Materials 2018, 11, 1033. https://doi.org/10.3390/ma11061033
Li T-T, Yan M, Jiang Q, Peng H-K, Lin J-H, Lou C-W. Characterization and Microstructure of Linear Electrode-Electrospun Graphene-Filled Polyvinyl Alcohol Nanofiber Films. Materials. 2018; 11(6):1033. https://doi.org/10.3390/ma11061033
Chicago/Turabian StyleLi, Ting-Ting, Mengxue Yan, Qian Jiang, Hao-Kai Peng, Jia-Horng Lin, and Ching-Wen Lou. 2018. "Characterization and Microstructure of Linear Electrode-Electrospun Graphene-Filled Polyvinyl Alcohol Nanofiber Films" Materials 11, no. 6: 1033. https://doi.org/10.3390/ma11061033





