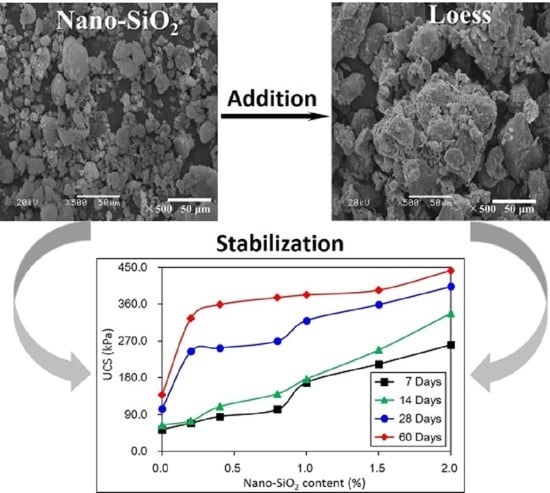
1. Introduction
Loess is a widespread surface deposit in many parts of the world. It is an important engineering material, for example as the material in a filled embankment. It is a typically problematic soil due to its propensity to collapse and subsidence after loading and wetting. It is also a typically hazardous material due to landslide and erosion prevalence. Therefore, it has a need for improvement by various stabilization methods, such as construction requirements and geohazard mitigation.
There has been considerable research into using various stabilizing agents to improve the performance of loess but much of this effort has focused on traditional chemical additives. Lime, fly ash, and cement are commonly used materials to reach more durable loess in infrastructure construction and geological barriers [
1,
2,
3,
4,
5,
6]. Overall, these chemical additives cause short-term physiochemical modification with a decrease in water content and an increase in density. The chemical reactivity then causes long-term pozzolanic stabilization with mineralogical and structural changes [
2,
6]. As a result, these chemical additives produce stronger and more applicative loess in mechanics and physics terms. However, they also produce some chemical changes in treated loess [
6]. Typically, these chemical additives cause much greater alkaline and saline environments, due to an increase in pH and salinity [
1,
4,
6]. In the soil improvement field, greater and more urgent attention has been paid to carbon emissions and treatment costs regarding various industries which involve lime, cement, and fly ash [
7,
8]. Hence, there has been great interest in replacing the generally-used chemical additives in soil improvement with alternative materials.
The interest in alternative materials has increased greatly in soil improvement works [
9,
10]. Nano-particles have also received special attention due to their unique properties [
11,
12]. Previous research has shown that nano-particles result in changes to physical, mechanical, and chemical properties. Taha and Taha [
13] investigated the effect of nano-particles on clay properties. They found that nano-clay, nano-alumina, and nano-copper additives strikingly restrain the expansive and shrinkage behaviors of clays and do not change its mineralogical properties. Research on nano-copper oxide and gamma-aluminum oxide powder on clay properties showed lower conductivity and higher shrinkage due to smaller pores and more flocculated fabric [
14,
15]. The addition of the nano-particles can reduce the development of desiccation cracks in clays [
13,
14]. At the same time, the addition of nano-clay causes a decrease in Atterberg limits of clays [
13,
16,
17]. Furthermore, a small amount of nano-SiO
2 addition can produce an obvious increase in the strength of treated clay [
17]. The aforementioned research work has focused on clays, even though silty and sandy soils are also often used in civil engineering. For these soils, increasing interest in the modification of their various properties following nano-particles addition has been conducted by several researchers. Gallagher and Mitchell showed that colloidal silica clearly decreases the risk of liquefaction of loose sand under seismic loading [
18]. Huang and Wang [
19] studied the impact of laponite on the strength of silty sand to mitigate liquefaction occurrence. Ren and Hu [
20] investigated the effect of nano-SiO
2 on silty soil properties. Tabarsa, Latifi [
21] evaluated the feasibility of loess improvement using nano-clay based on laboratory and field investigations. On the whole, the addition of nano-particles results in higher strength and density and lower conductivity, shrinkage, and Atterberg limit in various treated soils.
Nano-particles have been investigated as alternative additives for soil improvement purposes in replacing traditional stabilization agents. To some extent, their high cost limits their widespread application. However, nano-particles may generate lower total cost in practical engineering than traditional chemical additives, such as concrete and lime [
20]. Therefore, nano-particles have more advantages and greater potential to become cost-effective additives. In addition, the potential advantages in nano-particles would be a promising additive in soil improvement. Nevertheless, the use of nano-particles as a soil stabilizer is still uncommon [
15]. Until now, there have been very few attempts at using a nano-particle additive to treat loess [
21].
The present paper shows results from the addition of nano-SiO2 into loess by examining changes in mechanical, mineralogical, and structural properties with different nano-SiO2 contents and curing days. A series of tests were conducted on unconfined compressive strength, particle size distributions, SEM images, and XRD analyses. This study aims to understand the improvement of nano-SiO2 treated loess along with the complicated relations between macroscopic behaviors and microscopic characteristics. It is important for the improvement mechanisms and practical applications of treated loess.
2. Materials and Methods
2.1. Tested Materials
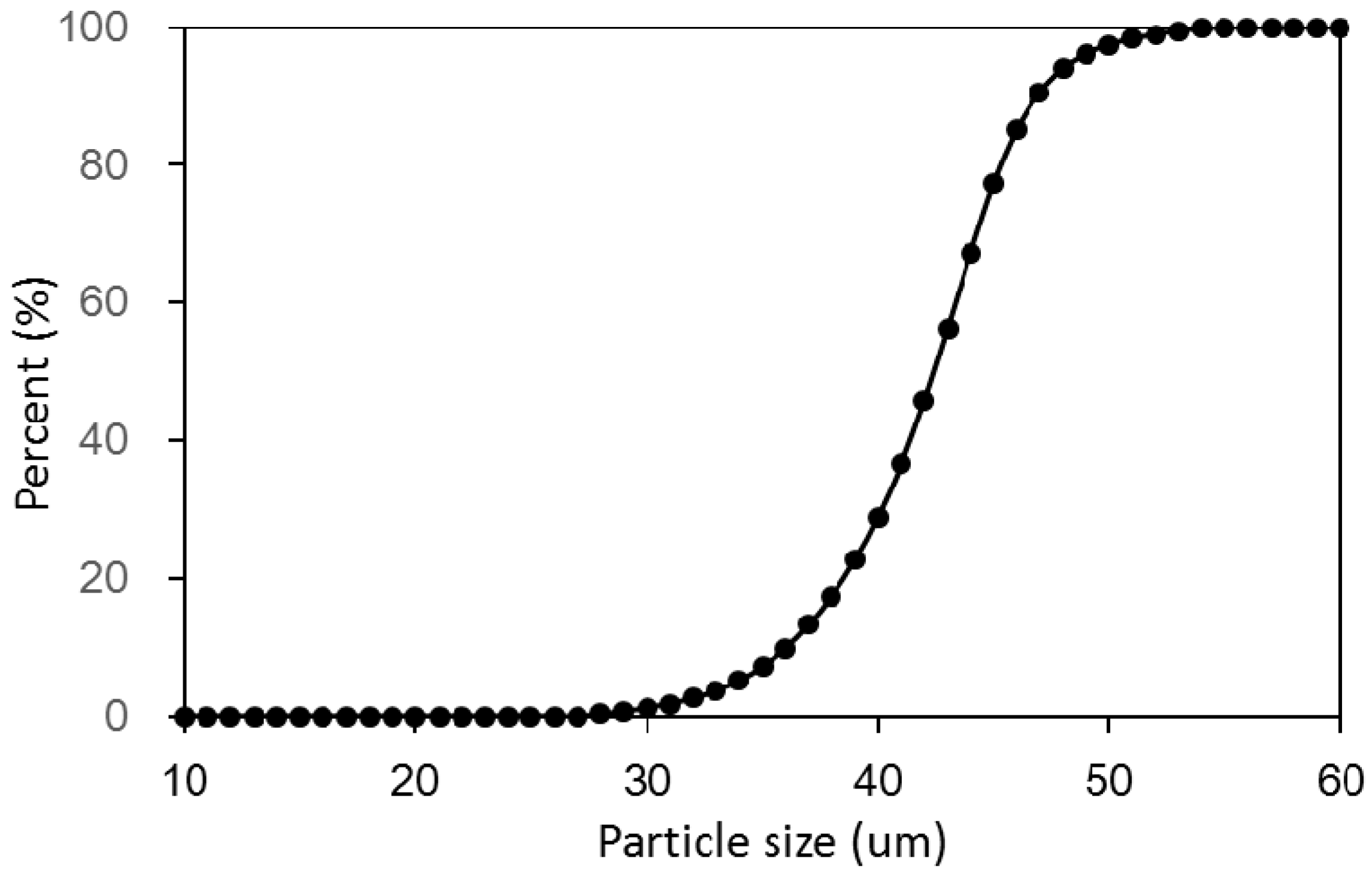
The sample examined in this study was deposited on Malan loess from the Quaternary age, which was taken from Lanzhou, China. This kind of loess is widely distributed in the Chinese Loess Plateau, of which it is a representative sample. The particle size curve of the loess is shown in
Figure 1. The loess consisted of predominantly silt (about 91.3%) with a small amount of clay and sand (about 8.7%). The mean particle diameter was 0.034 mm and the coefficient of uniformity was 3.5. The loess had low plasticity. Some physical properties and chemical compositions are listed in
Table 1.
The nano-SiO
2 used in this study is commercially available, and came from the manufacturing industry (Changsha, China).
Figure 2 shows the SEM images of nano-SiO
2. As shown in
Figure 2, the nano-SiO
2 were isolated particles and agglomerated particles. The average size of the agglomerated particles was about 30 μm, although the average diameter of the isolated particle was 30 nm. The basic properties of nano-SiO
2 are shown in
Table 2.
2.2. Sample Preparation
The loess was first allowed to air dry at room temperatures of about 20 °C, which was passed through a sieve with a 0.5 mm aperture. This sieve was selected because the particle size of all soil was less than 0.5 mm. Distilled water was first added to the oven-dried, disaggregated loess to reach an initial water content of 15%, which was selected to obtain a uniform sample during compaction. Afterward, the samples were sealed and stored for 12 h at room temperature, which ensured a uniform distribution of moisture before packing for the following sample preparation.
The amount of dry nano-SiO2 selected was 0.2%, 0.4%, 0.8%, 1.0%, 1.5% and 2.0% of the total dry weight of the loess. The mixed samples were placed in a designed steel cylinder 50 mm in diameter and 100 mm in height. The cylindrical samples were prepared using a static compacted method with the help of a hydraulic jack and a steel holder. To achieve a uniform density, the samples were placed in five layers and each layer was compacted so that a designated dry density of 1.47 g/cm3 was achieved. The cylindrical samples were sealed using a plastic film and were cured for 7 days, 14 days, 28 days, and 60 days at room temperature.
2.3. Test Procedures
To obtain the mechanical behavior of the untreated and nano-SiO2 treated loess, the cylindrical samples after acquiring respective curing days were placed on an automatic loading machine (Lanzhou city, Gansu Province, China) with a maximum loading capacity of 100 kN and they were compressed at a constant rate of 0.1 mm/min. The smooth perspex plate was placed at the bottom of each specimen during all tests to minimize end effects. To examine the quality of the specimens and prevent possible errors, the unconfined compressive strength (UCS) of the specific samples was repeatedly tested two or three times. The average value of the repeated samples was used in data analysis. After compressive strength tests, the fractured samples were carefully collected for composition analysis and structure tests.
The macro-structures and micro-structures of the untreated and treated samples were examined. The macroscopic particle size distributions of all samples were determined using a Microtrac S3500 laser diffraction instrument (Lanzhou, China). The micro-morphology and micro-size were observed on the powder samples after metallization with gold powder using a JSM-5600LV scanning electron microscope (SEM) (Lanzhou, China). Meanwhile, nitrogen adsorption BET was conducted with an ASAP 2020 Plus physisorption analyzer (Lanzhou, China) on powder for the untreated and treated samples for their microscopic pore size. All BET tested samples were outgassed after 12 h before running analyses at a maximum temperature of 22 °C.
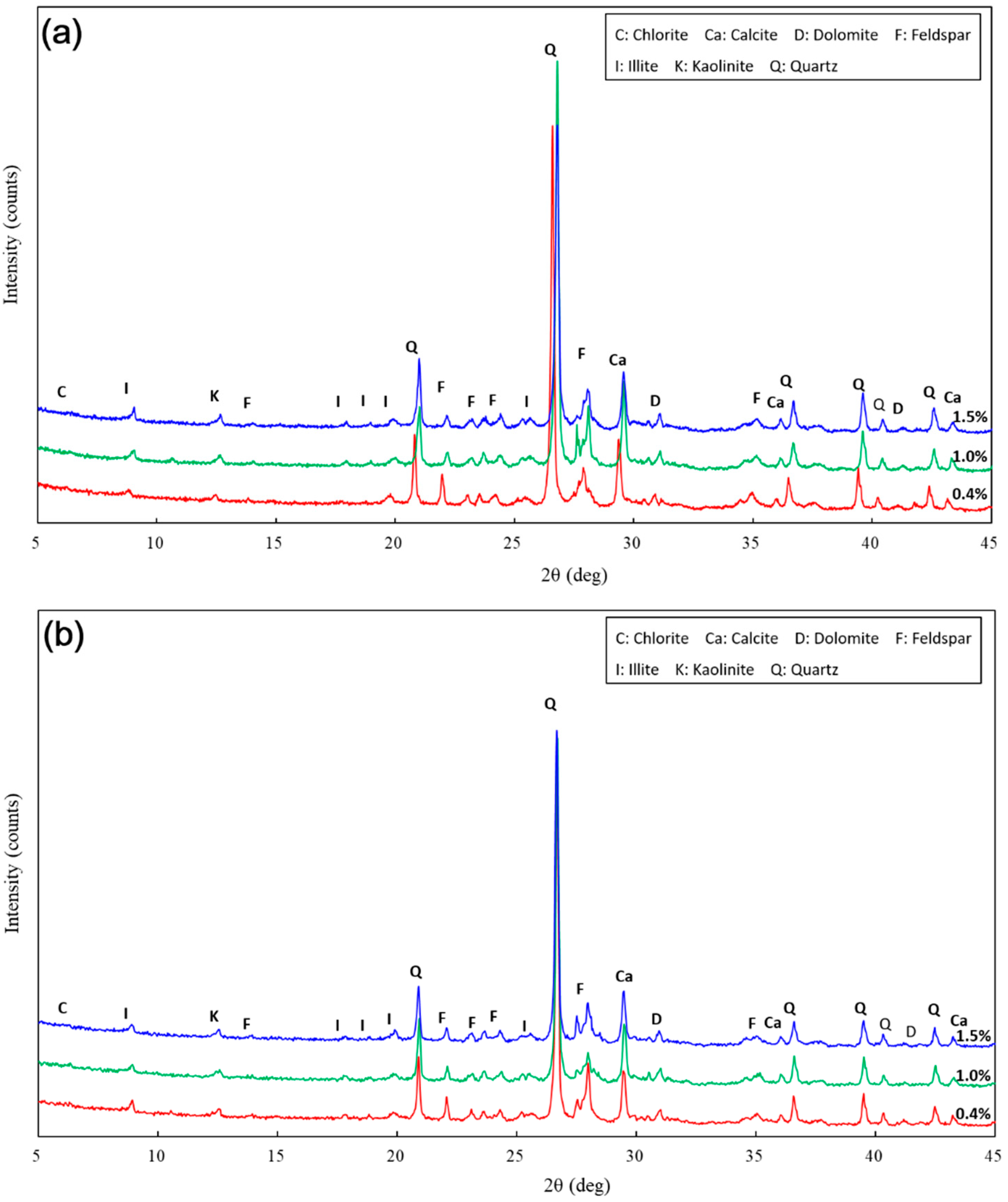
The mineralogical composition of the untreated and treated samples were examined by X-ray diffraction (XRD) for the specified samples. A Philips PW 3710 diffractometer (Lanzhou, China) was used for XRD analysis. The diffraction patterns were determined using Cu-Kα radiation with a Bragg angle (2θ) range of 5°–45°, running at a rate of 0.03°/s.
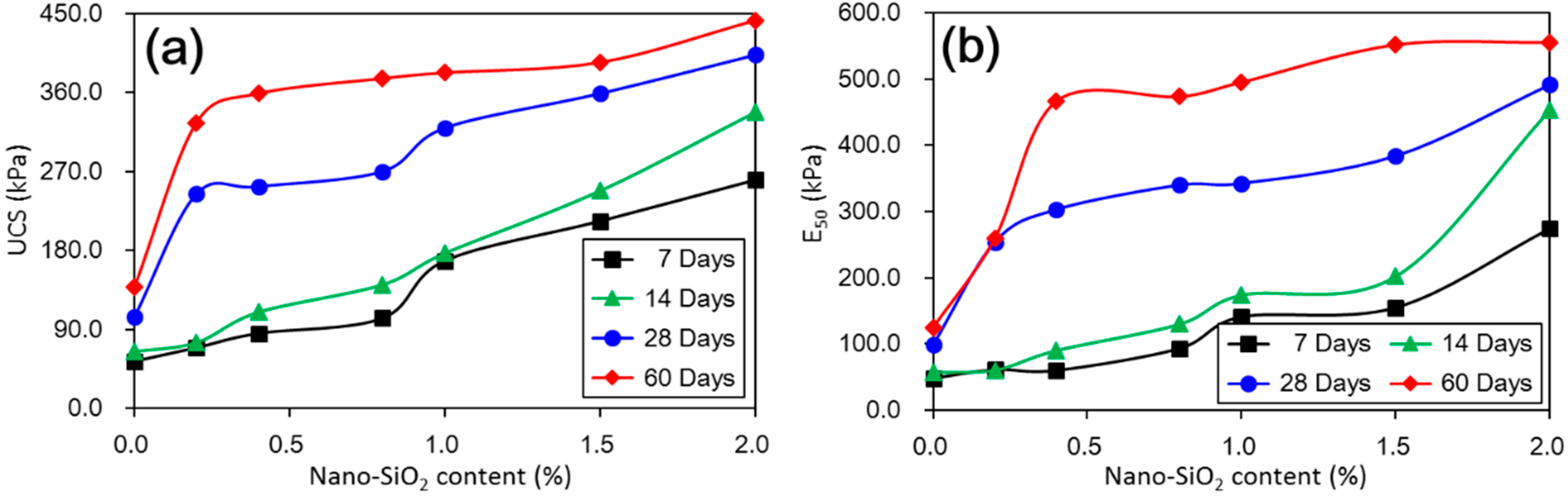
4. Discussion
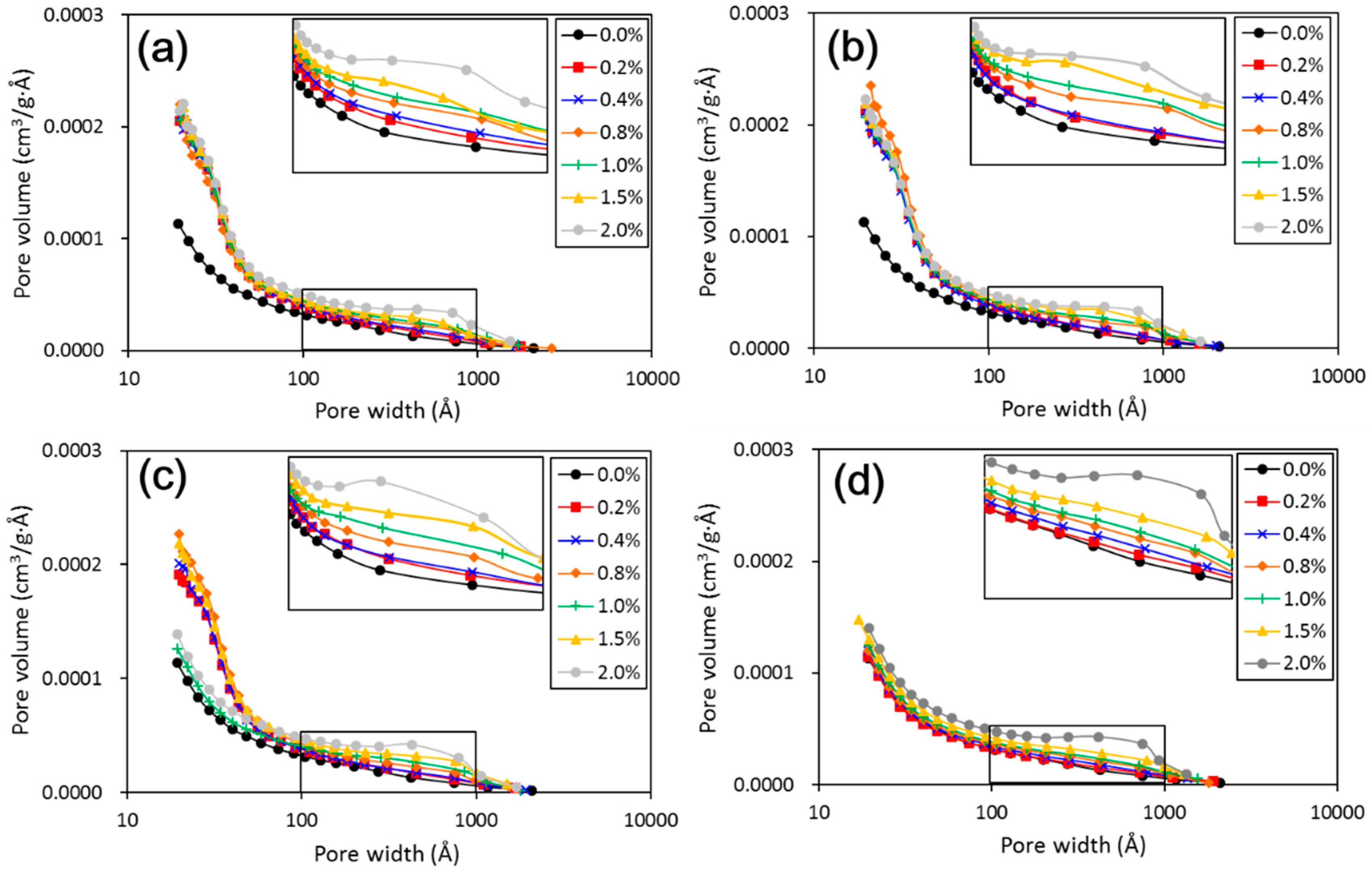
The above results have shown that the addition of nano-SiO
2 can strikingly change the properties of treated loess. The compressive strength of nano-SiO
2-treated loess increased with increasing nano-SiO
2 contents at different curing days (see
Figure 3). This resulted in an increase in USC and E
50 values (see
Figure 4). The decrease in water and volume supported the improvement of mechanical strength of nano-SiO
2-treated loess (see
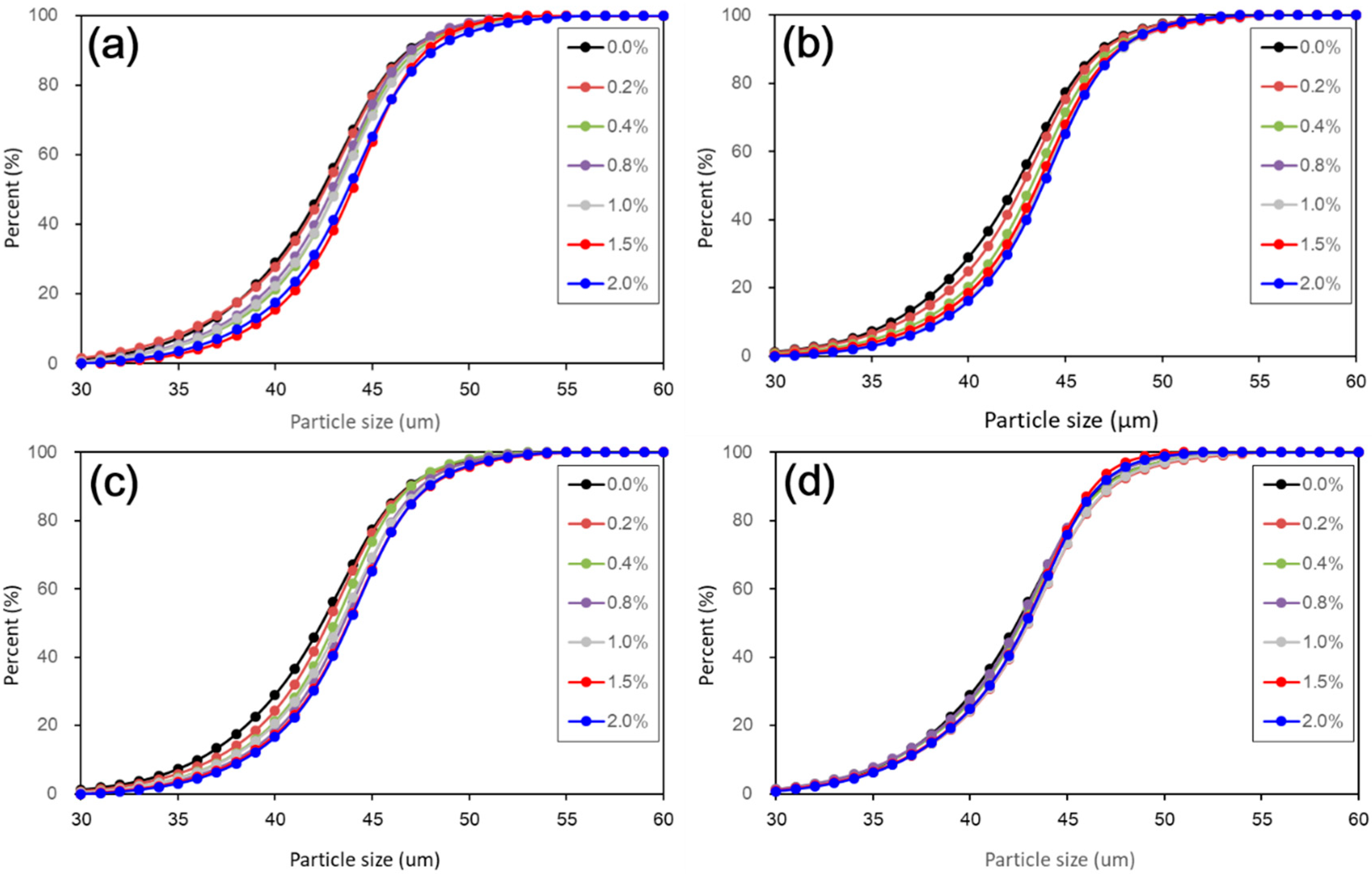
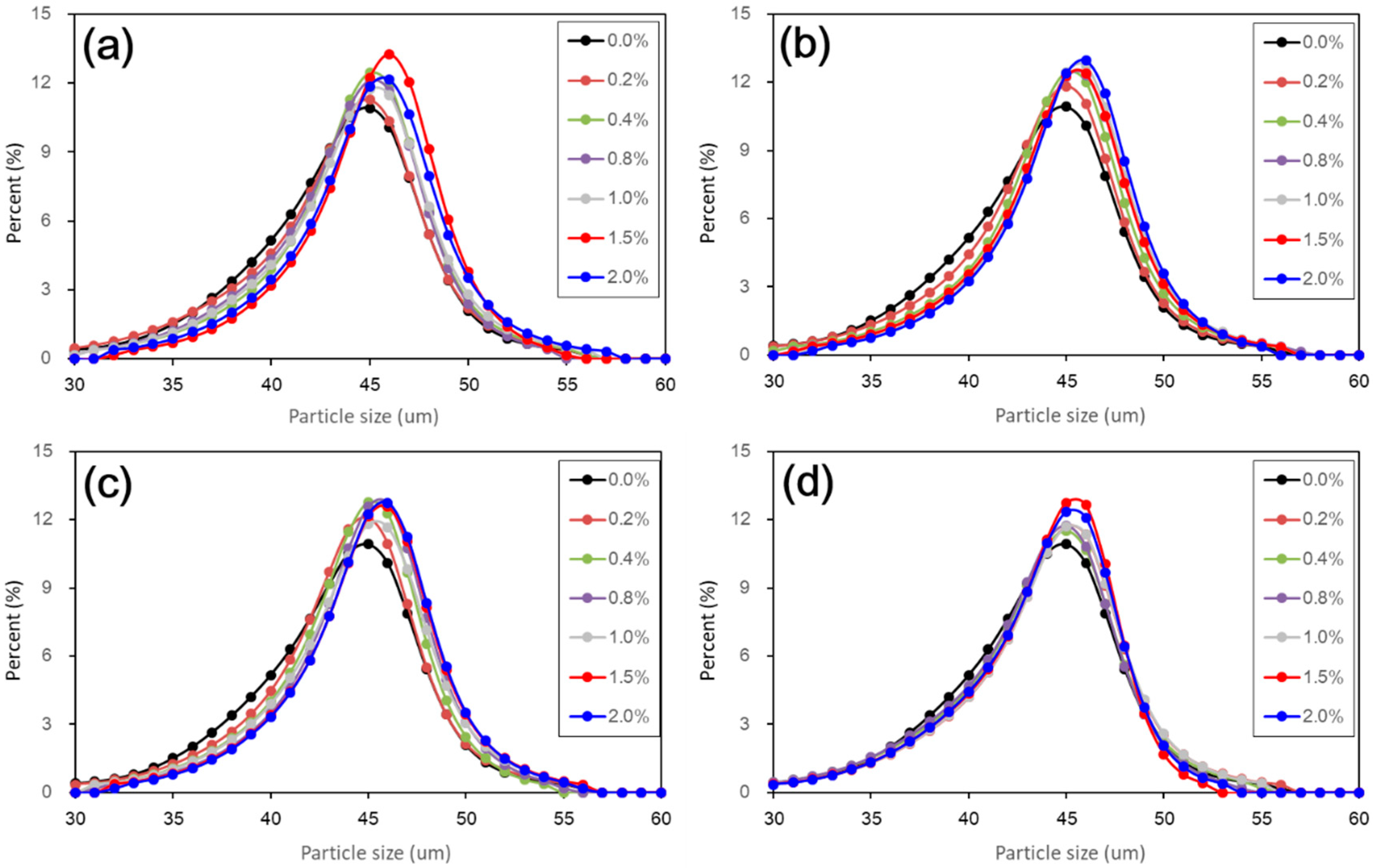
Figure 5). The results from particle size distributions and SEM images show there was an obvious modification in structure caused by coarser particles, denser packing, and smaller pores (see
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10). There was a similar trend in particle size and pore size after 28 days of curing. This means that the development of coarse particles and denser packing became weak with increasing curing duration. The particle size distributions and SEM images afforded a nice observation of macrostructure and microstructure, respectively. However, the BET analyses permitted better observation of the pore families and their evolution over curing duration. Meanwhile, the mineralogical components have proven that the changes in mechanical and structural properties of nano-SiO
2-treated loess are physical alterations rather than chemical reactions (see
Figure 11 and
Figure 12). The physical densification induced a strengthening effect on loess, which has also been observed its shear strength [
28].
There was a close relationship between macroscopic behaviors (such as, mechanical strength, density and water content) and microscopic characteristics (such as, structure and mineralogy) of treated soils [
25,
26,
27]. The SEM images results (see
Figure 8 and
Figure 9) confirmed that the addition of a nano-SiO
2 particle caused the filling of porous areas and the formation of greater aggregations and consequently caused coarser particle size and more interparticle contact. To some degree, the nano-SiO
2 particle itself was also beneficial to the aggregation formation [
13]. This agglomerated effect can be attributed to large surface areas and high surface reactivity of the nano-SiO
2 particle. As a result, the nano-SiO
2-treated loess produced stronger mechanical strength. Moreover, previous studies have found that loess with aggregates has a relatively higher strength [
2,
22].
There are two types of change trends in mechanical strength of treated loess. The different styles and trends are dependent on the reactive activity of nano-SiO
2 at different curing durations. Under short-term curing duration, the ductile behavior and linear increase in strength are related to the evolution of the structure due to nano-SiO
2 addition. As observed in
Figure 8, the nano-SiO
2 filling effect occurred first in pores between particles or aggregations and then came an obvious coating effect to single particle due to an ongoing addition. Nevertheless, the reactive activity of nano-SiO
2 was relatively weak to treated loess in the whole process. However, the structural changes were not a transient activity in processing. Under long-term curing duration, the brittle behavior and nonlinear increase in strength were attributed to stronger structure effect, which caused denser packing and smaller pores (
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10). We inferred that this is related to the nano-SiO
2 self-properties, due to large surface areas and high surface reactivity. The differences further show that various additives are time-dependent in improving the performance of treated soils. Therefore, for effective cost control in particle engineering, it is crucial that there is enough curing time to guarantee treated soils an appropriate addition content.
5. Conclusions
The present paper examined the changes in mechanical, mineralogical, and structural properties of nano-SiO2 treated loess with different contents and curing days. The compressive test results showed that mechanical strength of nano-SiO2-treated loess gradually improves by increasing content and curing days. The accumulative increase in mechanical strength can be attributed to the coarser particle, denser packing and smaller pore processes in nano-SiO2-treated loess. These changes in microscopic characteristics can be proven by the evidence from particle size, SEM, and BET. Furthermore, the changes in dry density and water content, along with the unchanged mineralogy, support the idea that the increase in mechanical strength of treated loess resulted from their physical structure modification rather than chemical alteration due to the addition of nano-SiO2. The results presented in this research have shown that nano-SiO2 may serve as a cost-effective additive in loess stabilization. Meanwhile, a small addition to seek full stabilization can be satisfied for nano-SiO2 treated loess when curing duration is long enough. Additionally, there was a close relationship between microscopic characteristics (mineralogy, microstructure and microspore) and macroscopic behaviors (strength, texture, state, etc.). The macroscopic behaviors of loess significantly depend on its microscopic characteristics. Thus, the linked relations can facilitate the understanding of the effect of nano-SiO2 on loess properties and their complicated interactions.
In addition, there is a strong need to conduct systematic laboratory experiments and particular field research into various nanomaterials that may be used as stabilizing additives to facilitate a better understanding for practical applications. Laboratory measurements are important for providing useful post hoc estimates for practical applications but field evaluations of the nano-materials are still not enough. Hence, there is a need to conduct an in-situ evaluation and a longer curing duration for nano-SiO2-treated loess.