Microstructure and Corrosion Resistance of Laser-Welded Crossed Nitinol Wires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Laser-Welding
2.2. Electrochemical Tests
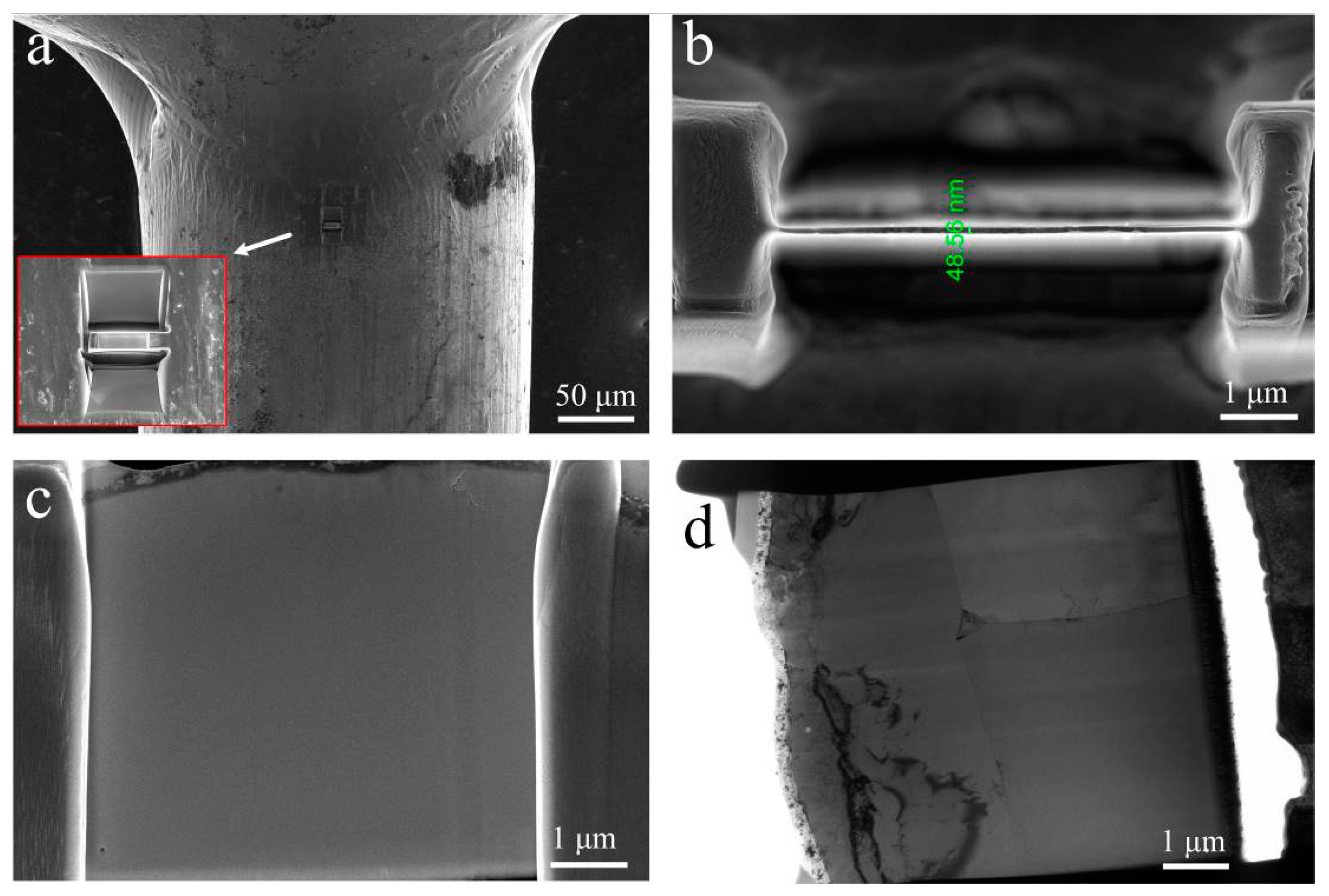
2.3. Microstructure Characterization
3. Results
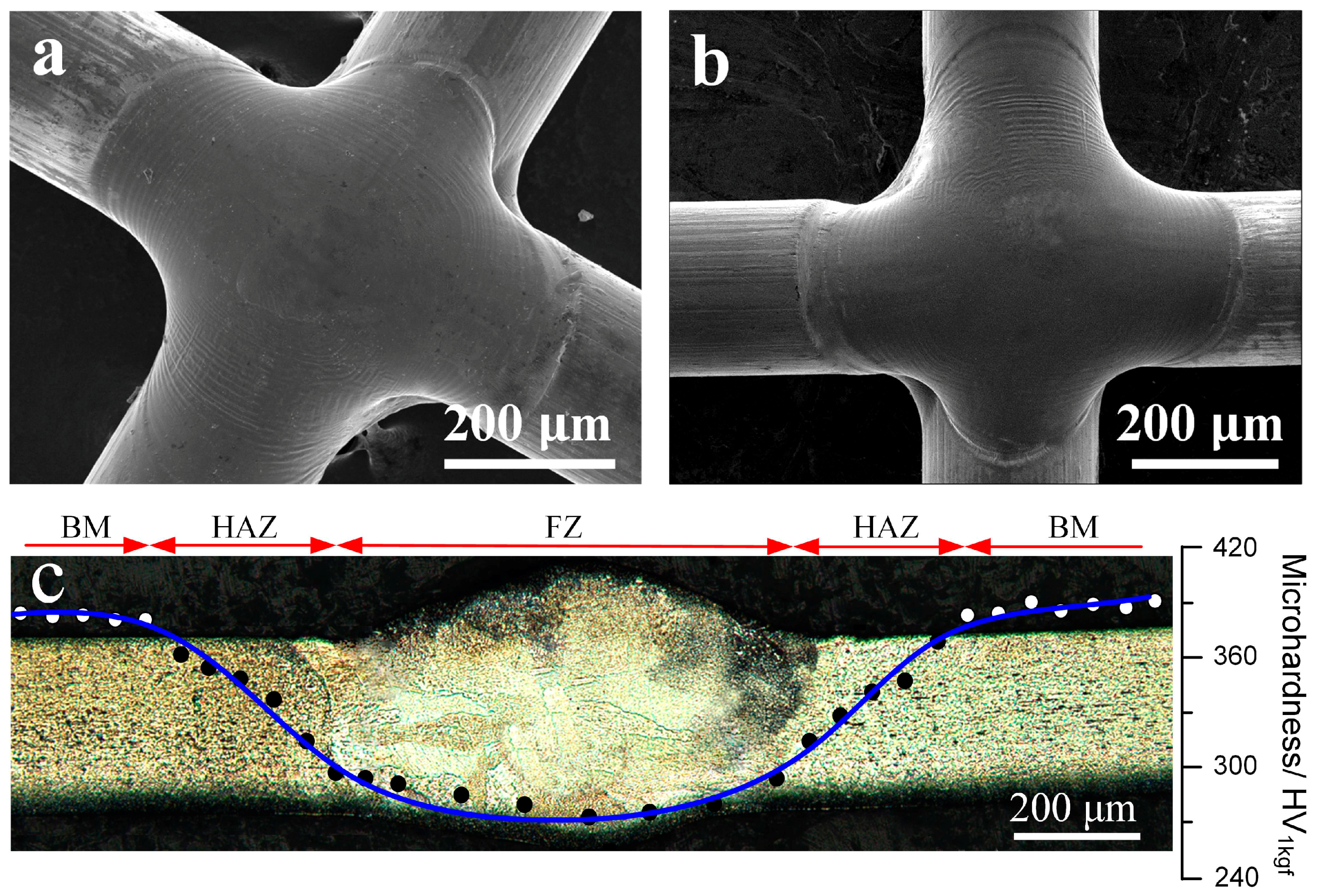
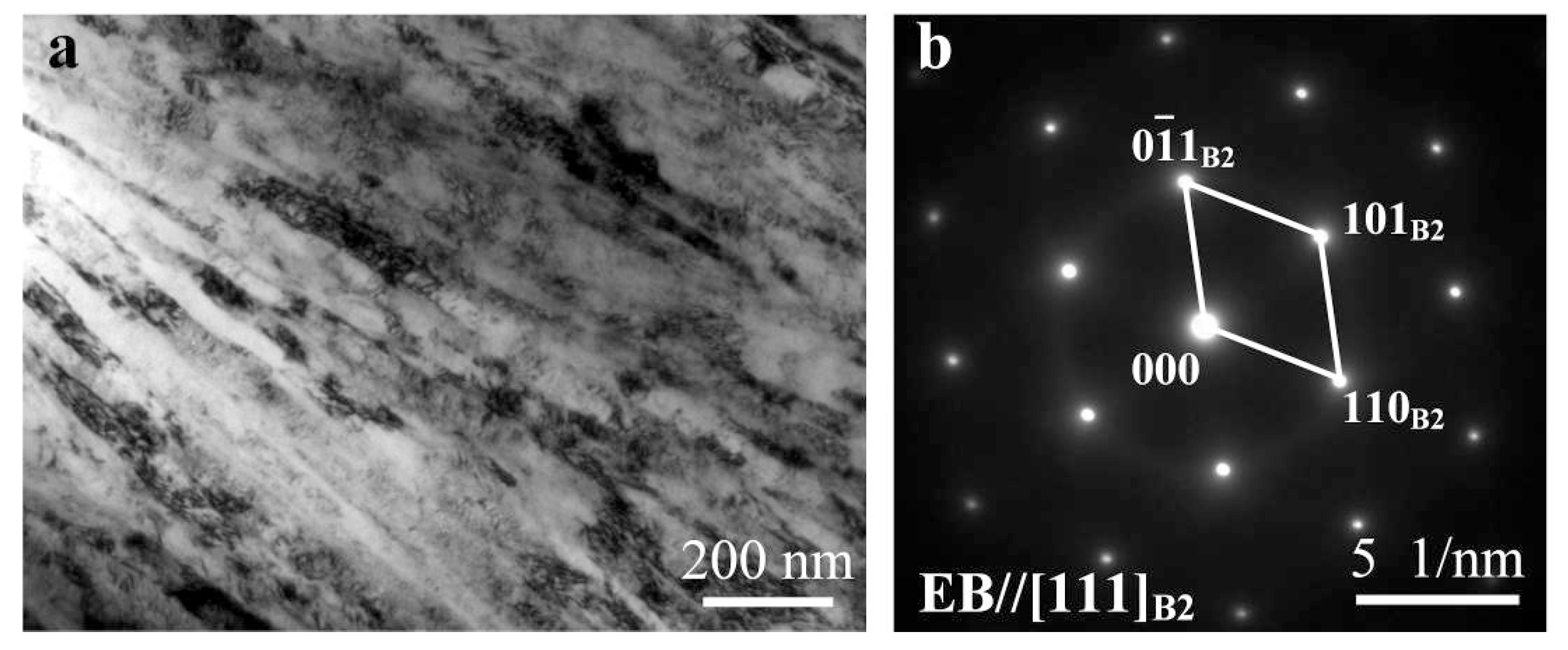
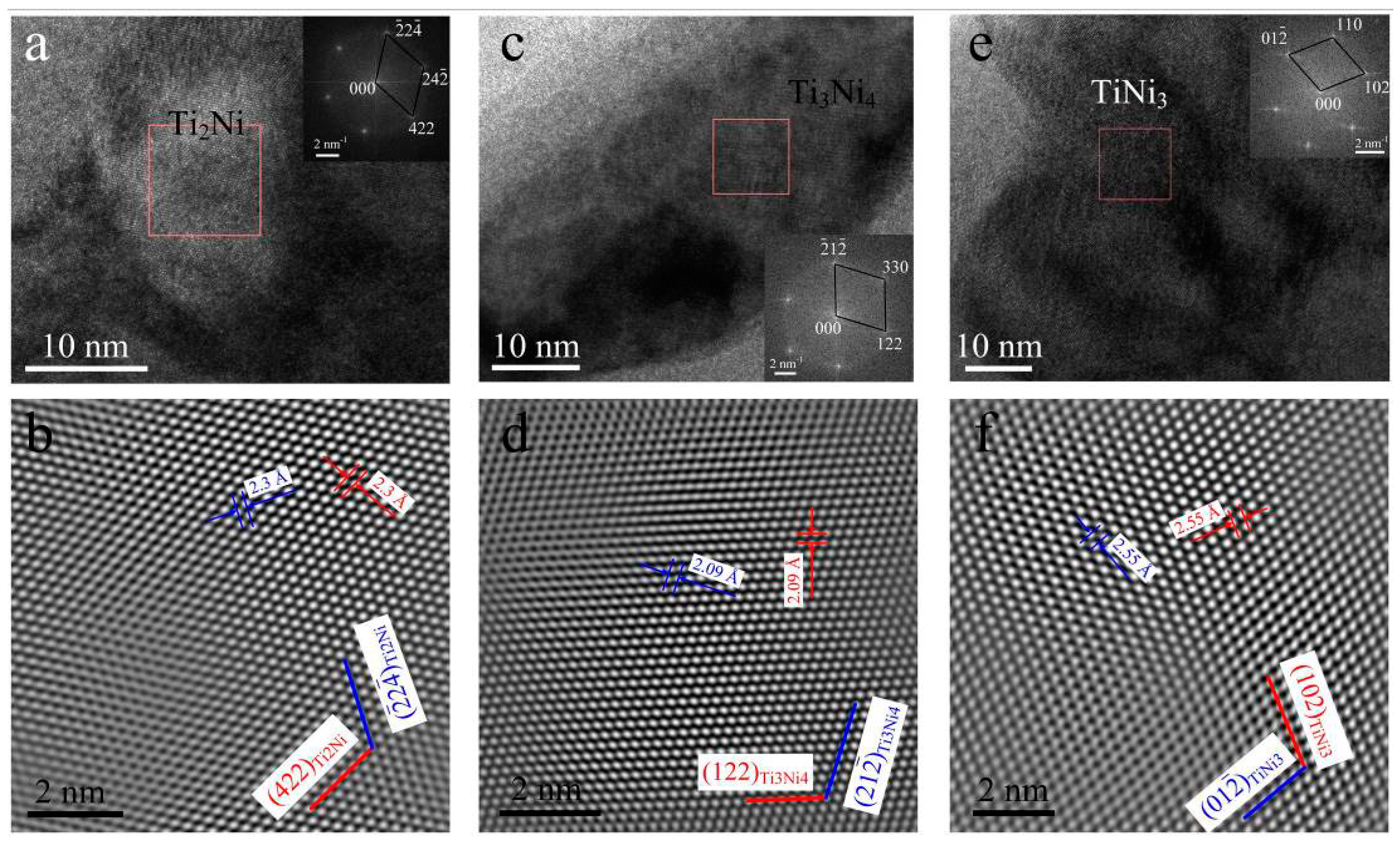
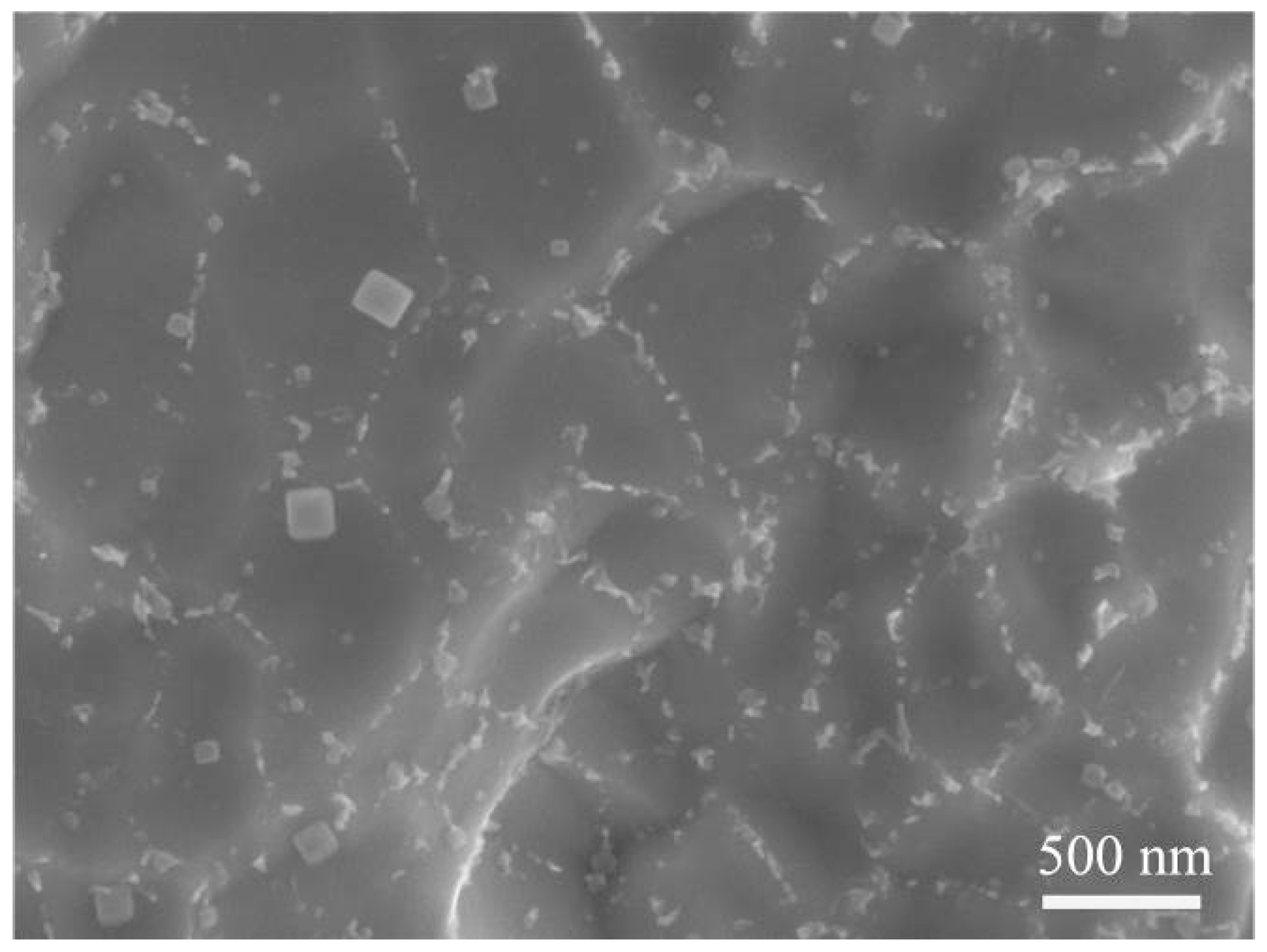
3.1. Microstructure Analysis
3.2. Corrosion Behavior of Nitinol Joints
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273, 149–160. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Petrini, L.; Migliavacca, F. Biomedical applications of shape memory alloys. J. Metall. 2011, 2011, 501483. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Miranda, R.M.; Braz Fernandes, F.M. Welding and joining of NiTi shape memory alloys: A review. Prog. Mater. Sci. 2017, 88, 412–466. [Google Scholar] [CrossRef]
- Kaiser, C.; Galatius, S.; Erne, P.; Eberli, F.; Alber, H.; Rickli, H.; Pedrazzini, G.; Hornig, B.; Bertel, O.; Bonetti, P.; et al. Drug-eluting versus bare-metal stents in large coronary arteries. New. Engl. J. Med. 2010, 363, 2310–2319. [Google Scholar] [CrossRef] [PubMed]
- Shabalovskaya, S.A.; Rondelli, G.C.; Undisz, A.L.; Anderegg, J.W.; Burleigh, T.D.; Rettenmayr, M.E. The electrochemical characteristics of native Nitinol surfaces. Biomaterials 2009, 30, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.N. Microjoining and Nanojoining; Woodhead: Cambridge, UK, 2008. [Google Scholar]
- Shabalovskaya, S.A. Surface, corrosion and biocompatibility aspects of Nitinol as an implant material. Bio-Med. Mater. Eng. 2002, 12, 69–119. [Google Scholar]
- Hsu, Y.T.; Wang, Y.R.; Wu, S.K.; Chen, C. Effect of CO2 laser welding on the shape-memory and corrosion characteristics of NiTi alloys. Metall. Mater. Trans. A 2001, 32, 569–576. [Google Scholar] [CrossRef]
- Yan, X.J.; Yang, D.Z. Corrosion resistance of a laser spot-welded joint of NiTi wire in simulated human body fluids. J. Biomed. Mater. Res. A 2006, 77, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Yang, D.Z.; Liu, X.P. Corrosion behavior of a laser-welded NiTi shape memory alloy. Mater. Charact. 2007, 58, 623–628. [Google Scholar] [CrossRef]
- Man, H.C.; Cui, Z.D.; Yue, T.M. Corrosion properties of laser surfice melted NiTi shape memory alloy. Scr. Mater. 2001, 45, 1447–1453. [Google Scholar] [CrossRef]
- Chan, C.W.; Man, H.C.; Yue, T.M. Susceptibility to stress corrosion cracking of NiTi laser weldment in Hanks’ solution. Corros. Sci. 2012, 57, 260–269. [Google Scholar] [CrossRef]
- Chan, C.W.; Man, H.C.; Yue, T.M. Susceptibility to environmentally induced cracking of laser-welded NiTi wires in Hanks’ solution at open-circuit potential. Mater. Sci. Eng. A 2012, 544, 38–47. [Google Scholar] [CrossRef]
- Chan, C.W.; Man, H.C.; Yue, T.M. Effect of post-weld heat-treatment on the oxide film and corrosion behaviour of laser-welded shape memory NiTi wires. Corros. Sci. 2012, 57, 158–167. [Google Scholar] [CrossRef]
- Tam, B.; Khan, M.I.; Zhou, Y. Mechanical and functional properties of laser-welded Ti-55.8 wt Pct Ni nitinol wires. Metall. Mater. Trans. A 2011, 42, 2166–2175. [Google Scholar] [CrossRef]
- Bataillard, L.; Bidaux, J.E.; Gotthardt, R. Interaction between microstructure and multiple-step transformation in binary NiTi alloys using in-situ transmission electron microscopy observations. Philos. Mag. A 1998, 78, 327–344. [Google Scholar] [CrossRef]
- Chatterjeee, S.; Abinandanan, T.A.; Chattopadhyay, K. Phase formation in Ti/Ni dissimilar welds. Mater. Sci. Eng. A 2008, 490, 7–15. [Google Scholar] [CrossRef]
- Liu, K.T.; Duh, J.G. Grain size effects on the corrosion behavior of Ni50.5Ti49.5 and Ni45.6Ti49.3Al5.1 films. J. Electroanal. Chem. 2008, 618, 45–52. [Google Scholar] [CrossRef]
- Demri, B.; Hage-Ali, M.; Moritz, M.; Muster, D. Surface characterization of C/Ti-6Al-4V coating treated with ion beam. Biomaterials 1997, 18, 305–310. [Google Scholar] [CrossRef]
- Cui, Z.D.; Man, H.C.; Yang, X.J. The corrosion and nickel release behavior of laser surface-melted NiTi shape memory alloy in Hank’s solution. Surf. Coat. Technol. 2005, 192, 347–353. [Google Scholar] [CrossRef]





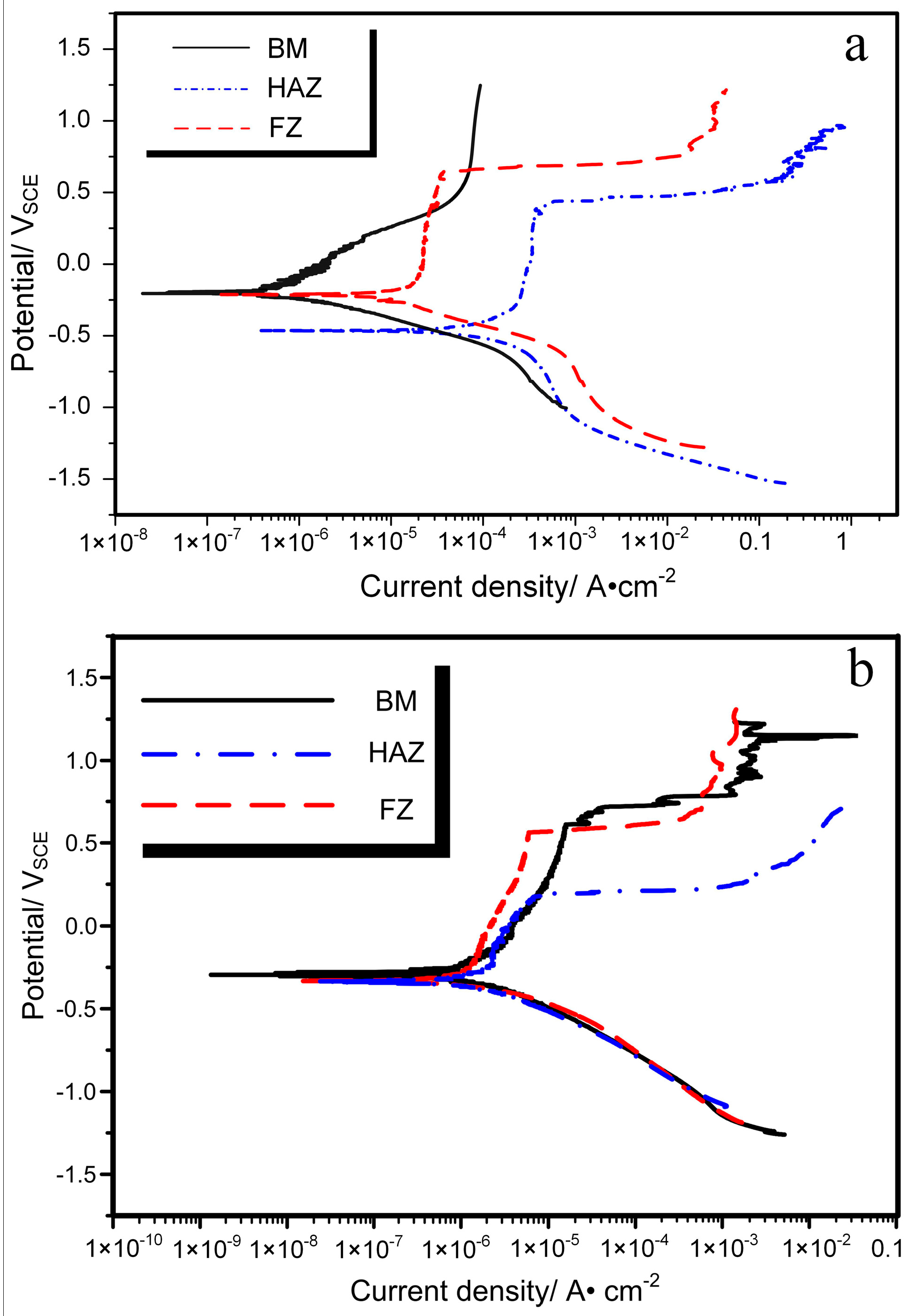

| Sample | Ecorr, mVSCE | Epit, mVSCE | Icorr, μA·cm−2 | Rcorr, μm·Year−1 |
|---|---|---|---|---|
| BM | −198 | - | 0.6 | 4.0 |
| FZ | −210 | 641 | 28.4 | 18.6 |
| HAZ | −468 | 404 | 111.3 | 739.8 |
| Sample | Ecorr, mVSCE | Epit, mVSCE | Icorr, μA·cm−2 | Rcorr, μm·Year−1 |
|---|---|---|---|---|
| BM | −302 | 703 | 0.7 | 4.5 |
| FZ | −328 | 568 | 1.2 | 7.6 |
| HAZ | −336 | 198 | 2.4 | 15.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.; Yao, R.; Yan, Z.; Yan, Z.; Wang, W.; He, X.; Zhou, J. Microstructure and Corrosion Resistance of Laser-Welded Crossed Nitinol Wires. Materials 2018, 11, 842. https://doi.org/10.3390/ma11050842
Dong P, Yao R, Yan Z, Yan Z, Wang W, He X, Zhou J. Microstructure and Corrosion Resistance of Laser-Welded Crossed Nitinol Wires. Materials. 2018; 11(5):842. https://doi.org/10.3390/ma11050842
Chicago/Turabian StyleDong, Peng, Runhua Yao, Zheng Yan, Zhifeng Yan, Wenxian Wang, Xiuli He, and Jun Zhou. 2018. "Microstructure and Corrosion Resistance of Laser-Welded Crossed Nitinol Wires" Materials 11, no. 5: 842. https://doi.org/10.3390/ma11050842





