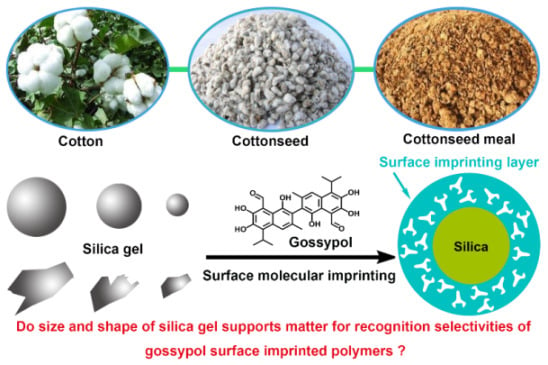
Influence of Size and Shape of Silica Supports on the Sol–Gel Surface Molecularly Imprinted Polymers for Selective Adsorption of Gossypol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
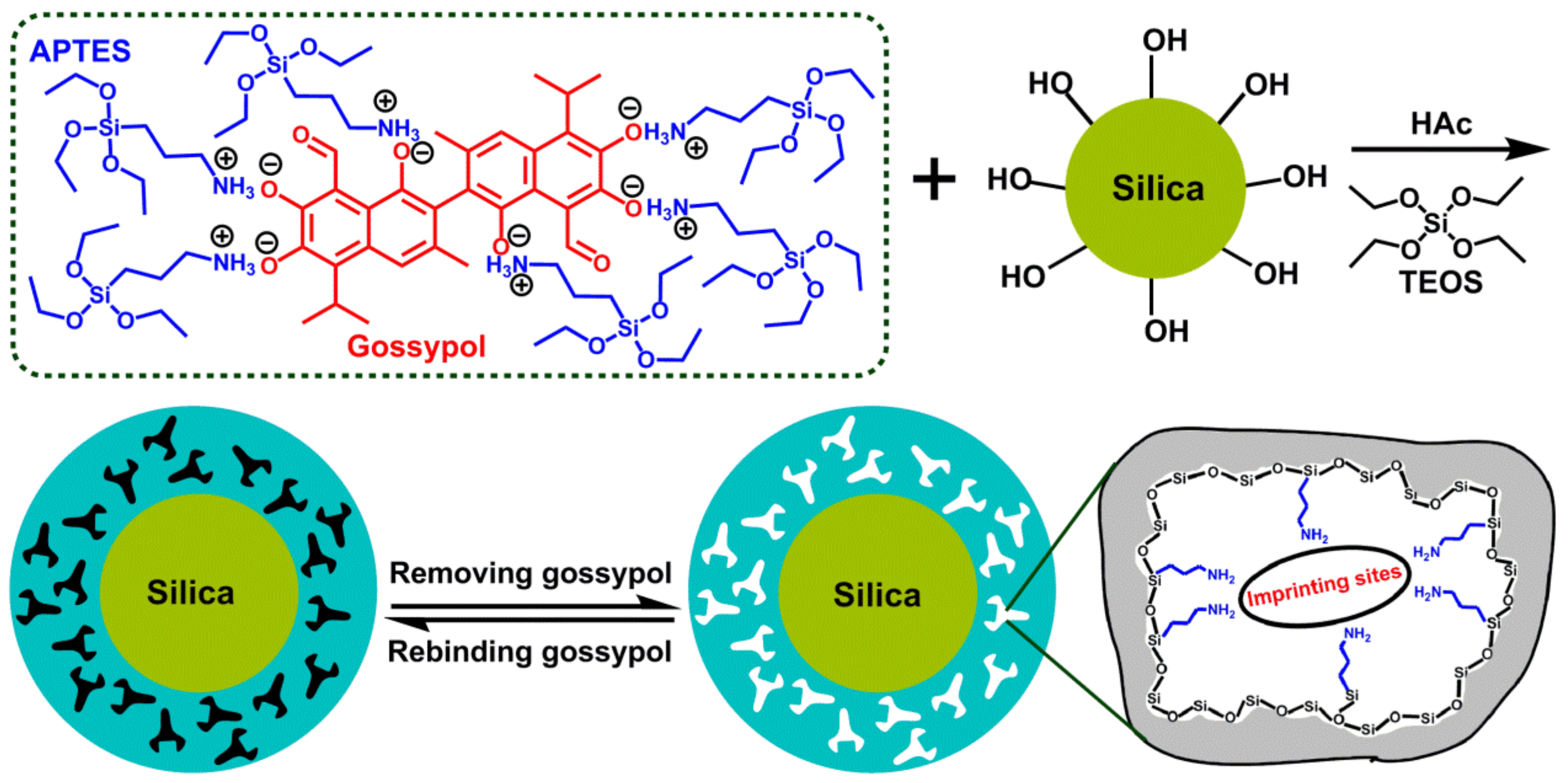
2.2. Preparation of Surface MIPs
2.3. Characterization
2.4. Binding Experiments
2.5. Reusability of MIPs
3. Results and Discussion
3.1. Preparation of the Gossypol-MIPs
3.2. Characterization of MIPs
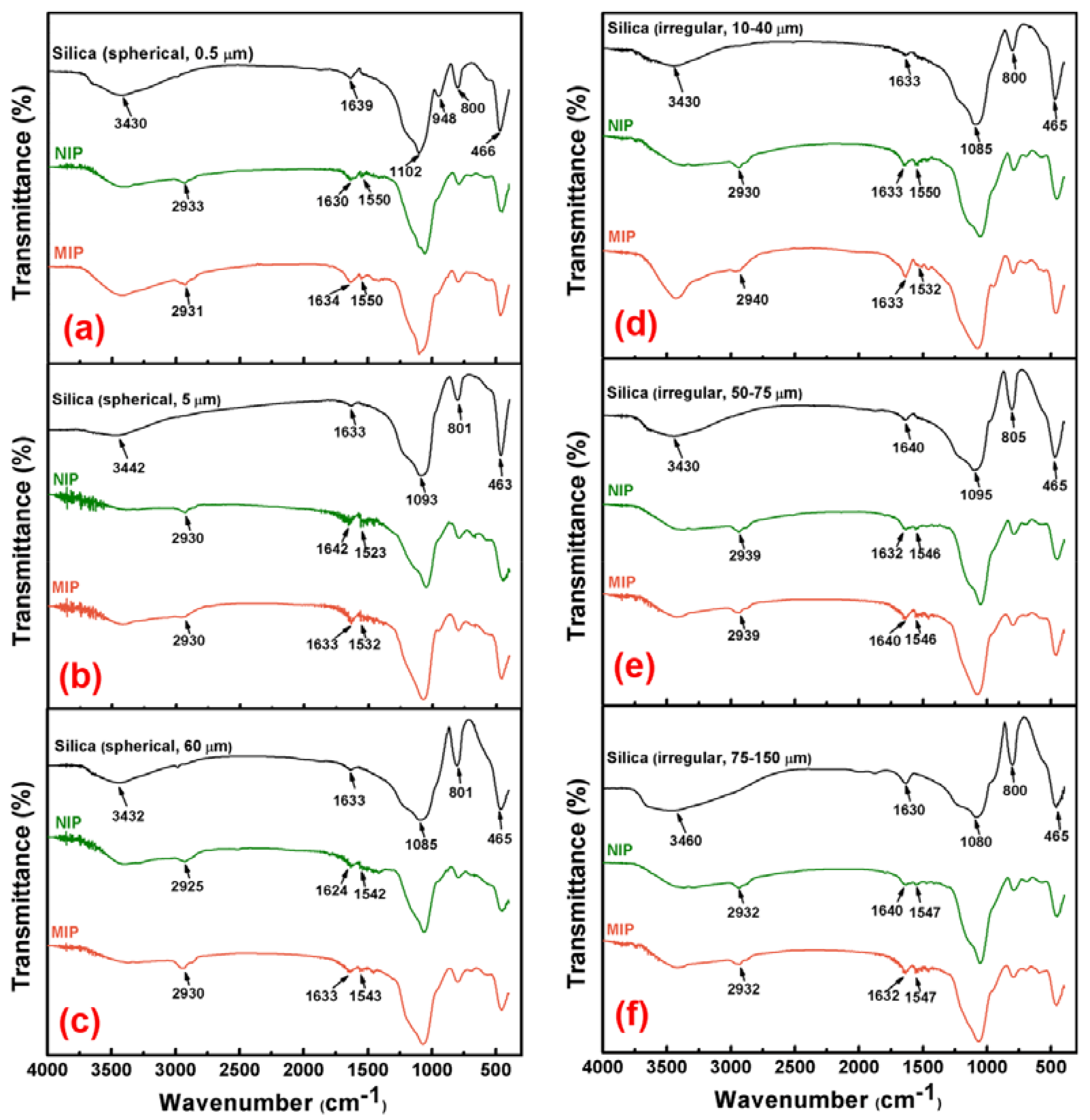
3.2.1. FTIR Analysis
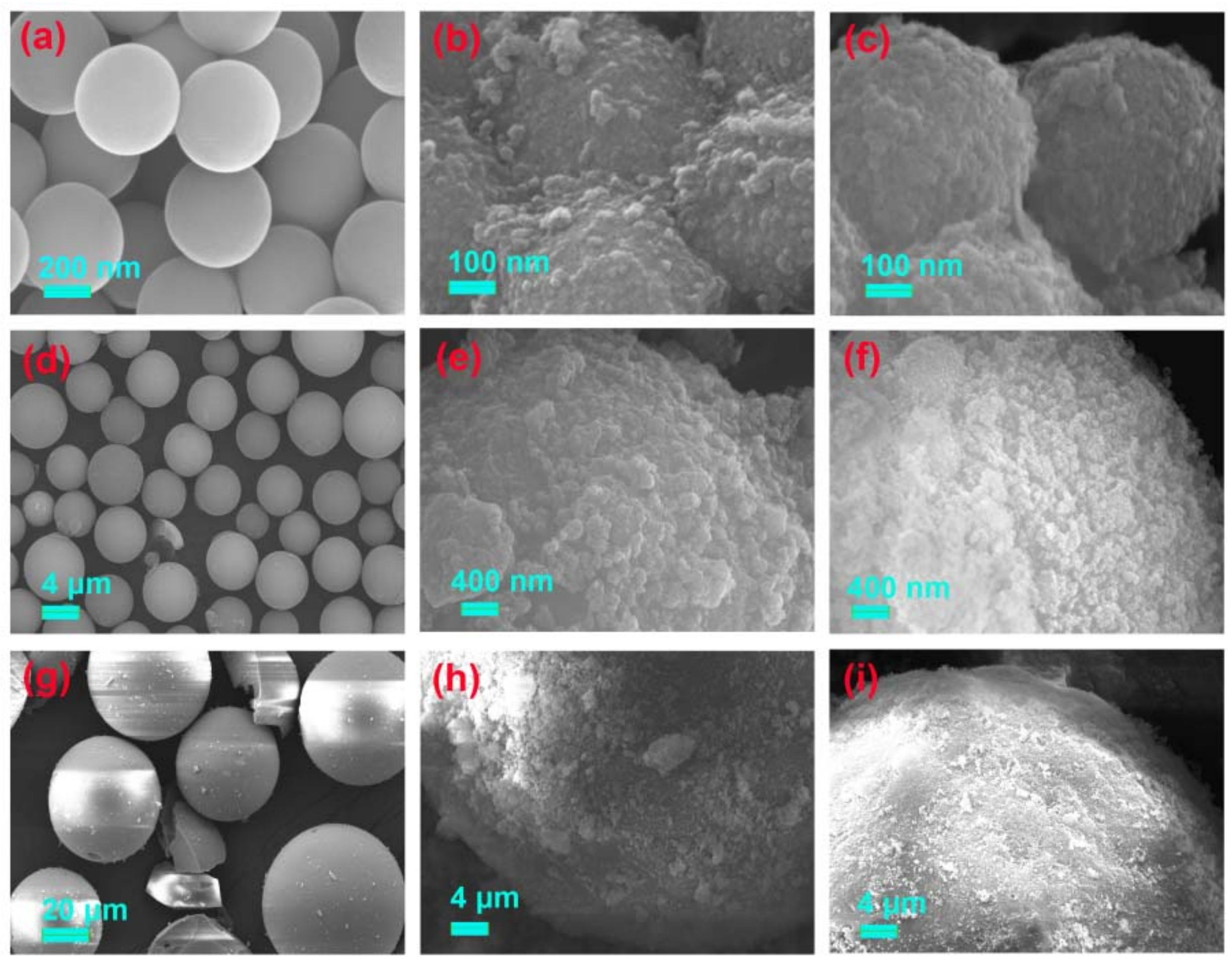
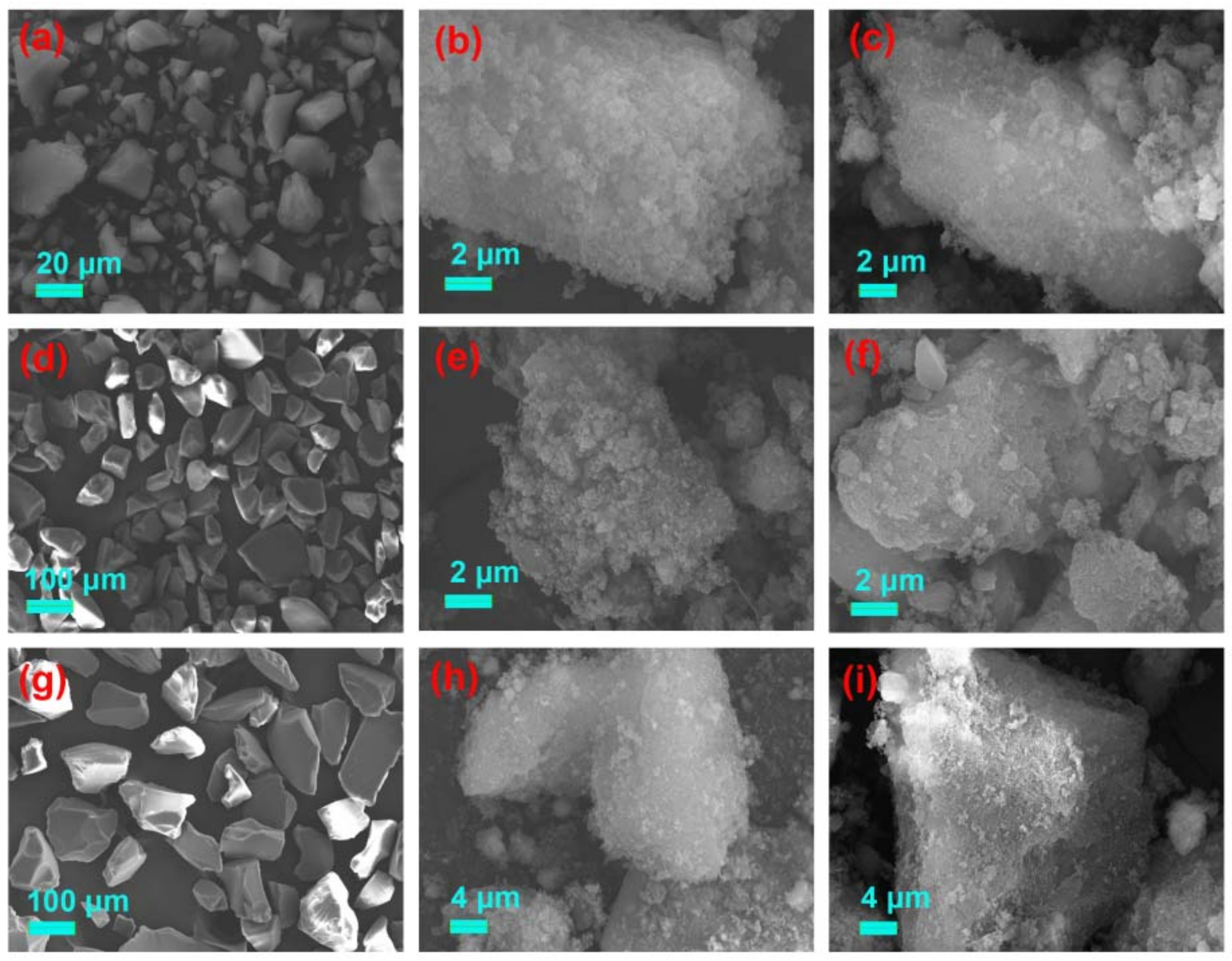
3.2.2. SEM Analysis
3.2.3. Standard BET Analysis
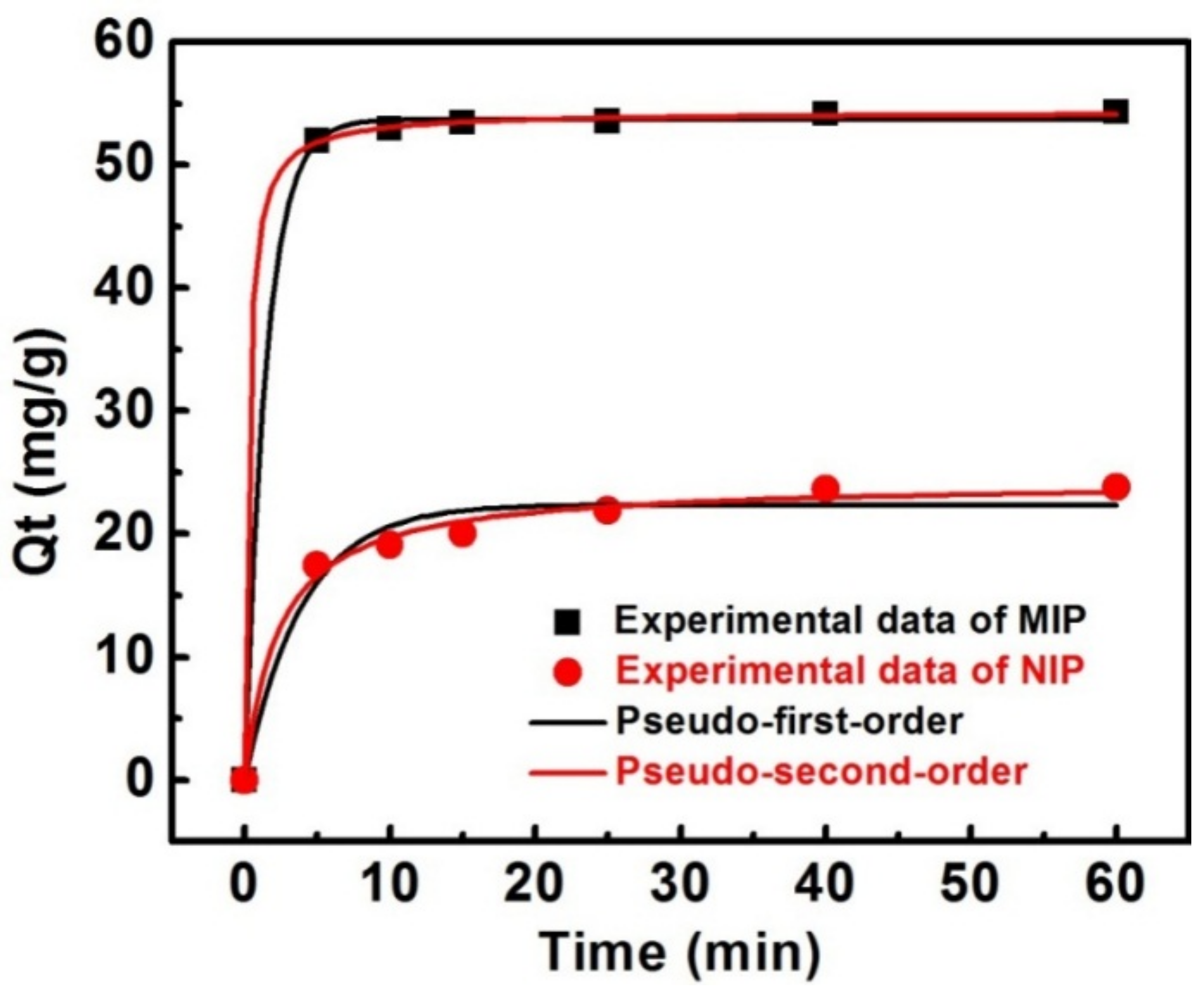
3.3. Adsorption Kinetics of MIPs
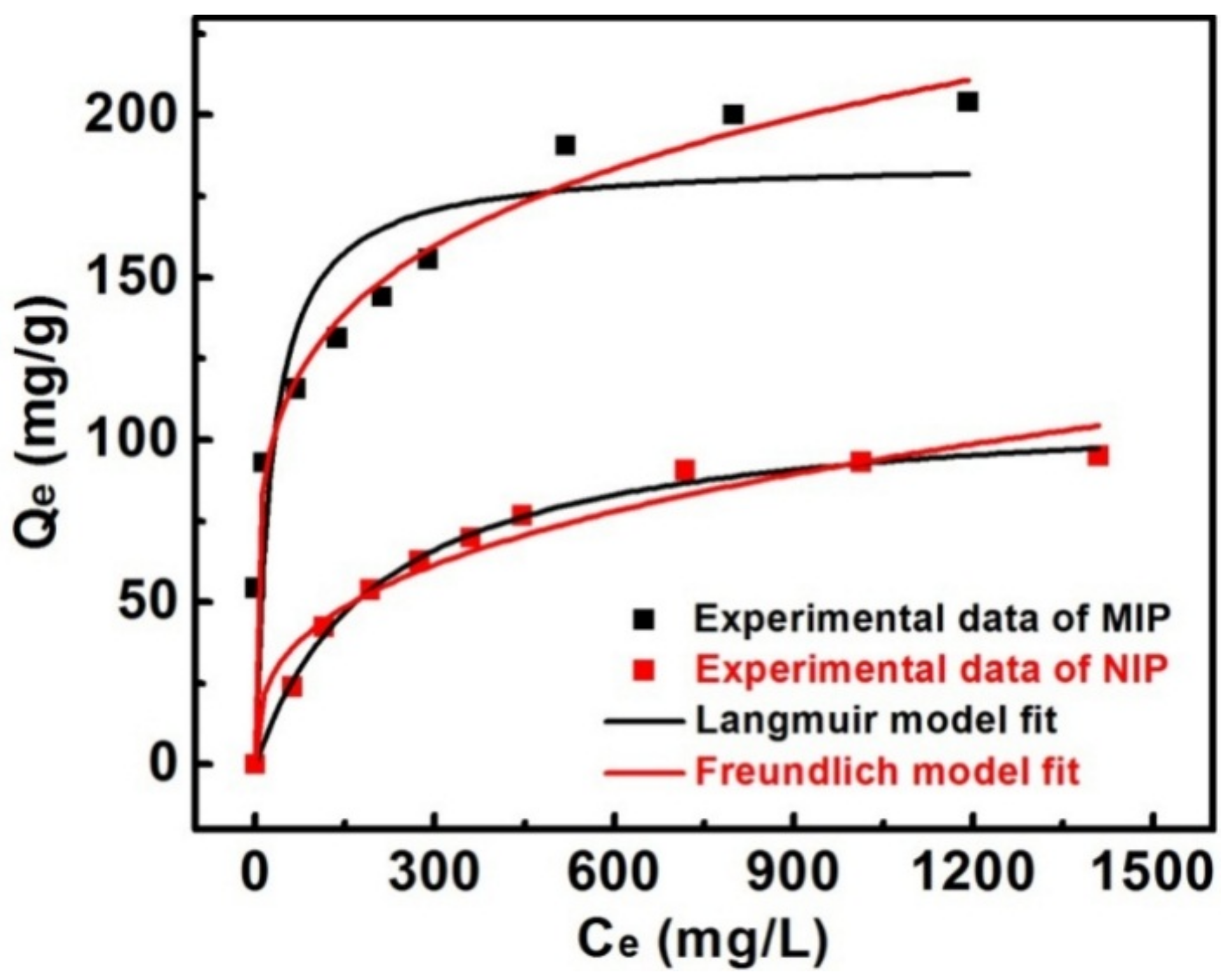
3.4. Adsorption Isotherms of MIPs
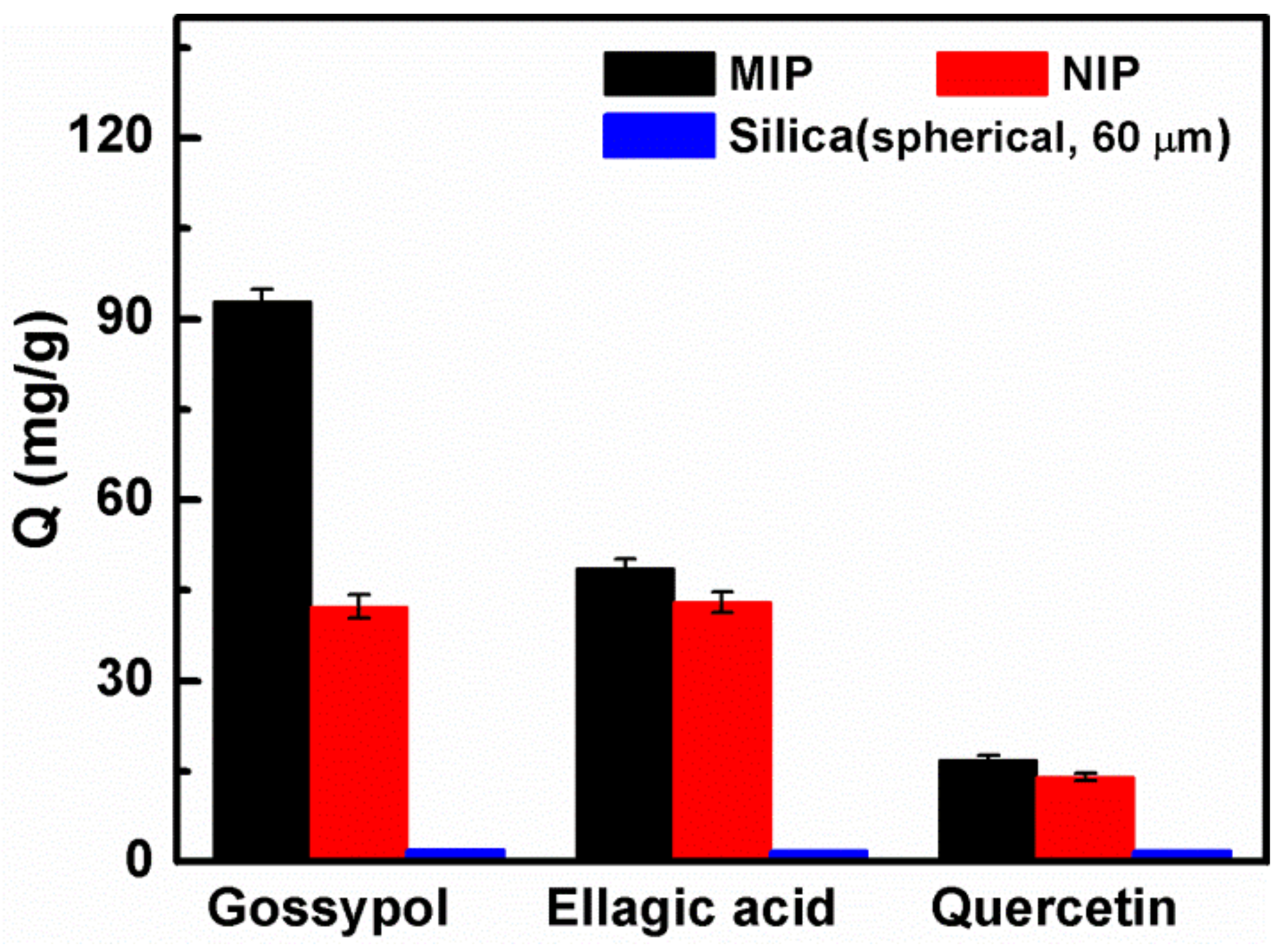
3.5. Adsorption Selectivity of the MIPs
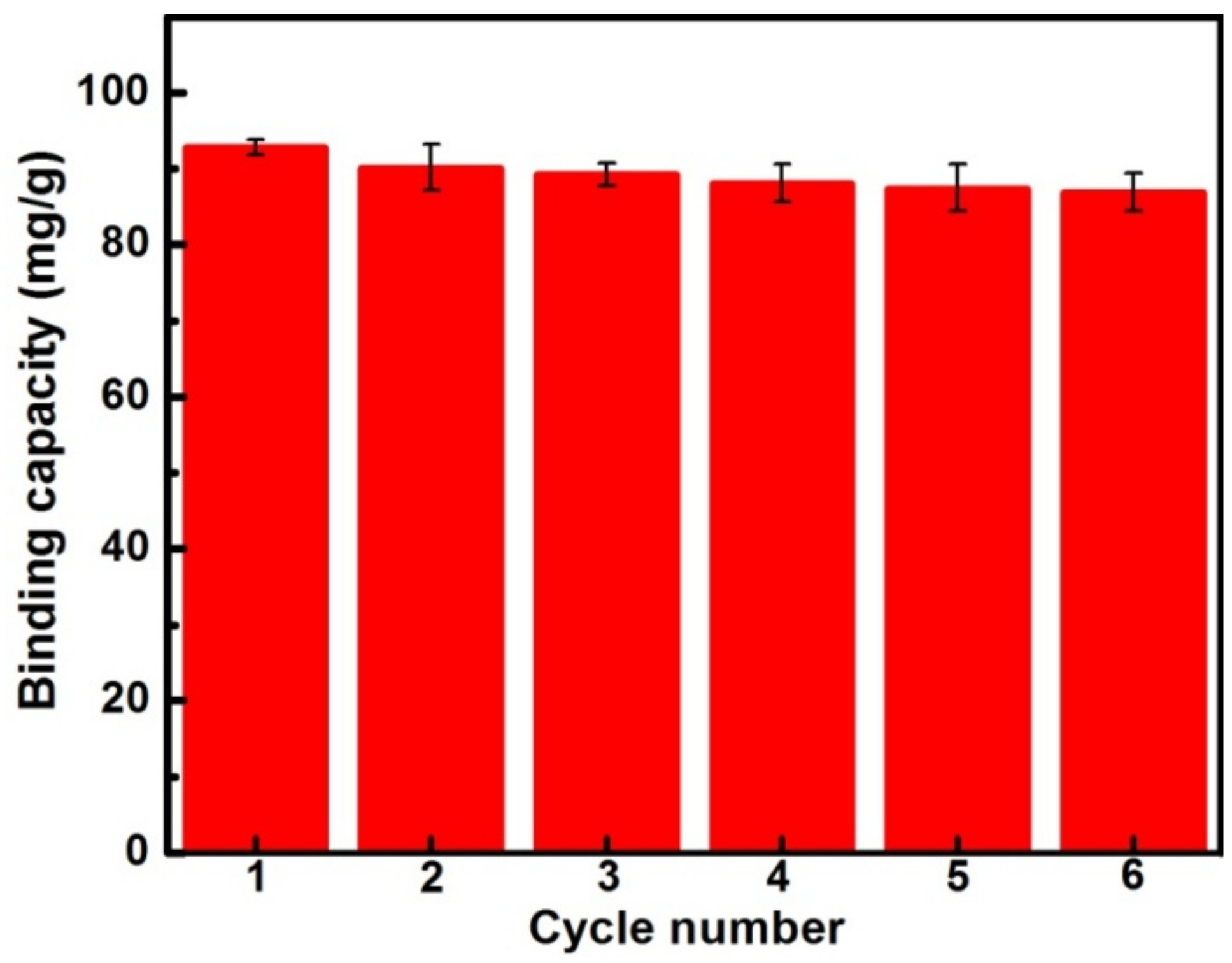
3.6. Regeneration and Stability of the MIPs
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wuff, G.; Sarhan, A. The use of polymers with enzyme-analogous structures for the resolution of racemate. Angew. Chem. Int. Ed. 1972, 11, 341–345. [Google Scholar]
- Andersson, L.; Sellergren, B.; Mosbach, K. Imprinting of amino acid derivatives in macroporous polymers. Tetrahedron Lett. 1984, 25, 5211–5214. [Google Scholar] [CrossRef]
- Sellergren, B.; Shea, K.J. Influence of polymer morphology on the ability of imprinted network polymers to resolve enantiomers. J. Chromatogr. A 1993, 635, 31–49. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Rodriguez, M.E.; Villar, P.; Vulfson, E.N. A new method for the introduction of recognition site functionality into polymers prepared by molecular imprinting: Synthesis and characterization of polymeric receptors for cholesterol. J. Am. Chem. Soc. 1995, 117, 7105–7111. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulff, G.; Liu, J. Design of biomimetic catalysts by molecular imprinting in synthetic polymers: The role of transition state stabilization. Acc. Chem. Res. 2012, 45, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş. Molecularly Imprinted Membranes: Past, Present, and Future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef] [PubMed]
- Schirhagl, R. Bioapplications for Molecularly Imprinted Polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; She, X.; Wang, L.; Fan, H.; Zhou, Q.; Huang, X.; Tang, J. Preparation, Characterization and Application of a Molecularly Imprinted Polymer for Selective Recognition of Sulpiride. Materials 2017, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Fan, F.; Zhang, Y.; Pei, Z.; Wang, W.; Pei, Y. A Facile Approach for Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin. Polymers 2017, 9, 135. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Z.; Wang, Y. Preparation and application of surface molecularly imprinted silica gel for selective extraction of melamine from milk samples. Talanta 2013, 116, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Jia, X.; Yang, Y.; Liu, X. Porous Carbon Microspheres: An Excellent Support To Prepare Surface Molecularly Imprinted Polymers for Selective Removal of Dibenzothiophene in Fuel Oil. Ind. Eng. Chem. Res. 2016, 55, 1710–1719. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Z.; Wu, M.; Xie, C.; Guan, G.; Wang, D. A surface functional monomer-directing strategy for highly dense imprinting of TNT at surface of silica nanoparticles. J. Am. Chem. Soc. 2007, 129, 7859–7866. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.T.C.; Dutra, R.F.; Noronha, J.P.; Sales, M.G.F. Myoglobin-biomimetic electroactive materials made by surface molecular imprinting on silica beads and their use as ionophores in polymeric membranes for potentiometric transduction. Biosens. Bioelectron. 2011, 26, 4760–4766. [Google Scholar] [CrossRef] [PubMed]
- Gauczinski, J.; Liu, Z.; Zhang, X.; Schönhoff, M. Surface Molecular Imprinting in Layer-by-Layer films on Silica Particles. Langmuir 2012, 28, 4267–4273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sheng, N.; Zhu, R.; Wei, F.; Cai, Z.; Zhai, M.; Du, S.; Hu, Q. Preparation of dummy template imprinted polymers at surface of silica microparticles for the selective extraction of trace bisphenol A from water samples. J. Hazard. Mater. 2010, 179, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Lofgreen, J.E.; Ozin, G.A. Controlling morphology and porosity to improve performance of molecularly imprinted sol-gel silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Wang, X.; Shen, D.; Chen, L. Quantum Dots Based Mesoporous Structured Imprinting Microspheres for the Sensitive Fluorescent Detection of Phycocyanin. ACS Appl. Mater. Interfaces 2015, 7, 9118–9127. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gao, Y.; Tan, K.; Wei, W.; Liu, X. Preparation of a Magnetic Molecularly Imprinted Graphene Composite Highly Adsorbent for 4-Nitrophenol in Aqueous Medium. ACS Sustain. Chem. Eng. 2016, 4, 3316–3326. [Google Scholar] [CrossRef]
- Zhu, R.; Zhao, W.; Zhai, M.; Wei, F.; Cai, Z.; Sheng, N.; Hu, Q. Molecularly imprinted layer-coated silica nanoparticles for selective solid-phase extraction of bisphenol A from chemical cleansing and cosmetics samples. Anal. Chim. Acta 2010, 658, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical Sensors Based on Molecularly Imprinted Sol-Gel Materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Raof, S.F.A.; Mohamad, S.; Abas, M.R. Synthesis and Evaluation of Molecularly Imprinted Silica Gel for 2-Hydroxybenzoic Acid in Aqueous Solution. Int. J. Mol. Sci. 2013, 14, 5952–5965. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of a Lower Toxic Approach Based on Green Synthesis of Water-Compatible Molecularly Imprinted Nanoparticles for the Extraction of Hydrochlorothiazide from Human Urine. ACS Sustain. Chem. Eng. 2017, 5, 3775–3785. [Google Scholar] [CrossRef]
- Withers, W.A.; Carruth, F.E. Gossypol—A toxic substance in cottonseed. A preliminary note. Science 1915, 41, 324. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Geissman, T.A.; Edwards, J.D. Gossypol, a pigment of cottonseed. Chem. Rev. 1960, 60, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Maugh, T.H. Male “pill” blocks sperm enzyme. Science 1981, 212, 314. [Google Scholar] [CrossRef] [PubMed]
- Montamat, E.E.; Burgos, C.; De Burgos, N.M.G.; Rovai, L.E.; Blanco, A.; Segura, E.L. Inhibitory action of gossypol on enzymes and growth of Trypanosoma cruzi. Science 1982, 218, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Randel, R.D.; Chase, C.C.; Wyse, S.J. Effects of gossypol and cottonseed products on reproduction of mammals. J. Anim. Sci. 1992, 70, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Barraza, M.L.; Coppock, C.E.; Brooks, K.N.; Wilks, D.L.; Saunders, R.G.; Latimer, G.W. Iron sulfate and feed pelleting to detoxify free gossypol in cottonseed diets for dairy cattle. J. Dairy Sci. 1991, 74, 3457–3467. [Google Scholar] [CrossRef]
- Hron, R.; Kuk, M.; Abraham, G.; Wan, P. Ethanol extraction of oil, gossypol and aflatoxin from cottonseed. J. Am. Oil Chem. Soc. 1994, 71, 417–421. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Z.; Zhao, S.; Sun, J.; Yang, X. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Anim. Feed. Sci. Tech. 2007, 135, 176–186. [Google Scholar] [CrossRef]
- Kuk, M.S.; Hronsr, R.J.; Abraham, G. Adsorptive gossypol removal. J. Am. Oil Chem. Soc. 1993, 70, 209–210. [Google Scholar] [CrossRef]
- Kuk, M.S.; Tetlow, R. Gossypol removal by adsorption from cottonseed miscella. J. Am. Oil Chem. Soc. 2005, 82, 905–909. [Google Scholar] [CrossRef]
- Zhi, K.; Wang, L.; Zhang, Y.; Zhang, X.; Zhang, L.; Liu, L.; Yao, J.; Xiang, W. Preparation and evaluation of molecularly imprinted polymer for selective recognition and adsorption of gossypol. J. Mol. Recognit. 2017, 9, e2627. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Zhang, B.; Tang, Y.; Zuo, X.; Wang, S.; Tang, J. Imprinted functionalized silica sol-gel for solid-phase extraction of triazolamin. Talanta 2011, 84, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, Z.; Ma, L.; Zhang, M.; Wang, J.; Dai, X.; Yan, Y. Selective Adsorption of Methylparaben by Submicrosized Molecularly Imprinted Polymer: Batch and Dynamic Flow Mode Studies. Ind. Eng. Chem. Res. 2012, 51, 14915–14924. [Google Scholar] [CrossRef]
- Reyes, J.; Wyrick, S.D.; Borriero, L.; Benos, D.J. Membrane actions of male contraceptive gossypol tautomers. Biochim. Biophys. Acta 1986, 863, 101–109. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, W.; Ma, J.; Wen, Q.; Wang, J.; Zhao, F. Synthesis and properties of bisphenol A molecular imprinted particle for selective recognition of BPA from water. J. Colloid Interface Sci. 2012, 367, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Shi, W.; Liu, W.; Yang, Y.; Liu, X.; Xu, B. Surface molecularly imprinted polymers grafted on ordered mesoporous carbon nanospheres for fuel desulfurization. RSC Adv. 2016, 6, 12504–12513. [Google Scholar] [CrossRef]
- Chang, Y.; Ko, T.; Hsu, T.; Syu, M. Synthesis of an Imprinted Hybrid Organic−Inorganic Polymeric Sol−Gel Matrix Toward the Specific Binding and Isotherm Kinetics Investigation of Creatinine. Anal. Chem. 2009, 81, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska, A.M.; Poliwoda, A.; Wieczorek, P.P. Surface molecularly imprinted silica for selective solid-phase extraction of biochanin A, daidzein and genistein from urine samples. J. Chromatogr. A 2015, 1392, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Mu, X.; Hao, Y.; Zhang, L.; Zhang, J.; Tang, Y. Combination of surface imprinting and immobilized template techniques for preparation of core–shell molecularly imprinted polymers based on directly amino-modified Fe3O4 nanoparticles for specific recognition of bovine hemoglobin. J. Mater. Chem. B 2014, 2, 1733–1741. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
| Samples | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Silica (spherical 0.5 μm) | 14.29 | 0.08242 | 23.07 |
| Spherical 0.5 μm NIP | 31.53 | 0.1757 | 22.29 |
| Spherical 0.5 μm MIP | 268.2 | 0.3716 | 5.542 |
| Silica (spherical 5 μm) | 280.9 | 0.8205 | 11.68 |
| spherical 5 μm NIP | 52.75 | 0.138 | 10.47 |
| spherical 5 μm MIP | 124.7 | 0.2206 | 7.077 |
| Silica (spherical 60 μm) | 431.4 | 0.8606 | 7.981 |
| spherical 60 μm NIP | 73.14 | 0.1755 | 9.598 |
| spherical 60 μm MIP | 112.2 | 0.3158 | 11.26 |
| Silica (irregular 10–40 μm) | 299.9 | 0.9128 | 12.18 |
| irregular 10–40 μm NIP | 85.36 | 0.2540 | 11.90 |
| irregular 10–40 μm MIP | 287.6 | 0.3775 | 5.251 |
| Silica (irregular 50–75 μm) | 275.9 | 0.8362 | 12.12 |
| irregular 50–75 μm NIP | 76.70 | 0.2248 | 11.73 |
| irregular 50–75 μm MIP | 130.4 | 0.2903 | 8.904 |
| Silica (irregular 75–150 μm) | 252.2 | 0.3120 | 4.947 |
| irregular 75–150 μm NIP | 91.66 | 0.2176 | 9.495 |
| irregular 75–150 μm MIP | 108.5 | 0.2610 | 9.626 |
| Supports | te (min) a | IF a | Qe (mg·g−1) b |
|---|---|---|---|
| Silica (spherical 0.5 μm) | 40 | 3.28 | 65.5 |
| Silica (spherical 5 μm) | 40 | 2.65 | 51.4 |
| Silica (spherical 60 μm) | 10 | 2.28 | 92.9 |
| Silica (irregular 10–40 μm) | 30 | 1.64 | 84.5 |
| Silica (irregular 50–75 μm) | 40 | 1.41 | 68 |
| Silica (irregular 75–150 μm) | 50 | 1.50 | 62.2 |
| Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| sample | Qe,exp (mg·g−1) | Qe,cal (mg·g−1) | k1 (min−1) | R2 | Qe (mg·g−1) | k2 (mg·g−1·min−1) | R2 |
| MIP | 54.3 | 53.67 | 0.6820 | 0.9994 | 54.30 | 0.07775 | 0.9999 |
| NIP | 23.8 | 22.36 | 0.2562 | 0.9669 | 24.27 | 0.01786 | 0.9917 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| sample | Qe,exp (mg·g−1) | Qm (mg·g−1) | KL (L·mg−1) | R2 | KF (mg·g−1) | n | R2 |
| MIP | 204 | 185.9 | 0.03761 | 0.8416 | 50.81 | 4.979 | 0.9903 |
| NIP | 95 | 111.6 | 0.00484 | 0.9959 | 8.826 | 2.936 | 0.9542 |
| Adsorbates | QMIP (mg·g−1) | QNIP (mg·g−1) | IF | α |
|---|---|---|---|---|
| Gossypol | 92.9 | 42.3 | 2.20 | - |
| Ellagic acid | 48.7 | 43 | 1.13 | 1.94 |
| Quercetin | 16.8 | 14 | 1.20 | 1.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, K.; Wang, L.; Zhang, Y.; Jiang, Y.; Zhang, L.; Yasin, A. Influence of Size and Shape of Silica Supports on the Sol–Gel Surface Molecularly Imprinted Polymers for Selective Adsorption of Gossypol. Materials 2018, 11, 777. https://doi.org/10.3390/ma11050777
Zhi K, Wang L, Zhang Y, Jiang Y, Zhang L, Yasin A. Influence of Size and Shape of Silica Supports on the Sol–Gel Surface Molecularly Imprinted Polymers for Selective Adsorption of Gossypol. Materials. 2018; 11(5):777. https://doi.org/10.3390/ma11050777
Chicago/Turabian StyleZhi, Keke, Lulu Wang, Yagang Zhang, Yingfang Jiang, Letao Zhang, and Akram Yasin. 2018. "Influence of Size and Shape of Silica Supports on the Sol–Gel Surface Molecularly Imprinted Polymers for Selective Adsorption of Gossypol" Materials 11, no. 5: 777. https://doi.org/10.3390/ma11050777